Académique Documents
Professionnel Documents
Culture Documents
PM TB Solutions C09
Transféré par
Vishwajeet UjhoodhaCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
PM TB Solutions C09
Transféré par
Vishwajeet UjhoodhaDroits d'auteur :
Formats disponibles
th
Physics Matters for GCE O Level (4 Edition): Full Solutions to Textbook Questions Chapter 9
Chapter 9 Kinetic Model of Matter
Test Yourself 9.1 & 9.2 (page 158)
1. In solids, the particles (atoms or molecules) are closely packed. In gases, the particles are far
apart. Since density is defined as the mass per unit volume, solids have higher density than gases
as there are many more particles packed closely together per unit volume compared to gases.
2. (a) Brownian motion refers to the observed random and irregular motion of particles in a fluid.
(b) As temperature is increased, the smoke particles will move faster (the movements will
become more vigorous and agitated).
(c) The larger smoke particles would move with slower speeds due to a larger inertia.
Test Yourself 9.3 (page 163)
1. (a) According to the kinetic model of matter, the air molecules in the container are in continuous
motion. When the air molecules hit the walls of the container, they exert forces on the wall.
Since pressure is the average force per unit area, the air molecules exert pressure on the
walls of the container.
(b) As more air is pumped into the tyre, more air molecules are introduced into the tyre. At the
same temperature and volume, there will be more air molecules colliding with the walls of the
tyre. Since the frequency of collision is increased, the average force exerted on the wall per
unit area is also increased and hence the pressure is increased.
2. Heating (increasing the temperature of) a gas results in the gas molecules moving with higher
speeds (possessing higher kinetic energies). If volume does not change, the gas molecules would
collide more violently and frequently with the walls of the container, which would increase the
pressure of the gas. Thus in order to maintain a constant pressure, the gas molecules move further
apart, resulting in an increase in volume.
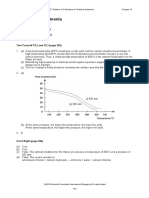
3.
Get It Right (page 165)
(a) True
(b) False
Smoke particles move randomly because air molecules are constantly bombarding them.
(c) True
(d) True
(e) (i) True
(ii) True
(iii) False
The pressure of a fixed mass of gas at constant temperature is inversely proportional to
its volume.
(f) True
2013 Marshall Cavendish International (Singapore) Private Limited
9.1
th
Physics Matters for GCE O Level (4 Edition): Full Solutions to Textbook Questions Chapter 9
Lets Review (pages 165166)
Section A: Multiple-Choice Questions
1. D
Liquid molecules rotate and vibrate randomly within the liquid whereas gas molecules move
randomly at much higher speeds, throughout its container.
2. C
If air temperature is decreased, the air molecules have lower kinetic energies and therefore move
at lower speeds. Since they are moving at lower speeds, they collide less frequently and less
forcefully with the smoke molecules. This causes the movements of the smoke particles to slow
down.
3. B
When a gas molecule hits the wall of a container, it exerts a force on the wall. The average force
on the wall per unit area is the gas pressure on the wall.
4. B
When the gas is heated, the kinetic energy of the gas molecules increase and therefore the
average speed of the molecules increase. They collide more frequently with the walls of the
container and hence, the gas pressure increases. Their average distance apart remains constant
because the number of molecules and the volume containing the molecules remain unchanged.
5. C
Since the number of molecules and the volume containing the molecules remain unchanged, the
number of molecules per unit volume remains unchanged.
6. C
Placing the flask in melting ice cools the gas inside the flask. As the gas is cooled down, the kinetic
energies of the gas molecules decrease and therefore the gas molecules move slower (decreased
speeds). They collide less frequently and less forcefully with the wall of the flask, thus the gas
pressure decreases.
7. D
When the gas is heated, the kinetic energies of the gas molecules increase and the molecules
move faster. The gas molecules collide more frequently and more forcefully with walls of the
container, resulting in an increased outward force. This leads to the expansion of the gas (i.e.
volume of gas increases), and hence the lowering of the gas pressure. The average distance apart
increases because the number of molecules remains constant while the volume increases.
Section B: Structured Questions
1. (a) Microscopic particles (either bright sparks of light in air or tiny particles suspended in liquid)
that are in continuous, random motion.
(b) The particle suspended in the fluid must have an inertia that is small enough to be accelerated
by the forces of collision of the fluid molecules. Since particles with the required small inertias
are too small to be seen by the naked eye, a microscope is needed.
(c) The kinetic model of matter describes matter as being made up of tiny molecules or atoms
that are in continuous motion. The random motion of the microscopic particles shows that the
particles are randomly bombarded, with unequal forces, on all sides by the fluid molecules (in
which the particles are suspended in). Hence, providing evidence that the fluid molecules are
in random and continuous motion.
2013 Marshall Cavendish International (Singapore) Private Limited
9.2
th
Physics Matters for GCE O Level (4 Edition): Full Solutions to Textbook Questions Chapter 9
2. (a) The trapped air molecules in the barrel are in continuous motion and they collide with the
inner wall of the pump. At any one time, there are numerous collisions taking place. As these
molecules collide with the wall, they exert a force on the wall. The average force acting per
unit area is the pressure exerted by the air on the wall. Since the washer is exposed to the
trapped air, like the wall of the pump, it also experiences a pressure by the air molecules.
(b) When the handle is slowly pushed in, the volume of the trapped air is reduced. Since the
mass of the gas is fixed, the number of air molecules per unit volume increases. The air
molecules collide more frequently with the wall (more collisions per unit time), and hence the
force acting per unit area increases. Therefore, the pressure increases.
Section C: Free-Response Questions
1. (a) The molecules in solids vibrate about fixed positions.
The molecules in liquids move about within the liquid.
The molecules in gases move about randomly and at high speeds.
(b) The strength of intermolecular forces between the particles in solids is strong.
The strength of intermolecular forces between the particles in liquids is weak.
The strength of intermolecular forces between the particles in gases is very weak.
(c) In solids, the intermolecular force of the particles is strong. The molecules are bonded closely
together and occupy minimal space. The strong bonds between the molecules limit the
movements of the molecules (they only vibrate about fixed positions) and therefore solids
have fixed volumes and shapes.
In liquids, the intermolecular force of the particles is weak. The molecules are free to move
about within the liquid. This gives liquids fixed volumes but not fixed shapes. The weak bonds
are not able to pull the molecules very close together and therefore liquid occupies a bigger
volume than solid.
In gases, the intermolecular force of the particles is negligible. The molecules are free to move
about at high speeds. Since the molecules move freely, the molecules can occupy any volume
and take any shape. Gases do not have fixed shapes or volumes.
2. (a) The kinetic theory of matter states that the particles that make up matter are in a state of
continuous motion. This means that the air in the tyre consists of many air molecules that are
in continuous motion. Since air is a type of gas, the air molecules in the tyre move freely with
great speeds. The collision of these molecules with the wall of the tyre produces a force on
the wall of the tyre. The force per unit area, exerted by the air molecules in the tyre, is the air
pressure.
(b) The pressure is equal at all points on the inside wall of the tyre because the number of the air
molecules per unit volume is the same throughout the tyre and the air molecules are in
random motion. There is no preferred direction in which the molecules move and therefore
there will be as many molecules colliding with one part of the wall, as there will be for other
parts.
(c) (i) If the temperature is increased, the air molecules in the tyre will gain kinetic energy and
move faster. The frequency of collisions between the molecules and the walls of the tyre
will increase, leading to an increase in pressure.
(ii) If air is pumped into the tyre, while maintaining a constant temperature and volume, the
number of molecules occupying the tyre is increased. This means that the number of
molecules colliding with the wall of the tyre per unit time also increases. This leads to an
increase in pressure.
2013 Marshall Cavendish International (Singapore) Private Limited
9.3
Vous aimerez peut-être aussi
- PM - TB Solutions - C09 PDFDocument3 pagesPM - TB Solutions - C09 PDFVishwajeet Ujhoodha59% (22)
- PM TB Solutions C11Document6 pagesPM TB Solutions C11Vishwajeet Ujhoodha100% (8)
- This Study Resource Was: WavesDocument5 pagesThis Study Resource Was: WavesAhmad Ahsan100% (2)
- Electromagnetic Induction: Test Yourself 22.1 (Page 430)Document7 pagesElectromagnetic Induction: Test Yourself 22.1 (Page 430)Jack KowmanPas encore d'évaluation
- PM TB Solutions C03Document5 pagesPM TB Solutions C03Vishwajeet Ujhoodha100% (5)
- PM TB Solutions C06Document7 pagesPM TB Solutions C06Vishwajeet Ujhoodha88% (8)
- PM TB Solutions C04Document5 pagesPM TB Solutions C04Vishwajeet Ujhoodha75% (4)
- PM TB Solutions C08Document4 pagesPM TB Solutions C08Vishwajeet Ujhoodha80% (5)
- PM TB Solutions C18 PDFDocument10 pagesPM TB Solutions C18 PDFAbdul moiz Waheed100% (5)
- This Study Resource Was: Current ElectricityDocument6 pagesThis Study Resource Was: Current ElectricityAbdul moiz Waheed100% (3)
- Measurement: Test Yourself 1.2 (Page 5)Document5 pagesMeasurement: Test Yourself 1.2 (Page 5)Vishwajeet Ujhoodha92% (12)
- PM TB Solutions C05Document6 pagesPM TB Solutions C05Vishwajeet Ujhoodha82% (11)
- PM TB Solutions C02Document10 pagesPM TB Solutions C02Vishwajeet Ujhoodha86% (14)
- PM TB Solutions C01Document5 pagesPM TB Solutions C01Vishwajeet Ujhoodha100% (2)
- Transfer of Thermal Energy: Test Yourself 10.1 (Page 168)Document5 pagesTransfer of Thermal Energy: Test Yourself 10.1 (Page 168)Jack Kowman100% (8)
- Physics Matters For GCEDocument4 pagesPhysics Matters For GCEno one46% (13)
- Practical Electricity: Test Yourself 19.1 (Page 365)Document5 pagesPractical Electricity: Test Yourself 19.1 (Page 365)Jack Kowman67% (6)
- CM TB Solutions C04Document2 pagesCM TB Solutions C04Robot Ninja67% (3)
- Magnetism: Test Yourself 20.1 (Page 388)Document6 pagesMagnetism: Test Yourself 20.1 (Page 388)Jack Kowman80% (5)
- Physics Matters For GCEDocument4 pagesPhysics Matters For GCEno one56% (18)
- Chemistry Matters Ch11 Textbk ANSDocument3 pagesChemistry Matters Ch11 Textbk ANSZeneon82% (17)
- The Mole: Test Yourself 9.1 and 9.2 (Page 139)Document10 pagesThe Mole: Test Yourself 9.1 and 9.2 (Page 139)Ahmad Ahsan40% (5)
- CM TB Solutions C06Document3 pagesCM TB Solutions C06Nisha75% (8)
- Chemistry Matters Ch04 Textbk ANSDocument2 pagesChemistry Matters Ch04 Textbk ANSZeneon67% (21)
- CM TB Solutions C01Document3 pagesCM TB Solutions C01MahamIsmail86% (7)
- Metals: Test Yourself 14.1 (Page 250)Document4 pagesMetals: Test Yourself 14.1 (Page 250)Jack Kowman100% (2)
- Chemistry Matters Ch06 Textbk ANSDocument2 pagesChemistry Matters Ch06 Textbk ANSZeneon79% (14)
- Chemistry Matters Ch16 Textbk ANSDocument2 pagesChemistry Matters Ch16 Textbk ANSZeneon63% (8)
- Ionic Bonding: Test Yourself 6.1 and 6.2 (Page 95) Number of Protons Number of Neutrons Number of ElectronsDocument2 pagesIonic Bonding: Test Yourself 6.1 and 6.2 (Page 95) Number of Protons Number of Neutrons Number of Electronskhalil rehman0% (1)
- Writing Chemical Equations: Test Yourself 8.1 (Page 130)Document1 pageWriting Chemical Equations: Test Yourself 8.1 (Page 130)khalil rehman100% (2)
- Writing Chemical Equations: Test Yourself 8.1 (Page 130)Document1 pageWriting Chemical Equations: Test Yourself 8.1 (Page 130)Zeeshan MunirPas encore d'évaluation
- Chemistry Matters Ch05 Textbk ANSDocument3 pagesChemistry Matters Ch05 Textbk ANSZeneon67% (12)
- An Introduction To Organic Chemistry: Test Yourself 21.1 (Page 414)Document3 pagesAn Introduction To Organic Chemistry: Test Yourself 21.1 (Page 414)Jack Kowman75% (8)
- Ammonia: Test Yourself 19.1 (Page 381)Document4 pagesAmmonia: Test Yourself 19.1 (Page 381)Jack Kowman100% (3)
- Chemistry Matters Ch01 Textbk ANSDocument3 pagesChemistry Matters Ch01 Textbk ANSZeneon85% (26)
- Chemistry Matters Ch07 Textbk ANSDocument4 pagesChemistry Matters Ch07 Textbk ANSZeneon67% (12)
- Chemistry Matters Ch13 Textbk ANSDocument3 pagesChemistry Matters Ch13 Textbk ANSZeneon50% (6)
- Speed of Reaction: Test Yourself 18.1 and 18.2 (Page 355)Document5 pagesSpeed of Reaction: Test Yourself 18.1 and 18.2 (Page 355)Jack Kowman25% (4)
- Chemical Calculations: Mass of Cucl .2H O Molar Mass of Cucl .2H O 3.42 64 + (2 ! 35.5) + (2 ! 18)Document5 pagesChemical Calculations: Mass of Cucl .2H O Molar Mass of Cucl .2H O 3.42 64 + (2 ! 35.5) + (2 ! 18)khalil rehmanPas encore d'évaluation
- Ecology: Test Yourself 21.1 (Page 405)Document4 pagesEcology: Test Yourself 21.1 (Page 405)leePas encore d'évaluation
- Pure Bio CH 14 Textbook Answers PDFDocument2 pagesPure Bio CH 14 Textbook Answers PDFlee0% (1)
- Alkanes and Alkenes: Test Yourself 22.1 (Page 429)Document7 pagesAlkanes and Alkenes: Test Yourself 22.1 (Page 429)Jack Kowman100% (1)
- Chemistry Matters Ch17 Textbk ANSDocument3 pagesChemistry Matters Ch17 Textbk ANSZeneon100% (3)
- Excretion in Humans: Test Yourself 11.1 (Page 223)Document2 pagesExcretion in Humans: Test Yourself 11.1 (Page 223)leePas encore d'évaluation
- The Mole: Test Yourself 9.1 and 9.2 (Page 139)Document8 pagesThe Mole: Test Yourself 9.1 and 9.2 (Page 139)Abdul moiz Waheed82% (11)
- CM TB Answers C17Document3 pagesCM TB Answers C17khalil rehman100% (1)
- Pure Bio CH 2 Textbook Answers PDFDocument2 pagesPure Bio CH 2 Textbook Answers PDFno one100% (3)
- Alcohols and Carboxylic Acids: Test Yourself 23.1 and 23.2 (Page 453)Document2 pagesAlcohols and Carboxylic Acids: Test Yourself 23.1 and 23.2 (Page 453)khalil rehman40% (5)
- Chemistry Matters Textbook Answers Chapter 3Document3 pagesChemistry Matters Textbook Answers Chapter 3MahamIsmail93% (15)
- Hormones: Test Yourself 15.1 (Page 287)Document3 pagesHormones: Test Yourself 15.1 (Page 287)leePas encore d'évaluation
- Homeostasis: Test Yourself 12.1 (Page 246)Document3 pagesHomeostasis: Test Yourself 12.1 (Page 246)lee100% (1)
- Gce o Level Physics MatterDocument8 pagesGce o Level Physics MatterBakhita Maryam100% (1)
- Chemistry Matters Ch19 Textbk ANSDocument3 pagesChemistry Matters Ch19 Textbk ANSZeneon100% (1)
- O Level Physics Unit 9: Kinetic Model of MatterDocument2 pagesO Level Physics Unit 9: Kinetic Model of MatterMc MurdoPas encore d'évaluation
- Latest Copy of Class 8 Physics Question Bank 1Document101 pagesLatest Copy of Class 8 Physics Question Bank 1KAMLESH PATIDARPas encore d'évaluation
- Answers To End-Of-Chapter Questions For Chapter 4, MoleculesDocument2 pagesAnswers To End-Of-Chapter Questions For Chapter 4, MoleculessarabPas encore d'évaluation
- Kinetic Model of MatterDocument6 pagesKinetic Model of MatterMayraaj KhaanamPas encore d'évaluation
- KMT & Gas Laws CompleteDocument47 pagesKMT & Gas Laws Completepenelope.ePas encore d'évaluation
- Kinetic Theory ProjectDocument11 pagesKinetic Theory Projectlavanya rajaPas encore d'évaluation
- KMT ActivityDocument2 pagesKMT Activitysrd9g6jj58Pas encore d'évaluation
- Building Control (Mandatory Guarantees) Regulations 2019Document2 pagesBuilding Control (Mandatory Guarantees) Regulations 2019Vishwajeet UjhoodhaPas encore d'évaluation
- The Non-Citizens (Property Restriction) (Amendment) Act 2016Document2 pagesThe Non-Citizens (Property Restriction) (Amendment) Act 2016Vishwajeet UjhoodhaPas encore d'évaluation
- 3-SJC Crane Study - Heights Above Buidling Limits (FT AGL) - 20210305Document1 page3-SJC Crane Study - Heights Above Buidling Limits (FT AGL) - 20210305Vishwajeet UjhoodhaPas encore d'évaluation
- Rda ActDocument5 pagesRda ActVishwajeet UjhoodhaPas encore d'évaluation
- National Savings Fund (Collection of Contributions) (Amendment) Regulations 2015Document3 pagesNational Savings Fund (Collection of Contributions) (Amendment) Regulations 2015Vishwajeet UjhoodhaPas encore d'évaluation
- API Provisional Explanatory NotesDocument5 pagesAPI Provisional Explanatory NotesVishwajeet UjhoodhaPas encore d'évaluation
- Membership Assessment Requirements OverviewDocument22 pagesMembership Assessment Requirements OverviewVishwajeet UjhoodhaPas encore d'évaluation
- IVSC Global Regulatory Convergence and The Valuation Profession May 2014Document6 pagesIVSC Global Regulatory Convergence and The Valuation Profession May 2014Vishwajeet UjhoodhaPas encore d'évaluation
- Xtreme Papers A Level MathematicsDocument3 pagesXtreme Papers A Level MathematicsVishwajeet Ujhoodha50% (2)
- Bh113 Bachelor of Applied Science Property and Valuation Honours Course BrochureDocument6 pagesBh113 Bachelor of Applied Science Property and Valuation Honours Course BrochureVishwajeet UjhoodhaPas encore d'évaluation
- CEM Programme Specification MSC Real EstateDocument13 pagesCEM Programme Specification MSC Real EstateVishwajeet UjhoodhaPas encore d'évaluation
- Investment Promotion (Invest Hotel Scheme) Regulations 2015Document10 pagesInvestment Promotion (Invest Hotel Scheme) Regulations 2015Vishwajeet UjhoodhaPas encore d'évaluation
- Membership Assessment Requirements Overview PDFDocument1 pageMembership Assessment Requirements Overview PDFVishwajeet UjhoodhaPas encore d'évaluation
- Enrolment and Access To International Valuation Standards 2017 ModuleDocument1 pageEnrolment and Access To International Valuation Standards 2017 ModuleVishwajeet UjhoodhaPas encore d'évaluation
- KP VCH 8325012017043855Document5 pagesKP VCH 8325012017043855Vishwajeet UjhoodhaPas encore d'évaluation
- Z Coq DC 025012017045708Document5 pagesZ Coq DC 025012017045708Vishwajeet UjhoodhaPas encore d'évaluation
- The Co-Operatives Act 2016Document88 pagesThe Co-Operatives Act 2016Vishwajeet UjhoodhaPas encore d'évaluation
- Ed NZ 61325012017044849Document2 pagesEd NZ 61325012017044849Vishwajeet UjhoodhaPas encore d'évaluation
- Roads Act Amendments 2015Document4 pagesRoads Act Amendments 2015Vishwajeet UjhoodhaPas encore d'évaluation
- Legal Supplement: The Construction Industry Development Board (Amendment) ActDocument14 pagesLegal Supplement: The Construction Industry Development Board (Amendment) ActVishwajeet UjhoodhaPas encore d'évaluation
- Roads Act Amendments 2015Document4 pagesRoads Act Amendments 2015Vishwajeet UjhoodhaPas encore d'évaluation
- Land (Duties and Taxes) (Amendment of Schedule) Regulations 2016Document2 pagesLand (Duties and Taxes) (Amendment of Schedule) Regulations 2016Vishwajeet UjhoodhaPas encore d'évaluation
- Increase Mauritius National Pensions 2015 RegulationsDocument2 pagesIncrease Mauritius National Pensions 2015 RegulationsVishwajeet UjhoodhaPas encore d'évaluation
- Access For Appliances and Fire-Fighting Facilities For Fire & Rescue Service UseDocument9 pagesAccess For Appliances and Fire-Fighting Facilities For Fire & Rescue Service UserowatersPas encore d'évaluation
- 160117-Smart City TrianonDocument5 pages160117-Smart City TrianonVishwajeet UjhoodhaPas encore d'évaluation
- Les AigrettesDocument42 pagesLes AigrettesVishwajeet UjhoodhaPas encore d'évaluation
- Les AigrettesDocument14 pagesLes AigrettesVishwajeet UjhoodhaPas encore d'évaluation
- Les AigrettesDocument14 pagesLes AigrettesVishwajeet UjhoodhaPas encore d'évaluation
- 160117-Smart City TrianonDocument5 pages160117-Smart City TrianonVishwajeet UjhoodhaPas encore d'évaluation
- Blup SavanneDocument3 pagesBlup SavanneVishwajeet UjhoodhaPas encore d'évaluation
- En458 PDFDocument1 168 pagesEn458 PDFpantocrat0r100% (1)
- About Meat Eating in Sikh DharmDocument4 pagesAbout Meat Eating in Sikh Dharmvijay123inPas encore d'évaluation
- LAB 2 SimulationDocument6 pagesLAB 2 SimulationArti Stic100% (1)
- TR Massage Therapy NC IIDocument162 pagesTR Massage Therapy NC IIAljon Fortaleza Balanag100% (4)
- ISO/IEC 20000 Lead Implementer Course (5 Days)Document3 pagesISO/IEC 20000 Lead Implementer Course (5 Days)rohitbanerjeePas encore d'évaluation
- Concrete Containing Fungal Treated Waste Foundry SandDocument6 pagesConcrete Containing Fungal Treated Waste Foundry SandFábio FriolPas encore d'évaluation
- Daily Aspirin Therapy Understand The Benefits and RisksDocument5 pagesDaily Aspirin Therapy Understand The Benefits and RisksNicholas OwensPas encore d'évaluation
- SSC 146G Summer 2016 Human Sexuality SyllabusDocument1 pageSSC 146G Summer 2016 Human Sexuality SyllabusJames SmithPas encore d'évaluation
- Minutes: Motion Was Submitted For ResolutionDocument29 pagesMinutes: Motion Was Submitted For Resolutionayen cusiPas encore d'évaluation
- Radio Journalism GuideDocument13 pagesRadio Journalism GuideMark June B. GonzagaPas encore d'évaluation
- The Transformation of PhysicsDocument10 pagesThe Transformation of PhysicsVíctor Manuel Hernández MárquezPas encore d'évaluation
- Ideation For Product Innovation What Are The BestDocument9 pagesIdeation For Product Innovation What Are The BestLIVIA MARILIA CHIARIPas encore d'évaluation
- Chapter 8 SQL Complex QueriesDocument51 pagesChapter 8 SQL Complex QueriesJiawei TanPas encore d'évaluation
- Tiger Rising Teachers' GuideDocument6 pagesTiger Rising Teachers' GuideCandlewick Press50% (2)
- Nokia CaseDocument28 pagesNokia CaseErykah Faith PerezPas encore d'évaluation
- Supplement BDocument65 pagesSupplement BAdnan AsifPas encore d'évaluation
- Intro - New Covenant TheologyDocument15 pagesIntro - New Covenant TheologyDavid SalazarPas encore d'évaluation
- Advertisement On Sunflowers Perfume by Elizabeth ArdenDocument18 pagesAdvertisement On Sunflowers Perfume by Elizabeth ArdenNur Fajarwati ZuchrifahPas encore d'évaluation
- Perceptiual - Cognitive SkillDocument17 pagesPerceptiual - Cognitive SkillGeovani AkbarPas encore d'évaluation
- Vaishali Ancient City Archaeological SiteDocument31 pagesVaishali Ancient City Archaeological SiteVipul RajputPas encore d'évaluation
- Phronesis Volume 7 Issue 1 1962 (Doi 10.2307/4181698) John Malcolm - The Line and The CaveDocument9 pagesPhronesis Volume 7 Issue 1 1962 (Doi 10.2307/4181698) John Malcolm - The Line and The CaveNițceValiPas encore d'évaluation
- Introduction To Logic Syllogisms-1: Class ExerciseDocument6 pagesIntroduction To Logic Syllogisms-1: Class ExercisePriyanshu PrakashPas encore d'évaluation
- The CIA Tavistock Institute and The GlobalDocument34 pagesThe CIA Tavistock Institute and The GlobalAnton Crellen100% (4)
- Defence TC 15Document30 pagesDefence TC 15Simran AroraPas encore d'évaluation
- Writing Statement of PurposeDocument1 pageWriting Statement of PurposeArunmani Arunachalam100% (1)
- SPE-21639-MSDocument8 pagesSPE-21639-MSehsanPas encore d'évaluation
- Unit 8Document2 pagesUnit 8The Four QueensPas encore d'évaluation
- Yu-Gi-Oh Nightmare Troubadour InfoDocument12 pagesYu-Gi-Oh Nightmare Troubadour InfoBrandon Bradley0% (1)
- Magnolia Residences Tower D Promo - 20% Downpayment OptionDocument1 pageMagnolia Residences Tower D Promo - 20% Downpayment OptionLiv ValdezPas encore d'évaluation