Académique Documents
Professionnel Documents
Culture Documents
TDR Competency Wheel by WHO
Transféré par
achint8kumarCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
TDR Competency Wheel by WHO
Transféré par
achint8kumarDroits d'auteur :
Formats disponibles
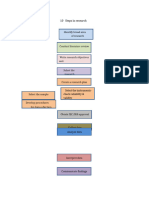
C OMPETENCY W HEEL
TDR Global Competency Framework for Clinical Research
Design & Planning of Research Safeguards
Health-related knowledge Ethics and human subject protection
Research methodology Risk and safety management
Developing a protocol Determining liability and insurance needs
Attracting funding
Quality Assurance
Protocol Operationalization Good Clinical (or other) Practice
Developing study plans and documents Working as per the QMS
Developing the QMS and SOPs Controlling quality of research
Developing the CRF and DMS
Regulations & Governance
Interpretation of Study Results Securing or maintaining approvals
Analysing data Securing or maintaining contracts
Disseminating Governance and
research organisational context
findings Research
regulations
Professional
skills
Cognitive skills
Strategic leadership
Interpersonal skills
Language & communication
Organisational skills
Record-keeping
Computer & IT skills
Work ethic
Oversight
Initiating study Data Flow
Closing study Creating and
Project management maintaining a database
Tracking study progress Collecting accurate data
Data management
Study Communications
Reporting Clinical & Laboratory Operations
Liaising or acting as a link
Providing clinical care
Facilitating or attending meetings
Ensuring appropriate use of IMPs
Staff Management Handling biomedical products
Human resources Performing laboratory assays
Creating or delivering training
Supervising or mentoring Interaction with Public & Participants
Resources Management Engaging with the community
Overseeing essential documents Enrolling and retaining participants
Logistics and facilities management Supporting and advising participants
Finances management throughout the informed consent process
Vous aimerez peut-être aussi
- Grant Management & ExecutionDocument19 pagesGrant Management & ExecutioneeshaPas encore d'évaluation
- Introduction To GHRP - Kuliah1Document17 pagesIntroduction To GHRP - Kuliah1Iik PandaranggaPas encore d'évaluation
- Research Methods MM Digital Business PT - 15012022 Session 1Document67 pagesResearch Methods MM Digital Business PT - 15012022 Session 1nandipha.kunenePas encore d'évaluation
- HealthMinds Corporate Presentation 2021Document11 pagesHealthMinds Corporate Presentation 2021sreejit nairPas encore d'évaluation
- Quality FocusDocument2 pagesQuality FocusGeorge FuryPas encore d'évaluation
- Data Governance Framework at Stony Brook University: ScopeDocument6 pagesData Governance Framework at Stony Brook University: ScopeJonatan Loaiza VegaPas encore d'évaluation
- Assignment 2 (Sandeep Behera-500118226)Document6 pagesAssignment 2 (Sandeep Behera-500118226)sumitjena180Pas encore d'évaluation
- Chapter 5Document42 pagesChapter 5Hanah NavaltaPas encore d'évaluation
- Business Research Methods (BRM) : 2014-2015 Pt-MbaDocument37 pagesBusiness Research Methods (BRM) : 2014-2015 Pt-MbaYash SoniPas encore d'évaluation
- Training Clinical Data Managers A Joint Industry-Academic PerspectiveDocument23 pagesTraining Clinical Data Managers A Joint Industry-Academic PerspectiveUjwal NandekarPas encore d'évaluation
- Pota Yawa GahaDocument6 pagesPota Yawa GahaBrix RamiterrePas encore d'évaluation
- Vaccinology Course Bangkok Jul 2017-For Participants 1 PDFDocument179 pagesVaccinology Course Bangkok Jul 2017-For Participants 1 PDFAnonymous RuVgQLPas encore d'évaluation
- Module Handbook OPS and Logistics 202122 LBS 2 1Document17 pagesModule Handbook OPS and Logistics 202122 LBS 2 1Reeya AgrawalPas encore d'évaluation
- Management StylesDocument11 pagesManagement StylesJanice AmlonPas encore d'évaluation
- Documented+information PPTDocument6 pagesDocumented+information PPTheshari wickramasinghePas encore d'évaluation
- Documents and Records: Presented By: Cardia FourieDocument52 pagesDocuments and Records: Presented By: Cardia FourieWael SaeedPas encore d'évaluation
- 16 P6 LipaDocument22 pages16 P6 LipaMahesh Patil KahatulPas encore d'évaluation
- Research TechnologistDocument1 pageResearch TechnologistjonhiidevPas encore d'évaluation
- Introduction To ResearchDocument46 pagesIntroduction To ResearchSambhavi SinghPas encore d'évaluation
- Process Diagrm 4. 3Document1 pageProcess Diagrm 4. 3Marilou GabayaPas encore d'évaluation
- System BuildingDocument13 pagesSystem BuildingNachyAloysiusPas encore d'évaluation
- Objectives and ResponsibilitiesDocument6 pagesObjectives and Responsibilitieshba hemfaPas encore d'évaluation
- Chapter - 4 - Knowledge ManagementDocument24 pagesChapter - 4 - Knowledge ManagementAshique RasoolPas encore d'évaluation
- Muhammad Farhan Abbasi - ResumeDocument2 pagesMuhammad Farhan Abbasi - ResumeFaari TvPas encore d'évaluation
- Hiv Drug Resistance Training: Standard Operating Procedures (Sops)Document44 pagesHiv Drug Resistance Training: Standard Operating Procedures (Sops)mehrezPas encore d'évaluation
- 3 - Research ProcessDocument8 pages3 - Research Processstephaniecaronan15Pas encore d'évaluation
- DOH JD For Nurse PositionsDocument19 pagesDOH JD For Nurse PositionsHarby Ongbay AbellanosaPas encore d'évaluation
- Powerpoint Presentation1330Document35 pagesPowerpoint Presentation1330Dilini Dakshika HerathPas encore d'évaluation
- Qualitative ResearchDocument5 pagesQualitative ResearchDeviMy RahayuPas encore d'évaluation
- Kuliah Kedua The Marketing Research ProcessDocument5 pagesKuliah Kedua The Marketing Research ProcesscepiPas encore d'évaluation
- Form 1. 2 Evidence of Current Competencies Acquired Related To Job-OccupationDocument2 pagesForm 1. 2 Evidence of Current Competencies Acquired Related To Job-Occupationjayrbayani14Pas encore d'évaluation
- Training Plan Course: Trainer's Methodology I Modality: Check Which Modality Will Be Applied. Multi-Modal Is AllowedDocument3 pagesTraining Plan Course: Trainer's Methodology I Modality: Check Which Modality Will Be Applied. Multi-Modal Is AllowedJean JeanPas encore d'évaluation
- Global Safety Standards: Anglogold AshantiDocument28 pagesGlobal Safety Standards: Anglogold AshantiJurandir GonçalvesPas encore d'évaluation
- Basic Course in Biomedical Research Handbook: March 2021Document89 pagesBasic Course in Biomedical Research Handbook: March 2021Ayush GuptaPas encore d'évaluation
- 2 DirectingDocument5 pages2 DirectingNonito Patrick GaleraPas encore d'évaluation
- Training Need IdentificationDocument10 pagesTraining Need IdentificationAkanshaPas encore d'évaluation
- 1635788393384new Download PDF V4.0Document6 pages1635788393384new Download PDF V4.0Jenny PrestonPas encore d'évaluation
- Craig Mclaughlin CV 2019Document5 pagesCraig Mclaughlin CV 2019api-230605727Pas encore d'évaluation
- Electives Module Pharmacology - 36Document2 pagesElectives Module Pharmacology - 36prasan bhandariPas encore d'évaluation
- Senior Doc Officer RDDocument4 pagesSenior Doc Officer RDKabitallah CompanyPas encore d'évaluation
- Unit 2Document28 pagesUnit 2Souvagya GangulyPas encore d'évaluation
- Research MethodologyDocument28 pagesResearch Methodologypraveer103100% (7)
- Module 2.2 - Concept of EBP PDFDocument24 pagesModule 2.2 - Concept of EBP PDFvincyPas encore d'évaluation
- Advance Research Methodologies and TechniquesDocument72 pagesAdvance Research Methodologies and TechniquesMarvin Sabesaje DaguploPas encore d'évaluation
- The Research Process: Characteristics of A Successful ResearcherDocument10 pagesThe Research Process: Characteristics of A Successful ResearcherCherry Ann Gaspar PequitPas encore d'évaluation
- 21st-Century-Skills-HILOT (Wellness Massage) MOI Basic Competencies 6Document4 pages21st-Century-Skills-HILOT (Wellness Massage) MOI Basic Competencies 6Victor De JesusPas encore d'évaluation
- Introduction To Competency-Based HRDocument19 pagesIntroduction To Competency-Based HREINSTEIN - Stephen Troy C. AlgenteraPas encore d'évaluation
- Background Triads Framework: Jan Clarkson, Craig Ramsay, Linda Young, Paula Elouafkaoui and Heather CassieDocument1 pageBackground Triads Framework: Jan Clarkson, Craig Ramsay, Linda Young, Paula Elouafkaoui and Heather CassieBibek RajPas encore d'évaluation
- Chapter 4Document13 pagesChapter 4delowerPas encore d'évaluation
- Initiating Planning Executing Monitor and Controlling Integration ScopeDocument4 pagesInitiating Planning Executing Monitor and Controlling Integration ScopenmrtpatilPas encore d'évaluation
- Prof. Bachelor Study Programme ''Business Process Management''Document4 pagesProf. Bachelor Study Programme ''Business Process Management''Hooria SajjadPas encore d'évaluation
- The Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerDocument20 pagesThe Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerMohammed HammedPas encore d'évaluation
- Guide To UX Research & Insights PlatformsDocument15 pagesGuide To UX Research & Insights PlatformslairisPas encore d'évaluation
- Steps in ResearchDocument1 pageSteps in Researchamr369Pas encore d'évaluation
- Creating An Effective Monitoring and Evaluation System at Organization LevelDocument23 pagesCreating An Effective Monitoring and Evaluation System at Organization Levelعدنان باحكيمPas encore d'évaluation
- 6CN010 HK Qualitative Data analysis+DS MODSDocument13 pages6CN010 HK Qualitative Data analysis+DS MODSnyw28Pas encore d'évaluation
- Code: CHEM2042 PRIYANKA Name: Priyanka Dattatray Kashid Subject: Chemistry Assignment: 01Document2 pagesCode: CHEM2042 PRIYANKA Name: Priyanka Dattatray Kashid Subject: Chemistry Assignment: 01Priyanka PawarPas encore d'évaluation
- Carriers of Information: A Canadian Approach to Records ManagementD'EverandCarriers of Information: A Canadian Approach to Records ManagementPas encore d'évaluation
- Collaborative Project Procurement ArrangementsD'EverandCollaborative Project Procurement ArrangementsPas encore d'évaluation
- Purba ChamparanDocument6 pagesPurba Champaranachint8kumarPas encore d'évaluation
- The Best Books On Investing - A Five Books InterviewDocument12 pagesThe Best Books On Investing - A Five Books Interviewachint8kumarPas encore d'évaluation
- National Vaccine Policy BookDocument23 pagesNational Vaccine Policy BookbajrangiPas encore d'évaluation
- 8 Privacy Tolls To Stay SecureDocument1 page8 Privacy Tolls To Stay Secureachint8kumarPas encore d'évaluation
- CRISIL Mutual Fund Ranking-Mar-2017Document32 pagesCRISIL Mutual Fund Ranking-Mar-2017achint8kumarPas encore d'évaluation
- What Book Should I Read To Gain Knowledge About Finance, The Stock Market, and Investing Topics - QuoraDocument11 pagesWhat Book Should I Read To Gain Knowledge About Finance, The Stock Market, and Investing Topics - Quoraachint8kumarPas encore d'évaluation
- Quality of Care Brief WashDocument4 pagesQuality of Care Brief Washachint8kumarPas encore d'évaluation
- Generation WarDocument18 pagesGeneration Warachint8kumarPas encore d'évaluation
- Purba ChamparanDocument6 pagesPurba Champaranachint8kumarPas encore d'évaluation
- Lancet Newborn Short PPT REVISED-Oct 7Document33 pagesLancet Newborn Short PPT REVISED-Oct 7achint8kumarPas encore d'évaluation
- Warren Buffetts Not To Do List TemplateDocument2 pagesWarren Buffetts Not To Do List Templateachint8kumarPas encore d'évaluation
- Stocks Most Popular Amongst Mutual FundsDocument14 pagesStocks Most Popular Amongst Mutual Fundsachint8kumarPas encore d'évaluation
- Quality of Care Brief ImplementationDocument8 pagesQuality of Care Brief Implementationachint8kumarPas encore d'évaluation
- Population Census in IndiaDocument38 pagesPopulation Census in Indiaachint8kumarPas encore d'évaluation
- National Vaccine Policy BookDocument23 pagesNational Vaccine Policy BookbajrangiPas encore d'évaluation
- CBD20LDocument1 pageCBD20LFelix StancioiuPas encore d'évaluation
- Presentation 2Document32 pagesPresentation 2Jackie LeePas encore d'évaluation
- Reversible Motors: Additional InformationDocument36 pagesReversible Motors: Additional InformationAung Naing OoPas encore d'évaluation
- Pathfinder House RulesDocument2 pagesPathfinder House RulesilililiilililliliI100% (1)
- 1st Activity in EthicsDocument2 pages1st Activity in EthicsAleiah Jane Valencia AlverioPas encore d'évaluation
- OOAD Documentation (Superstore)Document15 pagesOOAD Documentation (Superstore)Umâir KhanPas encore d'évaluation
- Labor Relations LawsDocument20 pagesLabor Relations LawsREENA ALEKSSANDRA ACOPPas encore d'évaluation
- Contractor Commissioning ProcedureDocument61 pagesContractor Commissioning ProcedureTaras Pompiliu100% (8)
- Cover LetterDocument16 pagesCover LetterAjmal RafiquePas encore d'évaluation
- Universal Declaration of Human RightsDocument36 pagesUniversal Declaration of Human RightsJanine Regalado100% (4)
- National Population PolicyDocument12 pagesNational Population Policymuthukumar100% (3)
- T8 B20 NEADS Trip 2 of 3 FDR - Transcript - NEADS Rome NY - DRM 2 - Dat 2 - PG 1-83 - Color-CodedDocument83 pagesT8 B20 NEADS Trip 2 of 3 FDR - Transcript - NEADS Rome NY - DRM 2 - Dat 2 - PG 1-83 - Color-Coded9/11 Document ArchivePas encore d'évaluation
- 1LG4253-4AA60 Datasheet enDocument1 page1LG4253-4AA60 Datasheet enanm bPas encore d'évaluation
- Why CPVC Pipes FailDocument12 pagesWhy CPVC Pipes FailNikita Kadam100% (1)
- Account Statement 060922 051222Document51 pagesAccount Statement 060922 051222allison squad xXPas encore d'évaluation
- Refill Brand Guidelines 2Document23 pagesRefill Brand Guidelines 2Catalin MihailescuPas encore d'évaluation
- SCH 415 Computer Applications in Chemistry: at The End of This Unit You Should Be Able To General ObjectiveDocument21 pagesSCH 415 Computer Applications in Chemistry: at The End of This Unit You Should Be Able To General ObjectiveFELIX ORATIPas encore d'évaluation
- DSP QBDocument8 pagesDSP QBNithya VijayaPas encore d'évaluation
- Maneesh Misra CV - 1Document3 pagesManeesh Misra CV - 1Rohit KarhadePas encore d'évaluation
- Accounting Graded AssignmentsDocument19 pagesAccounting Graded AssignmentsAnnela EasyPas encore d'évaluation
- PR Status ReportDocument28 pagesPR Status ReportMascheny ZaPas encore d'évaluation
- OIG ReportDocument43 pagesOIG ReportRohan M100% (1)
- US Navy Course NAVEDTRA 14342 - Air Traffic ControllerDocument594 pagesUS Navy Course NAVEDTRA 14342 - Air Traffic ControllerGeorges100% (4)
- Lab Manual: Department of Computer EngineeringDocument65 pagesLab Manual: Department of Computer EngineeringRohitPas encore d'évaluation
- Union of India v. Mohit Minerals Pvt. LTD.-GST Validity AnalysisDocument4 pagesUnion of India v. Mohit Minerals Pvt. LTD.-GST Validity Analysissandhya parimalaPas encore d'évaluation
- Overhead Line SolutionsDocument8 pagesOverhead Line SolutionsDomingo O Chavez PeñaPas encore d'évaluation
- Qualifications and Disqualifications of CandidatesDocument3 pagesQualifications and Disqualifications of CandidatesCARLO JOSE BACTOLPas encore d'évaluation
- N G Ày 2 0 T H Á NG B A N Ă M 2 0 2 0: Brand ManagementDocument10 pagesN G Ày 2 0 T H Á NG B A N Ă M 2 0 2 0: Brand ManagementThịnh NguyễnPas encore d'évaluation
- Valentine Carol Ann Duffy EssayDocument8 pagesValentine Carol Ann Duffy Essayafibybflnwowtr100% (1)
- Glass V Oral Surgeons of Virginia PLLC Vaedce-23-01246 0001.0Document80 pagesGlass V Oral Surgeons of Virginia PLLC Vaedce-23-01246 0001.0Sam OrlandoPas encore d'évaluation