Académique Documents
Professionnel Documents
Culture Documents
Penny Density Lab Questions
Transféré par
api-314050160Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Penny Density Lab Questions
Transféré par
api-314050160Droits d'auteur :
Formats disponibles
Joe Palaski, Luc Madonna, Anthony Rivetti
Penny Density Lab Questions
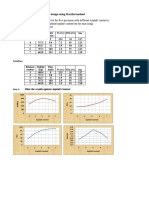
1. For both methods, the best match was iron. However, we know that this is incorrect for
pennies are not made of iron, therefore we made errors in our measurements and
calculations. One of the next best matches was copper though, so our calculations were
too far off.
2. Our densities are slightly different than the densities given on the chart because some of
our measurements were probably off by a little bit. When measuring the height of a stack
of pennies, it was up to the person measuring to determine if the stack was flush against
the zero mark and to determine how long the stack was. Since the stack was so small, it
was easy to make a mistake in measuring them. Also, when dropping the pennies into the
graduated cylinder, some water splashed out which altered the results.
3. This number is somewhat consistent with our data. The density that we measured is about
0.5 mL away from the actual density of the mixture. We think our number is different
because some of our measurements were off causing our calculations to be skewed.
4. Volume is the amount of space an object possesses. When an object is placed into water,
the water is displaced by the object since the object takes up a space causing the water
level to rise. You then can measure the amount of water that rose which is would be the
volume of the object.
5. Based on our data, the density that we measured by using the graduated cylinders is more
accurate. We believe this method was more accurate because the volume was not
calculated with a formula but by water displacement. We think that water displacement is
more accurate to calculate the volume because we did not have to measure the height and
diameter of the pennies, where there could be errors made.
6. We thought that instead of doing the experiment on stacks of pennies that we should just
do it on individual pennies. We had a difficult time keeping all the pennies of one group
with each other. Sometimes the stacks would fall next to each other and we did not which
ones belonged to each group. To fix this, we think that we should only do the experiment
on individual pennies. It would be much more efficient to measure and to keep track of
since there is only one and not a stack of five.
7. Before 1982, pennies were made of mostly about 95% copper and about 5% of zinc.
Pennies made after 1982 are made of a mixture of copper (2.5%) and zinc (97.5%). This
change in material was caused by the skyrocketing price of copper. The mints were
forced to make copper-plated-zinc pennies using less copper and more zinc.
Vous aimerez peut-être aussi
- Lab Write UpDocument3 pagesLab Write Upapi-297153504Pas encore d'évaluation
- Penny Lab Write Up: Procedure For Graduated CylinderDocument3 pagesPenny Lab Write Up: Procedure For Graduated Cylinderapi-310714444Pas encore d'évaluation
- Lab Write Up: 1. TITLE (1 Sentence)Document3 pagesLab Write Up: 1. TITLE (1 Sentence)api-296931788Pas encore d'évaluation
- Sample Lab ReportDocument3 pagesSample Lab Reportmamazookeepr63% (8)
- Pennylabwrite UpDocument2 pagesPennylabwrite Upapi-310727021Pas encore d'évaluation
- Lab Write UpDocument3 pagesLab Write Upapi-310714826Pas encore d'évaluation
- Science Project 1 1Document3 pagesScience Project 1 1api-459203541Pas encore d'évaluation
- Density ExplainedDocument3 pagesDensity Explainedapi-256236481Pas encore d'évaluation
- DensitylabDocument9 pagesDensitylabapi-239229276Pas encore d'évaluation
- Chem LabDocument2 pagesChem LabTrevor73Pas encore d'évaluation
- Lunar Crater ConclusionDocument2 pagesLunar Crater Conclusionapi-67689454Pas encore d'évaluation
- Experiment #6 / Unit 2 Using The Chemical Counting UnitDocument2 pagesExperiment #6 / Unit 2 Using The Chemical Counting Unitapi-368121935Pas encore d'évaluation
- Density LabDocument2 pagesDensity LababdallaaPas encore d'évaluation
- Penny LabDocument3 pagesPenny LabMolly BurnsPas encore d'évaluation
- Independent Practice - Intro UnitDocument1 pageIndependent Practice - Intro UnitCha-Cha Charlene Ynion MariñasPas encore d'évaluation
- Value Picking: A Data-Driven Guide to Inform Silver BuyingD'EverandValue Picking: A Data-Driven Guide to Inform Silver BuyingPas encore d'évaluation
- Density Determination LabDocument10 pagesDensity Determination LabJack MurphyPas encore d'évaluation
- Finding The Density of An Unknown Metal Using Indirect MeasurementsDocument3 pagesFinding The Density of An Unknown Metal Using Indirect MeasurementsDanny PopowskiPas encore d'évaluation
- Ch5 PDFDocument14 pagesCh5 PDFB.Pas encore d'évaluation
- Bronze NeedleDocument6 pagesBronze Needlejan skalaPas encore d'évaluation
- DIY Densimeter (Cek Ketulinan Silver Sendiri)Document6 pagesDIY Densimeter (Cek Ketulinan Silver Sendiri)Nita JebeePas encore d'évaluation
- Sorting Materials Into Groups Class 6 Extra Questions Short Answer Typ1 2Document7 pagesSorting Materials Into Groups Class 6 Extra Questions Short Answer Typ1 2Giridhar RagavasimhanPas encore d'évaluation
- Pow 5Document2 pagesPow 5api-285756441Pas encore d'évaluation
- Pennies LabDocument2 pagesPennies Labtwisted-revelation100% (1)
- Pennies Density LabDocument6 pagesPennies Density LabHope Shelby RangelPas encore d'évaluation
- Lab RepDocument3 pagesLab RepCharles PanuncilloPas encore d'évaluation
- Measurements LabDocument5 pagesMeasurements LabHarrison LeePas encore d'évaluation
- Chain Maille Wire Weaving: How to Make Chain Maille With Affordable Metals and Minimal ToolsD'EverandChain Maille Wire Weaving: How to Make Chain Maille With Affordable Metals and Minimal ToolsPas encore d'évaluation
- DENSITY My Teacher Once Asked Me, "Is Wool Heavier orDocument4 pagesDENSITY My Teacher Once Asked Me, "Is Wool Heavier orChristison AlorciousPas encore d'évaluation
- Is A Fart An Example of Diffusion, Expansion or Contraction?Document12 pagesIs A Fart An Example of Diffusion, Expansion or Contraction?Sharon RumminsPas encore d'évaluation
- Copper and Silver Nitrate Lab WriteDocument4 pagesCopper and Silver Nitrate Lab Writeapi-242497581Pas encore d'évaluation
- Lab Report DensityDocument3 pagesLab Report Densityapi-317559664Pas encore d'évaluation
- Lab Report Metallic Bonding-Matteo Giannetta Good Copy FinalDocument3 pagesLab Report Metallic Bonding-Matteo Giannetta Good Copy FinalMG - 11CJ 972501 Cawthra Park SSPas encore d'évaluation
- DensityDocument38 pagesDensityvectors academyPas encore d'évaluation
- Lab Write Up: 1. TitleDocument3 pagesLab Write Up: 1. Titleapi-297087367Pas encore d'évaluation
- Making Copper Wire Earrings: More Than 150 Wire-Wrapped DesignsD'EverandMaking Copper Wire Earrings: More Than 150 Wire-Wrapped DesignsÉvaluation : 5 sur 5 étoiles5/5 (6)
- Momentum 2Document6 pagesMomentum 2mykz24Pas encore d'évaluation
- Mariana Trujillo Salgado - Lara's (Template) Science Fair HOBDocument13 pagesMariana Trujillo Salgado - Lara's (Template) Science Fair HOBMariana Trujillo SalgadoPas encore d'évaluation
- Lirio - Quiz 2 and 3Document5 pagesLirio - Quiz 2 and 3Lance LirioPas encore d'évaluation
- Chapter 3-Student Reading: 234 Middle School Chemistry UnitDocument8 pagesChapter 3-Student Reading: 234 Middle School Chemistry Unitarpit mishraPas encore d'évaluation
- 3 Lab ReportDocument4 pages3 Lab ReportSasquatchCornPas encore d'évaluation
- Matter in Our SurroundingDocument7 pagesMatter in Our SurroundingVinod MalikPas encore d'évaluation
- CalculatingdensityDocument2 pagesCalculatingdensityapi-293263627Pas encore d'évaluation
- ConclusionsciencepdfDocument2 pagesConclusionsciencepdfapi-169518406Pas encore d'évaluation
- Relating Moles To Coefficients of A Chemical ReactionDocument4 pagesRelating Moles To Coefficients of A Chemical ReactionKhoa NguyenPas encore d'évaluation
- Chemistry Lab Pennies 1982Document5 pagesChemistry Lab Pennies 1982Ferzy11Pas encore d'évaluation
- Basics ICS GenerationDocument3 pagesBasics ICS GenerationJared YairPas encore d'évaluation
- Copper and Silver Nitrate LAbDocument3 pagesCopper and Silver Nitrate LAbyodadsface220% (1)
- Chem Sem 2 Unit 1 LAB PenniesDocument5 pagesChem Sem 2 Unit 1 LAB PenniesCharles Cao100% (1)
- Measuring the mass, volume, and density of a 5-peso coinDocument5 pagesMeasuring the mass, volume, and density of a 5-peso coinRejean Faith MallariPas encore d'évaluation
- Almaw Synthesis of An Oxide of CopperDocument5 pagesAlmaw Synthesis of An Oxide of Copperapi-299270243Pas encore d'évaluation
- Step by Step Wire Jewelry Fall 2009Document39 pagesStep by Step Wire Jewelry Fall 2009Carollyn Gulstone86% (7)
- Gcse Geography Rivers Coursework ExamplesDocument5 pagesGcse Geography Rivers Coursework Examplesmhzkehajd100% (2)
- Almaw Collecting A Precise Amount of CopperDocument5 pagesAlmaw Collecting A Precise Amount of Copperapi-299270243Pas encore d'évaluation
- GenChem.experiments 1 1Document11 pagesGenChem.experiments 1 1michaelangelsantos19Pas encore d'évaluation
- Separating Mixtures LabDocument3 pagesSeparating Mixtures Labapi-305324821Pas encore d'évaluation
- Chemistry Penny LabDocument3 pagesChemistry Penny LabRosie SteinbachPas encore d'évaluation
- SilentcalpresidencyDocument6 pagesSilentcalpresidencyapi-314050160Pas encore d'évaluation
- Luc Madonna Cover LetterDocument1 pageLuc Madonna Cover Letterapi-314050160Pas encore d'évaluation
- Augustine 2Document6 pagesAugustine 2api-314050160Pas encore d'évaluation
- CalvincoolidgepresidencyDocument7 pagesCalvincoolidgepresidencyapi-314050160Pas encore d'évaluation
- Napoleon 3Document5 pagesNapoleon 3api-314050160Pas encore d'évaluation
- EducationDocument2 pagesEducationapi-314050160Pas encore d'évaluation
- FinalannotatedDocument6 pagesFinalannotatedapi-314050160Pas encore d'évaluation
- The South Pole TelescopeDocument9 pagesThe South Pole Telescopeapi-305324400Pas encore d'évaluation
- StanthonymadonnaDocument6 pagesStanthonymadonnaapi-314050160Pas encore d'évaluation
- History EportfolioDocument2 pagesHistory Eportfolioapi-314050160Pas encore d'évaluation
- Napoleon BonaparteDocument9 pagesNapoleon Bonaparteapi-314050160Pas encore d'évaluation
- Gel Electrophoresis LucDocument5 pagesGel Electrophoresis Lucapi-314050160Pas encore d'évaluation
- Heresy NewDocument7 pagesHeresy Newapi-314050160100% (1)
- Songs That Relate To HamletDocument12 pagesSongs That Relate To Hamletapi-31405016033% (9)
- WW 1Document2 pagesWW 1api-314050160Pas encore d'évaluation
- Resume of Deliagonzalez34 - 1Document2 pagesResume of Deliagonzalez34 - 1api-24443855Pas encore d'évaluation
- TheEconomist 2023 04 01Document297 pagesTheEconomist 2023 04 01Sh FPas encore d'évaluation
- 10 1 1 124 9636 PDFDocument11 pages10 1 1 124 9636 PDFBrian FreemanPas encore d'évaluation
- RUJUKANDocument3 pagesRUJUKANMaryTibanPas encore d'évaluation
- SD8B 3 Part3Document159 pagesSD8B 3 Part3dan1_sbPas encore d'évaluation
- Cold Rolled Steel Sections - Specification: Kenya StandardDocument21 pagesCold Rolled Steel Sections - Specification: Kenya StandardPEng. Tech. Alvince KoreroPas encore d'évaluation
- Service and Maintenance Manual: Models 600A 600AJDocument342 pagesService and Maintenance Manual: Models 600A 600AJHari Hara SuthanPas encore d'évaluation
- Leaked David Fry II Conversation Regarding Loopholes and Embezzlement at AFK Gamer LoungeDocument6 pagesLeaked David Fry II Conversation Regarding Loopholes and Embezzlement at AFK Gamer LoungeAnonymous iTNFz0a0Pas encore d'évaluation
- FX15Document32 pagesFX15Jeferson MarceloPas encore d'évaluation
- Towards A Human Resource Development Ontology Combining Competence Management and Technology-Enhanced Workplace LearningDocument21 pagesTowards A Human Resource Development Ontology Combining Competence Management and Technology-Enhanced Workplace LearningTommy SiddiqPas encore d'évaluation
- TOGAF 9 Foundation Part 1 Exam Preparation GuideDocument114 pagesTOGAF 9 Foundation Part 1 Exam Preparation GuideRodrigo Maia100% (3)
- Rohit Patil Black BookDocument19 pagesRohit Patil Black BookNaresh KhutikarPas encore d'évaluation
- NLL - Elementary - Coursebook 2019 PDFDocument24 pagesNLL - Elementary - Coursebook 2019 PDFgilmolto100% (1)
- Sewage Pumping StationDocument35 pagesSewage Pumping StationOrchie DavidPas encore d'évaluation
- Unit-1: Introduction: Question BankDocument12 pagesUnit-1: Introduction: Question BankAmit BharadwajPas encore d'évaluation
- Logic and Set Theory PropositionDocument3 pagesLogic and Set Theory PropositionVince OjedaPas encore d'évaluation
- Udaan: Under The Guidance of Prof - Viswanathan Venkateswaran Submitted By, Benila PaulDocument22 pagesUdaan: Under The Guidance of Prof - Viswanathan Venkateswaran Submitted By, Benila PaulBenila Paul100% (2)
- Marshal HMA Mixture Design ExampleDocument2 pagesMarshal HMA Mixture Design ExampleTewodros TadessePas encore d'évaluation
- Postgraduate Notes in OrthodonticsDocument257 pagesPostgraduate Notes in OrthodonticsSabrina Nitulescu100% (4)
- Unit 1 - Gear Manufacturing ProcessDocument54 pagesUnit 1 - Gear Manufacturing ProcessAkash DivatePas encore d'évaluation
- CMC Ready ReckonerxlsxDocument3 pagesCMC Ready ReckonerxlsxShalaniPas encore d'évaluation
- Instrumentation Positioner PresentationDocument43 pagesInstrumentation Positioner PresentationSangram Patnaik100% (1)
- TDS Sibelite M3000 M4000 M6000 PDFDocument2 pagesTDS Sibelite M3000 M4000 M6000 PDFLe PhongPas encore d'évaluation
- Report Emerging TechnologiesDocument97 pagesReport Emerging Technologiesa10b11Pas encore d'évaluation
- GIS Multi-Criteria Analysis by Ordered Weighted Averaging (OWA) : Toward An Integrated Citrus Management StrategyDocument17 pagesGIS Multi-Criteria Analysis by Ordered Weighted Averaging (OWA) : Toward An Integrated Citrus Management StrategyJames DeanPas encore d'évaluation
- Annamalai International Journal of Business Studies and Research AijbsrDocument2 pagesAnnamalai International Journal of Business Studies and Research AijbsrNisha NishaPas encore d'évaluation
- PointerDocument26 pagesPointerpravin2mPas encore d'évaluation
- SEG Newsletter 65 2006 AprilDocument48 pagesSEG Newsletter 65 2006 AprilMilton Agustin GonzagaPas encore d'évaluation
- Maverick Brochure SMLDocument16 pagesMaverick Brochure SMLmalaoui44Pas encore d'évaluation
- Survey Course OverviewDocument3 pagesSurvey Course OverviewAnil MarsaniPas encore d'évaluation