Académique Documents
Professionnel Documents
Culture Documents
Week 1 Normal Cardiac Structure and Function - Odt
Transféré par
James Park0 évaluation0% ont trouvé ce document utile (0 vote)
8 vues9 pagesTitre original
Week 1 Normal Cardiac Structure and Function.odt
Copyright
© © All Rights Reserved
Formats disponibles
ODT, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme ODT, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
8 vues9 pagesWeek 1 Normal Cardiac Structure and Function - Odt
Transféré par
James ParkDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme ODT, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 9
-Finish notes
Normal Cardiac Structure and Function
Cardiac Anatomy and Histology
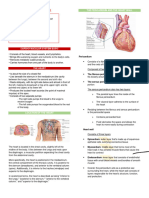
Pericardium
Heart and roots of the great vessels are enclosed by fibroserous sac called the pericardium
Consists of two layers: stronger outer fibrous layer and inner serous layer
Inner serous layer adheres to external wall of heart and is called the visceral pericardium
The visceral pericardium refects back on itself and lines the outer fibrous layer, forming the
parietal pericardium
Space between visceral and parietal layers contains a thin film of pericardial fluid that allows
heart to beat in a minimal friction environment
Pericardium is attached to the sternum and the mediastinal portions of the right and left pleurae
Its many connections to the surrounding structures keep the pericardial sac firmly anchored within the
htorax and thereby help to maintain the heart in its normal position.
Emanating from the pericardium superiorly are the aorta, the pulmonary artery, and the superior vena cava
The inferior vena cava projects through the pericardium inferiorly
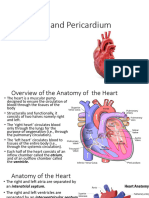
Surface Anatomy of the Heart
Heart is shaped like a cone and has four muscular chambers
Right and left ventricles are the main pumping chambers
Less muscular right and left atria deliver blood to their respective ventricles
Apex
Formed by the tip of the left ventricle, points inferiorly, anteriorly, and tot he left
Base/Posterior surface of the heart
Formed by atria, mainly the left, and lies between the lung hila
Anterior surface of the heart is shaped by the right atrium and ventricle.
Because the left atrium and ventricle lie more posteriorly, they form only a small strip of this anterior
surface
The inferior surface of the heart is formed by both ventricles, primarily the left
lies along the diaphragm; also referred to as the diaphragmatic surface
From an anteroposterior view (i.e. chest radiograph), four borders of the heart are apparent
Right border established by right atrium and almost in line with the superior and inferior venae cavae
Inferior border is nearly horizontal and is formed mainly by right ventricle, with a slight contribution
from left ventricle near the apex
Left ventricle and a portion of the left atrium make up the left border of the heart, whereas the
superior border is shaped by both atria
From this description, two basic rules of normal cardiac anatomy
1) right sided structures lie mostly anterior to their left-sided counterparts
2) Atrial chambers are located mostly to the right of their corresponding ventricles
Internal Structure of the Heart
Four major valves direct blood flow in a forward direction and prevent backward leakage
atrioventricular (AV) valves (tricuspid and mitral) separate the atria and ventricles, whereas the
semilunar valves (pulmonic and aortic) separate the ventricles from the great arteries.
All four heart valves are attached to fibrous cardiac skeleton, which is composed of dense
connective tissue.
Cardiac skeleton also serves as a site of attachment for the ventricular and atrial muscles
Figure 1.3: The four heart valves viewed from above with atria removed
Tricuspid and mitral valves are open and the semilunar valves (pulmonic and aortic) are closed
Each annulus fibrosus surrounding the mitral and tricuspid valves is thicker tha those
surroundign the pumonic and aortic valves
All four contribute to heart fibrous skeleton, which is composed of dense connective tissue.
Surface of heart valves and interior surface of the chambers are lined by a single layer of endothelial cells
aka endocardium.
Subendocardial tissue contains fibroblasts, elastic, and collagenous fibers, veins, nerves, and branches
of the conducting system
Continuous with the connective tissue of the heart muscle layer, the myocardium.
Myocardium is the thickest layer of the heart and has bundles of cardiac muscle cells
External to myocardium is a layer of connective tissue and adipose tissue through which pass the
larger blood vessels and nerves that supply the heart muscle
Epicardium is the outermost layer of the heart and is identical to, and just another term for, visceral
pericardium.
Right atrium and Ventricles
Superior and inferior venae cavae and coronoary sinus open into right atrium
Venae cavae return deoxygenated blood from systemic veins into the right atrium, whereas the
coronary sinus carries venous return from the coronary arteries.
Interatrial septum forms posterormedial wall of right atrium and separates it from th left atrium
Tricuspid valve is located in the floor of the atrium and opens into right ventricle
Right ventricle is roughly triangular in shape, and its superior aspect form a cone-shaped outflow ract,
which leads to the pulmonary artery.
Although the inner wall of the outflow tract is smooth, the rest of the ventricle is covered by a
number of irregular bridges (trabeculae carneae) that give right ventricular wall a spongelike
apperance.
A large trabecula that crosses the ventricular cavity is called the moderator band.
It carries a component of the right bundle branch of the conducting system to the
ventricular muscle.
Right ventricle contains three papillary muscles, which project into the chamber and via their
thin, stringlike chordae tendineae attach to the edges of the tricuspid valve leaflets.
The leaflets are attached to fibrous ring that supports the valve between the right atrium and
ventricles
Contraction of the ppapillary muscles prior to other regions of the ventricle tightens the
chordae tendineae, helping to align and restrain the leaflets of the tricuspid valve as they are
forced closed.
This prevents blood from regurgitating into right atrium during ventricular contraction.
At apex of right ventricular outflow tract is the pulmonic valve, which leads to the
pulmonary artery.
This valve consists of three cusps attached to a fibrous ring.
During relaxation of ventricle, elastic recoil of the pulmonary arteries forces blood back
torward the heart, distending the valve cusps toward one another.
This action closes the pulmonic valve and prevents regurigation of blood back into
right ventricle
Left Atrium and Ventricle
Four pulmonary veins enter the posterior half of left atrium
Wall of left atrium is ~ 2mm thick, slightly greater than that of the right atrium.
Mitral valve opens into left ventricle through inferior wall of the left atrium
Cavity of left ventricle is approximately cone shaped and longer than right ventricle's.
In healthy adult heart, wall thickness is 9 to 11 mm, ~ three times that of the right ventricle.
Aortic vestibulte is smooth walled part of left ventricular cavity, located inferior to the aortic valve.
Inferior to this region, most of the ventricle is covered by trabeculae carneae, which are finer and
more numerous than those in right ventricle.
Left ventricular chamber has two large papillary muscles
Larger than counterparts in right ventricle; their chordae tendineae thicker but less numerous
Chordae tendineae of each papillary muscle distribute to both leaflets of mitral valve.
Tensing of chordae tendineae during left ventricular contraciton helps restrain and align the
mitral leaflets, enabling them to close properly and preventing backflow.
Aortic valve sparates the left ventricle from aorta
Surrounding aortic valve opening is fibrous right, which has three cusps of the valve attached to
it.
Above right and left aortic valve cusps in the aortic wall are origins of the right and left coronary
arteries
Interventricular Septum
Thick wall between left and right ventricles.
Has muscular and membranous part
Margins of septum can be traced on surface of the heart by following the anterior and psoterior
interventricular grooves
Hydrostatic pressure of left ventricles causes muscular portion of septum to bulge toward right
ventricle.
Small, oval shaped membranous part of septum is thin and located inferior to cusps of aortic
valve.
Deoxygenated blood is delivered to heart through inferior and super venae cavae right atrium
tricuspid valve orifice right ventricle--> pulmonic valve pumonary artery and lungs (CO2
released/oxygen absorbed) oxygen rich blood returns to heart through pulmonary veins left
atrium mitral valve left ventricle aortic valve aorta distributed to all other tissues of
the body.
Impulse-Conducting System
Main components of cardiac conduction system: sinoatrial node, atrioventricular node, bundle of His,
right and left bundle branches, Purkinje fibers. Moderator band carries large portion of right bundle (IV,
interventricular)l
Impulse conducting system consists of specialized cells that initiate the heart beat and electrically
coordinate contractions of the heart chambers.
SA node is a small mass of specialized cardiac muscle fibers in wall of right atrium (located right of the
superior vena cava entrance and normally initiates the electricl impulse for contraction)
AV node is beneat the endocardium in inferiorposterior part of interatrial septum
Bundle of His is distal to AV node
perforates the interventricular septum posteriorly.
Within septum, bundle of His bifurcates into a compact, cablelike structure on the right side, known
as right bundle branch, and a broad sheet of fibers that continues over the left side of the septum, the
left bundle branch.
Right bundle branch is thick and deeply buried in muscle of interventricular septum and
continues toward apex.
Near junction of interventricular septum and anterior wall of right ventricle, right bundle branch
becomes subendocardial and birfurcates
One branch travels across right ventricular cavity in the moderator band,
Other branch continues toward tip of ventricle.
These branches evntually arborize into a finely divided anastomosing plexus that travels
throughout right ventricle.
Functionally, left bundle branch is divided into anterior and posterior fasciele and a small branch to
septum.
Anterior fascicle runs anteriorly toward apex, forming a subendocardial plexus in the area of anterior
papillary muscle.
Posterior fascicle travels to the area of the posterior papillary muscle
It then divides into a subendocardial pleux and spreads to the rest of the left ventricle
Subendocardial plexuses of both ventricles send distributing Purkinje fibers to the ventricular muscle.
Impulses within the His-Purkinje system are transmitted first to the papillary muscles and then
throughout the walls of the ventricles, allowing papillary muscle contraction to precede that of the
ventricles.
This coordination prevents regurgitation of blood flow through the AV valves.
Cardiac Innervation
Heart is innervated by both parasympathetic and sympathetic afferent and efferent nerves
Preganglioninc sympathetic neurons, with cell bodies located within upper five to six thoracic levels of
spinal cord, synapse with second-order neurons in the cervical sympathetic ganglia.
Traveling within cardiac nerves, these fibers terminate in the heart and great vessels.
Preganglioninc parasympathetic fibers orginiate in dorsal motor nucleus of medulla and pass as branches
of the vagus nerve to the heart and great vessels.
Here, fibers synapse with second-order neurons located in ganglia within these strutures.
Rich supply of vagal afferents from inferior and posterior aspects of venctricles mediates important
cardiac reflexes, whereas the abundant efferent fibers to the SA and AV nodes are active in
modulating electrical impulse intiation and conduction
Cardiac Vessels
consist of coronary arteries and veins and the lymphatics
Largest components of these structuers lie within the loose connective tissue in the epicardial fat.
Coronary arteries
Heart muscle is supplied with oxygen and nutrients by right and left coronary arteries, which arise
from root of aorta just above aortic valve cusps
After origin, they pass anteriorly, one on each side of the pulmonary artery.
Large left main cornary artery passes between left atrium and pulmonary trunk to reach AV groove.
It then divdes into left anterior descending (LAD) coronary artery and the circumflex artery.
LAD travels within anterior interventricular groove toward cardiac apex.
During its descent on anterior surface, the LAD gives off septal branches that supply the anterior
two thirds of the interventricular septum and the apical portion of the anterior papillary muscle
LAD also gives off diagnoal branhces that supply the anterior surface of left ventricle
Circumflex artery continues within left AV groove and passes around left order of heart to reach
the posterior surface
Gives off large obtuse marginal branches that supply the laterla and posterior wall of left
ventricle's
Right coronary artery (RCA) travels in right AV groove, passing posteriorly between right atrium and
ventricle. (perfuses the right venttricle and variable portions of the posterior left ventricle through its
terminal branches)
Posterior descending artery arises from RCA
Supplies blood to right ventricle via acute marginal branhces.
In most people, the distal RCA gives rise to a large branch, the posterior descending artery.
Travels from inferoposterior aspect of heart tot he apex
supplies blood to the inferior an dposterior walls of the ventricles and the posterior one third
of the interventricular septum.
Before posterior descnedin gbrnach, RCA gives off AV nodal artery.
This vessel travels from inferoposterior aspect of heart to the apex and suppleis blood to inferior
and posterior walls of the ventricles and the poterior one third of the interventricular septum
Just before giving off posterior descneding branch, RCA usually gives off the AV nodal artery.
Left main Coronary artery splits into circumfex artery and the anterior descneding artery
Anterior descending artery: perfuses left ventricle anterior wall (anterior portion of the
intraventricular septum and a portion of the anterior right ventricular wall)
Circumflex artery: Perfuses the lateral and psoterior regions of the left ventricle
Posterior descending and AV nodal arteries come from Right coronary artery in 85% of population
Right dominant population
8% posterior descending artery comes from circumfex artery instead
Left dominant circulation
Remaining 7%, posterior blood supply comes from both RCA and circumflex
Codominant circulation
Blood supply to SA node (70% of time) comes from RCA
25% of normal hearts, SA nodal artery comes from circu flex artery
5% of cases, both RCA and circumflex artery contribute to SA nodal artery
From epicardial locations, coronary arteries send perforating branches into ventricular muscle (richly
vascularizes all chambers
Capillaries arise from this that surround each cardiac muscle fiber
Thesbesian veins (tiny vascular channels) or directly from terminal branches of coronary arteries
supply muscle fibers just beneath endocardium (mostly papillary muscles and thick left ventricle)
Collateral connections (less than 200 micrometer in diameter) exist at subarteriolar level between
coronary arteries
Become larger and visible in atheroscleorotic disease
Coronary Veins
Return blood from myocardial capillaries to right atrium via coronary sinus.
Major veins lie in epicardial fat (superficial to arterial counterparts)
Thesbesian veins provide alternative route for small amount of blood return to cardiac chambers.
Lymphatic Vessels
Heart lymph drained by vessels in subendocardial connective tissue of all four chambers.
Drains into epicardial plexus (larger lymphatic vessels derive from this...follow coronary arteries
and veins).
Each larger vessels combines in AV groove to form single lymphatic conduit -->exits heart
mediastinal lymphatic plexus thoracic duct.
Histology of Ventricular Myocardial cells
Myocyte = Mature myocardial cell ~ 25 micrometer in diameter; 100 micrometer in length
Cross striated banding pattern like skeletal muscle
Unlike skeletal muscle, contain only one or two centrally located nuclei
Surrounded by connective tissue with rich capillary network
Myocardial cells contain myofibrils: long chains of individual sarcomeres (fundamental contractile units
of cell)
Sarcomere: two groups of overlapping filaments of contractile proteins
Myofilaments of myofibrils biochemically/physically interact muscle contraction
Sarcomere produce cross-striated bandign pattern
Density indicates concentration of contractile proteins
Sarcomere length: Z to Z distance ~ 2.2 and 1.5 micrometers during cardiac cycle
Larger dimension: ventricular filling
Smaller dimension: ventricular contraction
Figure 1.8 Myocardial cell
Multiple parallel myofibrils surrounded by mitochondria
T-tubules (invaginations of cell membrane the sarcolemme) increases surface area for ion transport
and transmission of electrical impulses
Intracellular SR has most of calcium, abuts the T tubules
Sarcomeres Myofibril = serially connected sarcomeres extend from one Z line to next
Composed of alternating (thin actin) and thick myosin myofilaments
Titin tetheres myosin to Z line and provides elasticity
Sarcolemma: myocardial cell membrane
Specialized area in membrane: intercalated disk
-distinct characteristic of cardiac muscle tissue
Seen as dark staining transverse lines that cross chains of cardiac cells at irregular intervals
represent gap junctions between cells; establishes structural and electrical continuity between
myocardial cells.
Transverse tubular system
another functional feature of cell membrane of myocardial cell
Deep fingerlike invaginations of sarcolemma
Also establish pathways for rapid transmission of electrical signals for contractions
Increases surface area of sarcolemma in contact with extracellular environment (allows
transmembrane ion transport that occurs with excitationa nd relaxation to occur quickly and in
sync).
SR (Sarcoplasmic reiculum
tubular membrane network complements T tubule system
SR abuts T tubules at right angles in lateral sacs (terminal cisternae)
terminal cisternae store calcium; release of stores links membrane excitation with
activation of contractile apparatus
Also abut lateral sacs and sarcolemma
35% of myocardial volume is mitochondria in order to sufficiently power heart cells.
Basic Electrophysiology
3 electrophysiologic types
Pacemaker cells (SA and AV node)
Ventricular and Atrial muscle cells
Specialized rapidly conducting tissues (Purkinje fibers)
Sarcolemma (membrane) of these three electrophysiologic types: phospholipid bilayer (largely
impermeable to ions)
Membrane proteins (ion channels, passive cotransporters, active transporters) = maintain voltage
difference.
Ca++ and Na++ are mostly outside cell, Potassium is mostly inside cell
Ion Movement and Channels
Passive movement of ions depends on
Energetic favorability
Permeability of membrane for the ion
Energetics
Concentration gradient and transmembrane potential (voltage) drive passive ion flux.
i.e. 145 mM Na++ outside; 14 mM Na++ inside...Na goes from high to low
Transmembrane potential of myocyte ~ -90mV (so Na+ is attracted to inside negative charge).
Permeability
Voltage determines how many channels are open at a given time ~ voltage determines permeability
voltage sensitive
i.e. Fast sodium channel: transmembrane protein assumes various conformations based on
voltage
Open state only occurs for few thousandths of second
Slow depolarization of cardiac fast sodium channels (and constant membrane that is less negative than
usual ~ -70 mV).
inactivation of channels occurs without initial opening and current flow
Closed inactive channels cannot recover to resting state
typical case in SA/AV nodes (fast sodium channels persistnetly unable to conduct Na+ ions).
This is why sodium channels do not play a role in SA/AV nodes
Potassiium and Calcium channels are also voltage sensitive, but behave differently than sodium
channels (channel closed; inactivation gate open channel open; inactivation gate open channel
open; inactivation gate closed)
Figure 1-10: ion channels, cotransporters, active transporters of myocyte
Sodium entry responsible for action potential in non pacemaker cells
Calcium channels effective in phase 2 of purkinje fibers and muscle cells AP
Potassium exits to repolarize cell; open potassium channels resting membrane potential of
nonpacemaker cells
sodium-calcium exchange protein keeps intracellular calcium low
Sodium-potassium atpase maintains gradient for ion
Active calcium tranporters remove calcim to external environment and into sarcoplasmic reticulum.
Figure 1-11: Fast sodium channels
4 covalently linked transmembrane domains (I, II, III, IV)
Resting Potential
In nonpacemaker cells, electrical charge differential between inside and outside of cell = resting
membrane potential
Na K ATPase pump: 3 NA out, 2 K in--> keeps potassium ions high inside cell and sodium low
Cardiac myocytes: Inward rectifier potassium channels open in resting state while other gates are
closed (sodium and calcium). Cardiac myocytes are therefore more permeable to potassium. Potassium
leaves, and causes cardiac myocytes to be highly negative inside.
K eventually is re-attracted to highly negative interior: concentration gradient is opposed by
electrostatic force.; at equilibrium they are equal and there is zero K + movement
-26.7 ln (K in/ Kout)
Action Potential
Table 1-1
Transmembrane Cardiac Ionic Currents
If
Pacemaker current
Responsible for phase 4 depolarization in pacemaker cells
Ina
Na++ current
Responsible for phase 0 rapid depolarization in nonpacemaker cells
Ica.L
Slow, long lasting Ca++ current
Responsible for phase 0 depolarization in pacemaker cells,
major contributor to inward current during phase 2 of nonpacemaker cells
Iki
Maintain resting potential
Current of inward rectifying potassium channel
Ito
Transient outward potassium current
Responsible for phase 1 of action potential
Iks, Ikr
Delayed rectifier potassium currents of slow (Iks) and rapid (Ikr) types; repolarizing currents
that are active during phases 2 and 3 of action potential.
Cardiac Muscle cell
Figure 1-13
Depolarization: Na+ (Ina) = rapid upstroke of phase 0
Transient outward potassium current (Ito) partial repolarization during phase 1
Slow Ca++ influx (Ica. I) balanced by K+ efflux (Ikl) = plateau of phase 2
Final rapid repolarization: from further K+ efflux phase 3
Phase 4 maintained by Ikl inward rectifier potassium channels
Phase 4: resting state
phase 0
At resting membrane, sodium/calcium channels are closed
As voltage becomes more positive, more and more sodium channels open
Sodium flows down concentration gradient as more channels open
At threshold potential, enough of Na+ channels have opened generates self sustaining
inward current that exceeds open rectifier potassium channels efflux
Leads to rapid upstroke
Sodium channel sopen for one thousandths of a second then quickly inactivated, which is
why action potential is short lived.
Phase 1
Brief current of repolarization returns membrane potential from positive to zero by transient
outward potassium channels (Ito)
phase 2
phase 3
phase
Specialized conduction system
Pacemaker cells
Figure 1-14
Refractory Period
Figure 1-15
Impulse Conduction
Normal Sequence of Cardiac Depolarization
Figure 1-16
Excitation-Contraction Coupling (electrical action potential leads to contraction of cardiac muscle
cells....chemical energy in high phosphate bonds is translated into mechanical energy of myocyte
contraction)
Contractile Proteinsi n the Myocyte
Main proteins: Myosin and Actin...regulated by troponin and tropomyosin
Myosin
Thick filaments ~ 300 molecules...globular heads evenly spaced.
Contains myosin atpase (required for contraction)
Actin
smaller molecule (thin filaments alpha helix two strands that interdigitate between thick myosin
filaments
Titin
Connects myosin to Z line of sarcomere; provides elasticity
Tropomyosin
double helix; lies in grooves between actin filmaments
in resting state, ihibits myosin-actin interaction; prevents contraction during relaxed state
Troponin
Sits on actin filmaents
three subunits (TnT); links troponin to actin and tropomyosin
troponin I (TnI) inhibits ATPase activity of actin-myosin
Troponin C (TnC) binds calcium ions that regulate contractile process
Calcium-Induced Calcium-InducedRElease and the Contractile Cycle
Calcium influx couples electrical activation to physical contractions
phase 2 calcium influx by L-type Ca++ channels leads to small amount of calcium to enter
This triggers Calcium release from sarcoplasmic reticulum
T tubule invaginations of sarcolemal membrane l type channels close with specialized
Calcium release receptors into SR (ryanodine receptors).
When calcium binds ryanodine receptor, receptor open conformation much greater
release of Calcium into cytosol from terminal cisternae of SR.
This is called calcium induced calcium release (l type triggers calcium release from
sarocplasmic reituclum)
After larger calcium influx, calcium binds to troponin C (TnC) and allows contractions
Calcium is sthen put back into sarcoplasmic reticulum via SERCA (sarcoendoplasmic
reticulum calcium ATPASE).
PL inhibits SERCA. When PL is dephosphorylated, it inhibits Calcium uptake.
Excessive calcium released back into extracellular space via
Sodium-calcium exchange
Sarcolemmal Ca++ - ATPase (to a smaller degree).
As calcium binds to TnC, TnI is inhibited; so it cannot inhibit actin-myosin interaction, which opens up
active site between actin-myosin interface.
Contraction: myosin heads bind to actin filaments and flex
Cross bridge: When actin-myosin are together
1st step: activationof myosin head via hydrolysis of ATP (ADP + Pi + myosin head binds to actin)
2nd step: ADP + Pi + myosin head binds to actin formation of cross bridge conformation change
of myosin head, pulls actin filament inward
3rd step: ADP is released, new ATP binds to mosin head, causing it to release actin filament
Progressive coupling + uncoupling causes muscle fiber to shorten by increasing overlap between
myofilaments within each sarcomere.
As long as there is ATP, contraction continues as long as calcium concengtrationn is high to in order
to bind TnC and prevent inhibition by tropomyosin
Myocyte relaxation is also coupled with electrical activity
Torward end of phase 2, L-type channels ianctivate arrests inlfuex of calcium, abolishes trigger for
Calcium induced calcium release
Calcium is pumped back into sarcoplasmic reticulum via Ca ++ ATPase (SERCA)
Calcium is pumped outside by Na+ -Ca+ exchanger and to a lesser extent ATP-consumign calcium
pump, sarcolemmal Ca++ - ATPase
As calcium levels fall, Calcium dissociates from TnC, so tropomyosin is reactivated, and inhibts
myosin-actin interface relaxation of contracted cell.
Contraction-relaxation cycle repeats with next action potential.
Introduction to Cardiac Signaling Systems
Beta-Adrenergic and Cholinergic Signaling
Concentration of Calcium in cytosol = major determinant of force of cardiac contraction with each
heartbeat
Beta-Adrenergic stimulation = enhances calcium fluxes in myocyte strengths fore of ventricular
contractions
Catecholamines (e.g. norepinephrine) bind to myocyte B1 adrenergic receptor Gstimulatory (inner
surface of membrane ) membrane bound adenylate cyclase cAMP made from ATP cAMP
activates PKA's phosphorylation of cellular proteins (i.e. L-type calcium channels) Increase in
Calcium ions in sarcoplasmic reticulum increase in force of contractions
B-adrenergic stimulation also enhances myocyte relaxation
Phospholamban (PL) returns Ca++ to cytosol of SR
SR membrane bound protein
When dephosphorylyzed, PL inhibits Ca++ uptake by SR via SERCA
However, Beta adrenergic activation of PKA inhibits PL's inhibitory effect
Greater intake of Calcium ion increases Calcium removal from cytosol myocyte relxation
Phosphorylation of TnI also due to increased cAMP activity (inhibits actin-myosin
interaction further relxation of cell)
Cholinergic Signaling (PNS from vagus nerve) opposes Beta-Adrenergic stimulation.
Acetylcholine muscarinic M2 receptor G proteins Gi (G inhibitory) inhibits adenyl cyclase
activity reduces cAMP
At sinus node: heart rate decreases
Myocardium: counteract force of contraction induced by beta-adrenergic signaling
Ventricular cells are less sensitivity to cholinergic signaling than atrial cells (due to different degrees
of G protein coupling)
Basically, adrenergic receptor enhances contraction, while cholinergic opposes enhancement.
Vous aimerez peut-être aussi
- Cardiovascular SystemDocument18 pagesCardiovascular SystemBinta Elsa JohnPas encore d'évaluation
- General Anatomy 5.thoracic Cavity (Intro To Cardivascular and Respiratory)Document49 pagesGeneral Anatomy 5.thoracic Cavity (Intro To Cardivascular and Respiratory)amrokhalefa21Pas encore d'évaluation
- Anatomy of Heart: Mr. Binu Babu MBA, M.Sc. (N) Asst. Professor Mrs. Jincy Ealias M.Sc. (N) LecturerDocument71 pagesAnatomy of Heart: Mr. Binu Babu MBA, M.Sc. (N) Asst. Professor Mrs. Jincy Ealias M.Sc. (N) Lecturerjyoti kunduPas encore d'évaluation
- Anatomy of CVS: 2. The HeartDocument68 pagesAnatomy of CVS: 2. The Heartsultan khabeebPas encore d'évaluation
- The Cardiovascular SystemDocument57 pagesThe Cardiovascular Systemkrmgxc8p4fPas encore d'évaluation
- HeartDocument28 pagesHeartZahidKhanPas encore d'évaluation
- Cardiovascular - HeartDocument57 pagesCardiovascular - HeartCla NuvalPas encore d'évaluation
- Kuliah The Heart, Pericardium, and Coronary VesselsDocument62 pagesKuliah The Heart, Pericardium, and Coronary VesselsRafianza AkbarPas encore d'évaluation
- المحاضرة الثانية عشر - مادة التشريح العام - المرحلة الاولىDocument10 pagesالمحاضرة الثانية عشر - مادة التشريح العام - المرحلة الاولىAbdullah ThePas encore d'évaluation
- 11 The Cardiovascular SystemDocument27 pages11 The Cardiovascular SystemHarshika KDGPas encore d'évaluation
- The Heart and PericardiumDocument40 pagesThe Heart and PericardiumMartha Orendu Oche AttahPas encore d'évaluation
- Null 1Document37 pagesNull 1Esdras DountioPas encore d'évaluation
- Anatomy of The Heart - Lecture PDFDocument88 pagesAnatomy of The Heart - Lecture PDFDiane MontecerPas encore d'évaluation
- PHA618 Ex 31 Revised PDFDocument20 pagesPHA618 Ex 31 Revised PDFPATRICIA ROSE SORIANOPas encore d'évaluation
- Hear Structure NotesDocument27 pagesHear Structure NotesRaifa FathimaPas encore d'évaluation
- Anatomy 2nd-U-1 CVSDocument121 pagesAnatomy 2nd-U-1 CVSsinte beyuPas encore d'évaluation
- Anaphy HeartDocument8 pagesAnaphy HeartVivien LancinPas encore d'évaluation
- Week 11 THE HEART 2021Document60 pagesWeek 11 THE HEART 2021Huffy PotterPas encore d'évaluation
- Anatomy of HeartDocument65 pagesAnatomy of HeartSadab AlamPas encore d'évaluation
- The Cardiovascular To MhsDocument25 pagesThe Cardiovascular To MhsikuwagePas encore d'évaluation
- Cardiovascular System - The HeartDocument77 pagesCardiovascular System - The HeartZiraili VidalPas encore d'évaluation
- Cardiovascular System Heart ModuleDocument19 pagesCardiovascular System Heart Modulebadodong700Pas encore d'évaluation
- Osup TTDocument23 pagesOsup TTKIM EUNHAPas encore d'évaluation
- Cardiovascular System: - HeartDocument58 pagesCardiovascular System: - HeartFaraz AhmedPas encore d'évaluation
- Introduction To CVSDocument24 pagesIntroduction To CVSKjoPas encore d'évaluation
- HeartDocument27 pagesHeartJeevitha VanithaPas encore d'évaluation
- Development of The HeartDocument19 pagesDevelopment of The HeartYusuf UmarPas encore d'évaluation
- Heart Structure - 1Document28 pagesHeart Structure - 1Daniel McfarlanePas encore d'évaluation
- Week Objectives 1-5Document295 pagesWeek Objectives 1-5qvqck4zmnnPas encore d'évaluation
- Cardiovascular SystemDocument18 pagesCardiovascular SystemDeep RoyPas encore d'évaluation
- Special Cardiobascular SystemDocument24 pagesSpecial Cardiobascular SystemBijay Kumar MahatoPas encore d'évaluation
- 1.02 Thorax - Heart - Dr. MalakDocument31 pages1.02 Thorax - Heart - Dr. MalakJedoPas encore d'évaluation
- Chapter 15 - Cardiovascular SystemDocument89 pagesChapter 15 - Cardiovascular SystemOliver Namyalo100% (1)
- Sistem Kardio 2022Document51 pagesSistem Kardio 2022Silyas KhanPas encore d'évaluation
- Lecture 4 - HeartDocument87 pagesLecture 4 - Heartsamabakr2003Pas encore d'évaluation
- Cardiovascular SystemDocument26 pagesCardiovascular SystemGaurav SinghPas encore d'évaluation
- Heart 1Document52 pagesHeart 1yashodharaPas encore d'évaluation
- Anatomy and Physiology Ii-Dm Cardiovascular Sys-1Document64 pagesAnatomy and Physiology Ii-Dm Cardiovascular Sys-1Mathews showsPas encore d'évaluation
- Heart Anatomy For BSC NursingDocument57 pagesHeart Anatomy For BSC NursingVIJAYA KUMAR YPas encore d'évaluation
- The Cardiovascular System: A. KabweDocument117 pagesThe Cardiovascular System: A. KabwePeter ChipatelaPas encore d'évaluation
- Anatomy of HeartDocument63 pagesAnatomy of HeartClement NjingiPas encore d'évaluation
- Cardiovascular SystemDocument53 pagesCardiovascular SystemPelin OktayPas encore d'évaluation
- Introduction of Angiology and HeartDocument58 pagesIntroduction of Angiology and Heart9999999999999-567421Pas encore d'évaluation
- Circulatory SystemDocument96 pagesCirculatory SystemuzumakiyashPas encore d'évaluation
- Cardio NewDocument204 pagesCardio NewHaleema SaadiaPas encore d'évaluation
- Circulatory Syst Ana 241Document34 pagesCirculatory Syst Ana 241giftabumere54Pas encore d'évaluation
- Cardiovascular System (2015 - 06 - 09 22 - 21 - 55 Utc)Document89 pagesCardiovascular System (2015 - 06 - 09 22 - 21 - 55 Utc)DiazPas encore d'évaluation
- Cardiac Anatomy and PhysiologyDocument57 pagesCardiac Anatomy and PhysiologyRheecha JoshiPas encore d'évaluation
- CARDIODocument11 pagesCARDIOJenica SorianoPas encore d'évaluation
- CVS Up To Cardiac Output-1Document198 pagesCVS Up To Cardiac Output-1mokshachapaneriPas encore d'évaluation
- The Circulatory SystemDocument114 pagesThe Circulatory SystemKhant Si ThuPas encore d'évaluation
- Embryology of CVSDocument63 pagesEmbryology of CVSAdan Iman100% (1)
- The Heart: Dr. Silvia BoyajianDocument44 pagesThe Heart: Dr. Silvia BoyajianAsem YousefPas encore d'évaluation
- Cardiovascular System SonDocument36 pagesCardiovascular System SonsmilingjudgePas encore d'évaluation
- Pericardium and HeartDocument26 pagesPericardium and HeartNunmoy HnialumPas encore d'évaluation
- 11 The Pericardium and The HeartDocument39 pages11 The Pericardium and The HeartJeff D'erique Ozil TettehPas encore d'évaluation
- AnatomyDocument8 pagesAnatomyOM BAWNEPas encore d'évaluation
- Cardiovascular System: Prepared by Samjhana GautamDocument71 pagesCardiovascular System: Prepared by Samjhana GautamSamjhana GautamPas encore d'évaluation
- Cardiovascular System: Dr. Mohanad R. AlwanDocument46 pagesCardiovascular System: Dr. Mohanad R. Alwanmohanad11Pas encore d'évaluation
- A Case Study On Hypertensive Urgency - Group 2 Section 3BDocument48 pagesA Case Study On Hypertensive Urgency - Group 2 Section 3BhernandezmariannerosePas encore d'évaluation
- Antepartum Fetal SurveillannceDocument78 pagesAntepartum Fetal SurveillannceYibelu BazezewPas encore d'évaluation
- MRCP PearlsDocument682 pagesMRCP Pearlssherbiny alnaghyPas encore d'évaluation
- Haimovicis Vascular SurgeryDocument1 219 pagesHaimovicis Vascular SurgeryaquijadaPas encore d'évaluation
- Dialysis in Malaysia: EditorialDocument3 pagesDialysis in Malaysia: EditorialfisplPas encore d'évaluation
- Autoimmune Hemolytic AnemiaDocument4 pagesAutoimmune Hemolytic AnemiaSamuel WibowoPas encore d'évaluation
- Heart FailureDocument94 pagesHeart FailureAnusha Verghese100% (1)
- 33 - Ma. Lourdes S. Florendo V Philam Plans Inc.Document5 pages33 - Ma. Lourdes S. Florendo V Philam Plans Inc.Patrice ThiamPas encore d'évaluation
- Referred PainDocument10 pagesReferred Paindina sharafPas encore d'évaluation
- Anesthesia TESTDocument8 pagesAnesthesia TESTvelo100% (1)
- Exercisies in Word Prefix Suffix Root CombinationDocument8 pagesExercisies in Word Prefix Suffix Root CombinationN KPas encore d'évaluation
- Prakash Et Al 2023 Factor Xi Xia Inhibitors For The Prevention and Treatment of Venous and Arterial Thromboembolism A 1Document8 pagesPrakash Et Al 2023 Factor Xi Xia Inhibitors For The Prevention and Treatment of Venous and Arterial Thromboembolism A 1Gabriela PachecoPas encore d'évaluation
- Carotid Stenosis (Carotid Artery Disease)Document5 pagesCarotid Stenosis (Carotid Artery Disease)Pieter SteenkampPas encore d'évaluation
- STUDY OF ILLNESS CONDITION FormatDocument2 pagesSTUDY OF ILLNESS CONDITION FormatChryst Louise SaavedraPas encore d'évaluation
- IsoxsuprineDocument1 pageIsoxsuprineAndrean EnriquezPas encore d'évaluation
- Kostis William - ACE Inhibitor-Induced Angioedema - A ReviewDocument7 pagesKostis William - ACE Inhibitor-Induced Angioedema - A ReviewluongcongthucPas encore d'évaluation
- Corrigan's SignDocument2 pagesCorrigan's SignAsyraf SafwanPas encore d'évaluation
- What Is Obesity and How Does It Affect The UDocument10 pagesWhat Is Obesity and How Does It Affect The UavahajPas encore d'évaluation
- Arab Board Rheumatology Questions 2004Document8 pagesArab Board Rheumatology Questions 2004little luluPas encore d'évaluation
- Hypoglycemia in NeonatesDocument23 pagesHypoglycemia in NeonatesNashirah Frinzz HauziPas encore d'évaluation
- Urine Analyzer IEEEDocument6 pagesUrine Analyzer IEEESamin ZarifPas encore d'évaluation
- 9 Assessment of Gastrointestinal Function and Management of Gastrointestinal DisordersDocument61 pages9 Assessment of Gastrointestinal Function and Management of Gastrointestinal DisordersNoor MajaliPas encore d'évaluation
- Dissertation Kurzendorfer TanjaDocument197 pagesDissertation Kurzendorfer TanjaASIK1144Pas encore d'évaluation
- NCM 116 Lab Activity 2 NCP 1 Git PabrnmanDocument6 pagesNCM 116 Lab Activity 2 NCP 1 Git Pabrnmanjericho dinglasanPas encore d'évaluation
- Desescalada de FluidosDocument3 pagesDesescalada de FluidosWendy JuampoPas encore d'évaluation
- Lymphatic System MCQsDocument3 pagesLymphatic System MCQsNimra86% (7)
- Review of Literature: (Bharucha and Kuruvilla, 2003)Document11 pagesReview of Literature: (Bharucha and Kuruvilla, 2003)jainslotusPas encore d'évaluation
- Diuretics: Chris Hague, PHDDocument29 pagesDiuretics: Chris Hague, PHDranachamanPas encore d'évaluation
- Interior Root WordsDocument3 pagesInterior Root WordsPaula McAuliffePas encore d'évaluation
- Wellen's Syndrome: Mohan GameshDocument28 pagesWellen's Syndrome: Mohan Gameshmyolie wuPas encore d'évaluation