Académique Documents
Professionnel Documents
Culture Documents
CO2 Capture Paper
Transféré par
Maslia Olfah06Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
CO2 Capture Paper
Transféré par
Maslia Olfah06Droits d'auteur :
Formats disponibles
ISSN: 2321-4902
Volume 1 Issue 2
Online Available at www.chemijournal.com
International Journal of Chemical Studies
Influence of Some Phosphates on The Rate of Calcium Sulfate
Dihydrate Crystalistion in sodium Chloride Solution
N. S. Yehia 1, W. K. Saif elyazal 2, A. M. Heneash 3, I. A. Ibrahem 4
1. Chemistry Department, Faculty of Science, Minufiya University.
2. Nubaria power station, chemistry departement , electricity ministry.
3. Hydrobiology, Marine invironment,National institute oceashonsography andfishriese.
4. Chemistry Department, Faculty of Science, Alazhar University.
Crystallization of calcium sulfate dihydrate (CaSO4.2H2O) in sodium chloride solutions at different supersaturation
( = 1.22.49), pH =3, ionic strength (I = 0.15 M) and at 25C was studied. The influence of disodium hydrogen
phosphate and sodium tripolyphosphate and disodium dihydrogen phosphate having very low concentrations (10 -7
mol dm-1) on the rate of crystallization at different supersaturation was investigated. The rate of crystallization was
found to be dependent of the stirring rate suggesting diffusion mechanism. The addition of all additives retarded the
rate of crystallization to an extent proportional to their amounts present. Furthermore, the retardation effect was
enhanced as the supersaturation decreases. The results also revealed that the increase in both pH (3 10) and
crystallization temperature (20 80 oC) brought about an increase in calcium sulfate crystallization rate.
Keyword: Crystallization; Additives,Calcium Sulfate; Solution, Phosphates.
1. Introduction soluble salts are therefore, of considerable
Calciumsulfate minerals (i.e., gypsum, anhydrite interest, especially the influence of anions and
and hemi-hydrate)are common scale-deposit cations which may exert a marked effect on the
minerals inwater treatment plants[14] and oil and rate of precipitation, either through adsorption or
gas industry[5]. Crystallization of calcium sulfate by lattice substitution[15]. The present work aims
dihydrate (gypsum) is of importance in view of at studying the crystallization of gypsum under
their applications in a number of industrial and conditions simulating the cation exchanger
environmental precipitation processes. With regeneration system in case absence and presence
increasing temperature, the solubility of all some phosphates and polyphosphate additives .
calcium sulfate forms decreases. This is the cause
of calcium sulfate scale formation on heat 2. Methodology
transfer surface [6]. Crystallization can take place 2.1. Materials
on foreign substance or dust particles in the Calcium chloride CaCl2 and sodium sulfate
solution and it is very difficult to reproduce the Na2SO4 (BDH, England) were used to prepare
results of such studies[7]. Earlier, many authors seed crystals. Hydrochloric acid HCl (MERCK,
studied the growth of seed crystals of gypsum in Germany) was used to adjust pH. (di-sodium
super-saturation solutions[812], crystallization of hydrogen phosphate , sodium tri-poly phosphate ,
gypsum on other crystal surfaces[13] and the di-sodium di-hydrogen pyrophosphate,
precipitation on heated metal surface[14]. The respectively. Sodium chloride NaCl from El-Nasr
factors that govern this mechanism of Pharmaceutical chemicals (ADWIC, Egypt) was
precipitation and dissolution of the sparingly chemical reagent grade. All solutions were
Vol. 1 No. 2 2013 www.chemijournal.com Page | 68
International Journal of Chemical Studies
prepared with deionized water of high quality and sampling. At the beginning of the
(conductivity < 0.1 S cm-1). A closed experiment, the pH of the supersaturated
thermostatic double-walled vessel of 300 cm3 solutions was adjusted to the desired value (3) by
was used for all the experiments. The the controlled addition of hydrochloric acid
experiments were performed in the temperature standard solutions. The pH of solutions was
range of 23-25 C, and the systems were measured using a combined glass electrode
mechanically agitated with a flat-bladed stirrer at standardized before and after each experiment
a constant rate (200 rpm). with NBS primary standard buffer solution.
The mineralization reactions were initiated by
2.2. Preparation of polycrystalline calcium inoculation with dry seed crystals in the reaction
sulfate dihydrate vessel. The crystallization rate followed
Calcium sulfate dihydrate crystals were prepared potentiometric with time using metrohm titrano .
by precipitation from calcium chloride and The degree of supersaturation maintained at
sodium sulfate solutions, as described previously constant levels by the simultaneous addition of
by Lui and Nancollas[16]. The obtained solid was ionic species. Inhibitor solutions were also
aged at least for one month before being filtered introduced as titrans in order to compensate for
to obtain the dry crystals that were used in crystal dilution effects. In addition, samples were
growth experiments. The crystals were identified periodically withdrawn and filtered at the reaction
as gypsum by X-ray powder diffraction temperature through Millipore filters (0.22 m),
(Shimadzu XD-3 diffractometer) and IR analysis prior to solution and solid-phase analysis. The
by scanning electron microscopy. The data confirmed that the lattice-ion and inhibitor
crystallization of calcium sulfate dihydrate concentrations were constant to within 1%.
carried out under various conditions was done in For the calcium sulfate dihydrate crystallization
a thermostatted double-walled Pyrex glass vessel growth experiments, the seed crystals of calcium
of 500 mL capacity and were adjusted to the sulfate dihydrate were prepared by a dropwise
required temperatures by circulating addition of 500 cm3 of a 0.6 mol dm-3 CaCl2
thermostatted water through the outer jacket. solution to 500 cm3 of a 0.6 mol dm-3 Na2SO4
Stemming was effected using a variable-space solution with a continuous magnetic stirring at
magnetic stirrer with a Teflon stirring bar. 70oC over a period of 2 hours. The crystals
Nitrogen gas was first bubbled into a solution of obtained were filtered through a 0.22 mm
the electrolyte at the temperature of the reaction membrane filter and washed repeatedly with
for saturation with water vapor and then into the deionized water until becoming free of NaCl.
reaction vessel throughout the duration of the Then, the crystals were dried in an electric oven
experiment. at about 105C overnight.
R dm / dt Rs n ....( 2 )
3. Results and Discussion
2.3. Supersaturated Solutions of Calcium The concentrations of free ionic species in the
Sulfate Dihydrate solutions were computed by successive
Supersaturated solutions of calcium sulfate approximations for the ionic strength I, as
dihydrate were prepared by the addition of described previously[17] using activity coefficients
thermostatted known volumes of calcium calculated from the extended form of the Debye
chloride solutions followed by careful addition of Hckel equation proposed by Davies[18]. The rate
the appropriate amounts of sodium sulfate of precipitation, R, may be expressed by[19]
solutions in the cell which adjusted to the
R=dm/dt=R s n (1)
required ionic strength (0.5 M) with sodium
chloride solutions. The reaction vessel was fitted in which m is the number of moles precipitated in
with a Teflon cover with holes for the electrodes time t, R a rate constant, n the effective order of
Vol. 1 No. 2 2013 www.chemijournal.com Page | 69
International Journal of Chemical Studies
reaction, and s proportional to the number of where "" are the activity coefficients for the
growth sites available on the seed crystals. The Zvalent ions and I is the solution ionic strength.
degree of saturations is defined in terms of ionic The value of Ksp was calculated as a function of
products and solubility products for the calcium temperature by means of the following
sulfate dihydrate salt by the following relationship obtained by Marshall and Shlusher[21]
expression[20]. for calcium sulfate dihydrate in aqueous solutions
IP 1 / 2 K1/ 2
SO .....
from 0 to 110C.
12,545.62
K 1 / 2 log sp 390.9619 152.6246 log 0.0818493
SO ..(2)
(5)
where the ionic products, IP, and the solubility Crystal growth experimental conditions are
product, KSO , are expressed in terms of the summarized in Table 1 in which [Ca]t and [SO4]t
appropriate activities of the ionic species are the total molar concentration of calcium and
sulfate, respectively. Typical time plots of the
Ca SO
2 2
4
1/ 2
amount of gypsum per moles precipitate,
at time t and at equilibrium, respectively. calculated from the titrants addition.
The degree of supersaturated () of the solutions, The results cited in Table 1 show that the rate of
which is defined as the ratio of the activity crystal growth of calcium sulfate dihydrate was
products divided by the thermodynamic solubility proportional to the mass of seed crystals used to
product of the mineral (Ksp), is where initiate the reaction. The suggestion of a
predominantly diffusion mechanism over a range
4
Ca 2 SO 2
1/ 2
1
of relative supersaturations may also be supported
sp by the observed dependence of the experimental
,.. (3) rate of precipitation on changes in fluid
parentheses denote activities of the respective dynamics, as shown in Table 1 (compare
ions and Ksp is the thermodynamic solubility experiments a, b,c and d), which conclude that
product of the precipitating solid. The activity the reaction is a mass transfer limited[22]. A
coefficients of divalent cations and anions were similar mechanism for the crystal growth of
assumed equal and were obtained using the calcium sulfate dihydrate has been observed [22, 23].
extended Debye-Huckel equation as proposed by The effective order of reaction was determined
Davies[15]: from the slope of typical plots of -log R against
1 / 2 log , as depicted in Figure 1 which confirms a
log f 0.5115 2 0.3
1 1/ 2 ,. (4)
first-order dependence upon
supersaturation (n = 1) in Eq. (1).
relative
Table 1: Crystallization of calcium sulfate dihydrate crystals, Tca :Tso4 = 1 : 1 at t = 25 oC, 50 mg seed, 200 rpm, ionic
strength = 0.15 mol L-1 (NaCl) and pH = 3
wt. of
T ca2+/106 mol L-1 X10 (r /104 ) mol min-1 m-2
seed/mg
9.482 12 50 1.8
10 13.2 50 2.0
10.775 15 50 2.315
11.637 17 50 2.56
12.93 20 50 2.92
15.04 24.9 50 3.7
(a) 10 13.2 50 2.2
(b) 10 13.2 50 2.4
(c) 10 13.2 50 2.51
(d) 10 13.2 50 2.623
(a): Stirring rate 300 rpm(b): Stirring rate 400 rpm(c): Stirring rate 500 rpm (d): Stirring rate 600 rpm
Vol. 1 No. 2 2013 www.chemijournal.com Page | 70
International Journal of Chemical Studies
Fig 1: Plots of Log R against Log
Additives play an important role in the theory of and Na2H2P2O7 upon the rate of crystallization of
crystallization and dissolution. Recently, it is gypsum in sodium chloride solution was studied
found that the presence of metal ions in the at 25C; stirring speed of 200 rpm, weight of seed
reaction medium inhibited the rate of crystals of gypsum 50 mg at solution (pH = 3),
precipitation of calcium sulfate dihydrate. The and a relative supersaturation ( =1.32) results
effects of phosphates and polyphosphate as di- obtained are illustrated in Figure (2).
sodium hydrogen phosphate and sodium tri- It is clear from Figure (2) that the addition of a
polyphosphate and di-sodium di-hydrogen very small amount of these compounds retarded
pyrophosphate on the rate of crystallization of the rate of crystallization to an extent
calcium sulfate dihydrate crystals were studied. proportional to the amount added. The decrease
Previous studies showed that di-sodium hydrogen was, however, more pronounced in the case of
phosphate at close similarity conditions and Na2PO4 and stpp and sod.pyrophosphate
substantiate my results in the percentage of sequentially. In fact, the presence of 10-7mol
inhibition of calcium sulphate dihydrate (reduce decreased the crystallization rate to 61.21, 56.3,
the rate of crystalizalisation )[24]. 42.5 % respectively.
3.1. Effect of Concentration of the Inhibitors
Concentration: Na2Hpo4 and stpp (Na5P3O10)
Fig 2: Effect of Add1 and Add2 and Add3 on rates of crystallization of calcium sulfate dihydrate crystals at = 1.32, I =
0.15 mol dm-3,PH=3and 50 mg seed.
Vol. 1 No. 2 2013 www.chemijournal.com Page | 71
International Journal of Chemical Studies
The mechanism of phosphates and poly and pyro the treated of CaSO4.2H2O by disodium hydrogen
phosphates in retardation of the rate of phosphate show that dissappeared some peaks at
crystallization of calcium sulfate dihydrate has 2 (11.56)and 22.99.the decreases the crystanility
been studied via carrying out the crystallization of CaSO4.2H2O when treated by disodium
process at 25C, pH = 3, I = 0.15 mol dm-3, and hydrogen phosphate with increase of particle size
= 1.32 in the presence of 10-7mol dm-3 of the .this means that disodium hydrogen phosphate
phosphate compounds . Calculated volumes of effect on the crystanility and particle siz
CaCl2, NaCl, phosphate additives, respectively, (4)Scanning electron micrographs of calcium
are added in the same order. After the end of sulfate dihydrate (A) in absence of Additives and
reaction, the solution was filtered and the (b,c,d) in the presence of 10-7 M additives 1,2,3
produced solid was characterized using XRD and respectively.
SEM techniques. Fig(4) show the scaning electron microscopic
Figure (3) shows both of the diffractograms of (SEM) of CaSO4.2H2O and treated with different
solid left after the formation of calcium sulfate in types of phosphates . this fig. show that the
absence of additives and the diffractogram of CaSO4.2H2O more crystaalline when compared
calcium sulfate in presence of a known amount of with modified additives . the comparison between
additives 10-7mol dm-3). additives treated with different types of
It is seems from Figure (3) show the XRD of phosphates show that particles are smaller and
CaSO4.2H2O and modified by disodium hydrogen simillare when treated by disodium hydrogen
phosphate, sodium tripolyphosphate, Disodium phosphate. The marphology structure will be
pyrophosphate this fig. show that, the crystanility confirmed with XRD analysis . this means that
of CaSO4.2H2O partially effect when treated with the best additive is the CaSO4.2H2O with
tripoly phosphate and pyrophosphate, however, disodium hydrogen phosphate.
Vol. 1 No. 2 2013 www.chemijournal.com Page | 72
International Journal of Chemical Studies
Fig (3): XRD analysis of calcium sulfate dihydrate (A) in absence of all additives (B),(c) ,(d) in the presence of 10-7 M of
additives 1,2,3 respectively
A B
Vol. 1 No. 2 2013 www.chemijournal.com Page | 73
International Journal of Chemical Studies
C D
Fig 4: Scanning electron micrographs of calcium sulfate dihydrate (A) in absence of Additives and (b,c,d) in the presence of
10-7 M additives 1,2,3 respectively.
3.2. Effect of Supersaturation Degree () on 2.49. The other parameters were fixed at an ionic
calcium sulfate dihydrate crystallization rateThe strength solution (I) of 0.15 mol dm-3, reaction
degree of inhibition maybe interpreted in terms of temperature of 25C, stirring speed of 200 rpm,
asimple Langmuir adsorption isotherm[25]. To and weight of seed crystals of gypsum of 50 mg
investigate the effect of supersaturation degree at solution (pH = 3). The experimental results are
() on calcium sulfate dihydrate crystallization given in Figure (5) as a relation between degree
rates in sodium chloride solution, the of supersaturation and calcium sulfate dihydrate
crystallization of calcium sulfate in absence and crystallization rate.
in the presence of additives was investigated at
different degrees of supersaturation from 1.2 to
Fig (5): Effect of supersaturation degree () on the rate of crystallization of calcium sulfate dihydrate crystals at pH=3, T=
25 c, I = 0.15 mol dm -3, and 50 mg seed in absence of inhibitors and in presence of additives
Vol. 1 No. 2 2013 www.chemijournal.com Page | 74
International Journal of Chemical Studies
Fig 6: Effect of pH on the rate of crystallization of calcium sulfate dihydrate crystals at = 1.32, T = 25 c, I = 0.15 mol dm
-3
, and 50 mg seed in absence of inhibitors and in presence of inhibitors
From the figure, it is clear that, as the degree of From the above figure, it is clear that with the
supersaturation increased from 1.2 to 2.49, the increase of pH from 37, the calcium sulfate
gypsum crystallization rate increased from 1.8 to dihydrate crystallization rate slightly increased
3.7 mol min-1 m-2 in absence of inhibitors and from about 2 to 2.06 mol min-1 m-2 in absence of
from 2 to 0.776 and from 2 to 0.874 and from 2 to inhibitors and from 0. 776,0.87,1.15 to
1.15 mol min -1 m-2 in presence of three inhibitors 0.81,0.91,1.19 mol min -1 m-2 in presence of
respectively. The order in the presence of Additives 1,2,3 respectively. This means that pH
additives was found to be equal to (n=1), which over a wide range (3-7) does not affect the
suggests diffusion mechanism. calcium sulfate dihydrate [26,27,28]. Further increase
in solution pH from 7 to 10 led to increase the
3.3. Effect of pH on Calcium Sulfate Dihydrate calcium sulfate dihydrate rates crystallization
Crystallization Rate from 2.06 to 2.13 mol min-1 m-2 and from
The effects of solution pH on the crystallization 0.81,0.91,1.19 to 0.92,1.13,1.38 mol min-1 m-2 in
growth of calcium sulfate dihydrate in the absence and in presence of Additives[29,30,31]. This
absence and in the presence of additives were may be due to the increase in degree of
studied at different pH range (310). Other deprotonation[32, 33].
experiments conditions were fixed at a reaction
temperature of 25C, stirring speed of 200 rpm, 3.4. Effect of Ionic Strength (I) on Calcium
weight of seed crystals of calcium sulfate Sulfate Dihydrate Crystallization Rate
dihydrate 50 mg at ionic strength ( = 0.15 M), The effect of ionic strength of the crystallization
and a relative supersaturation ( =1.32). The medium on the calcium sulfate dihydrate
experimental results are plotted in Figure (6) as a crystallization rate in sodium chloride solution is
relation between pH and the rates of studied; several experiments are carried out in
crystallization of calcium sulfate dihydrate absence and in the presence of Additives at
crystals. different ionic strength from 0.1 to 0.5 M. The
Fig (6) Effect of pH on the rate of crystallization other parameters are fixed at reaction temperature
of calcium sulfate dihydrate crystals at = 1.32, of 25C, stirring speed of 200 rpm, weight of
T = 25 c, I = 0.15 mol dm -3, and 50 mg seed in seed crystals of gypsum 50 mg at solution (pH =
absence of inhibitors and in presence of inhi 3) and a relative supersaturation ( =1.32). The
Vol. 1 No. 2 2013 www.chemijournal.com Page | 75
International Journal of Chemical Studies
experimental results are given in Figure (7) as a sulfate dihydrate crystallization rates increased
relation between ionic strength and the rates of from about 1.8 to 2.8 mol min-1 m-2 in absence of
crystallization Fig (7): Effect of ionic strength (I) inhibitors while it decreased from 0.82,0.9,1.2 to
on the rate of crystallization of calcium sulfate 0.0.63,0.79,0.95 mol min-1 m-2 in presence of
dihydrate crystals at = 1.32, pH=3, T = 25 c Additives 1,2,3 respectively. This means that, the
and 50 mg seed in absence of inhibitors and in effect of ionic strength on the rate of
presence of phosphate inhibitors . crystallizations indicates that the reaction is ionic
From the figure (7) , it is clear that as the ionic in its nature.
strength increases from 0.1 to 0.5 M, the calcium
Fig 7: Effect of ionic strength (I) on the rate of crystallization of calcium sulfate dihydrate crystals at = 1.32, pH=3, T = 25
c and 50 mg seed in absence of inhibitors and in presence of phosphate inhibitors
3.5. The Effect of Temperature on Calcium Figure (8) shows clearly that by increasing the
Sulfate Dihydrate Crystallization Rate temperature from 20 to 80 C, the rate of
The rate of crystallization of calcium sulfate crystallization increases from 2 to 2.06 mol min-1
dihydrate is studied in absence of inhibitors and m-2 in absence of inhibitors while it increases
in the presence of additives at temperature range from 0.776,0.87,1.15 to 0.84,0.93,1.21 mol min-1
of 20 to 80C. The experimental results are given m-2 in presence of additives 1.2.3 respectively.
in Figure (8) as a relation between temperature The growth rates of calcium sulfate dihydrate
and the rates of crystallization of calcium sulfate crystals markedly increase with the increase in
dihydrate crystals of calcium sulfate dihydrate the growth temperature.
crystals.
Fig 8: Effect of temperature on the rate of crystallization of calcium sulfate dihydrate crystals at = 1.32, pH=3, I = 0.15
mol dm-3 and 50 mg seed in absence of inhibitors and in presence of inhibitors.
Vol. 1 No. 2 2013 www.chemijournal.com Page | 76
International Journal of Chemical Studies
4. Conclusion 10. P.G. Klepetsanis, E. Dalas, P.G. Koutsoukos,
The analysis of the results shows that the Langmuir 15 (1999)1534.
11. L. Amathiu, R. Boistelle, J. Crystal Growth 88
presence of disodium hydrogen
(1988) 183.
phosphate,sodium tripolyphosphateand 12. E. Badens, S. Veeler, R. Boistelle, J. Crystal
disodiumdihydrogenpyrophosphate in the Growth 196 (1999) 704.
reaction medium inhibited the rate of 13. H. Muller-Steinhagen, O. Zhao, A. Zadeh, X. Ren,
precipitation of calcium sulfate dihydrate Cana J. Chem. Eng. 78 (2000) 12.
(gypsum). The degree of inhibition depends on 14. Z. Amjad, Environ TreatControl MP/November
(1989) 52.
the concentration of additives and degree of 15. S.K. Hamdona, M.S. Seif, S.M. Hamza,
saturation. Precipitation is inhibited and a Desalination 133 (2001) 205
precipitation rate is decreased in the following 16. S.T. Liu and G. H. Nancollas, The kinetics of
order: disodium hydrogen phosphate > sodium crystal growth of calcium sulfate dihydrate, J.
tripolyphosphate> Disodium pyrophosphate. Crystal Growth, 6 (1970) 281
Complete inhibition of precipitation of gypsum 17. G.H. Nancollas, Interactions in Electrolyte
Solution, Elsevier, Amsterdam, 1966.
was not found in any concentration of these metal 18. C.W. Davies, Ion Association, Butterworths,
ions and degree of saturations. The degree of London, 1952.
inhibition of additives (inhibitors) increases in 19. G.H. Nancollas, Adv. Colloid Interface. Sci. 10
acidic medium, lower ionic strength, higher (1979) 215.
degree of supersaturation, and lower temperature 20. Y. Yoshikawa, G.H. Nancollas, J. Crystal Growth
64 (1983) 222
21. W. L. Marshall and R. Slusher, Thermodynamics
5. References of calcium sulfate dihydrate in aqueous sodium
chloride solution, 0-110C, J. Phys, Chem., 70
1. D.H. Kim, B.M. Jenkins, J.H. Oh, Gypsum scale (1966) 4015
reduction and collection from drainage water in 22. E. etin, I. Erogleu, S. zkar, Kinetics of gypsum
solar concentration, Desalination 265 (2011) formation and growth during the dissolution of
140147. colemanite in sulfuric acid, J. Crystal Growth,
2. H.A. El Dahan, H.S. Hegazy, Gypsum scale 231 (2001) 559
control by phosphate ester, Desalination 127 23. O. Schierholz, The crystallization of calcium
(2000) 111118. sulfate dihydrate, Cana J. Chem., 36 (1958)
3. S.K. Hamdona, O.A. Al Hadad, Influence of 1057
additives on the precipitation of gypsum in 24. S.K.hamdona,S.M.Hamza,influence of some
sodium chloride solutions, Desalination 228 phosphates and polyphosphate on the
(2008) 277286. precipitation of calcium sulfate dehydrate in
4. M. Uchymiak, E. Lyster, J. Glater, Y. Cohen, sodium chloride solution,J.taibah university for
Kinetics of gypsum crystal growth on a reverse science,2(2009)44-51.
osmosis membrane, J. Membr. Sci. 314 (2008) 25. P. Koutsoukos, Z. Amjad, G.H. Nancollas, J. Collid
163172. Interface Sci. 83(1981) 599.
5. D. Bosbach, J.L. Junta-Rosso, U. Becker, M.F. 26. S. T. Liu, G. H. Nancollas, The crystal growth of
Hochella, Gypsum growth in the presence of calcium sulfate dihydrate in the presence of
background electrolytes studied by scanning additives, J. Colloid Interface Sci. 44 (1973) 422.
force microscopy, Geochim. Cosmochim. Acta 27. P. G. Klepetsanis, P. G. Koutsoukos, Precipitation
60 (1996) 32953304. of calcium sulfate dihydrate at constant calcium
6. O.D. Linnikov, Desalination 128 (2000) 35. activity, J. Cryst. Growth 98 (1989) 480.
7. J.S. Gill, G.H. Nancollas, J. Crystal Growth 48 28. M.C. van der Leeden, G.M. van Rosmalen,
(1980) 34. Aspects of additives in precipitation processes:
8. M.R. Christoffersen, J. Christoffersen, M.P.C. Performance of polycarboxylates in gypsum
Weijnen, G.M. Van Rosmalen, J. Crystal Growth growth prevention Desalination 66 (1987) 35.
58 (1982) 585. 29. M. P. C. Weijnen, G. M. van Rosmalen, The
9. S.M. Hamza, S.K. Hamdona, J. Crystal Growth influence of various polyelectrolytes on the
125 (1992) 591. precipitation of gypsum, Desalination 54
(1985) 239.
Vol. 1 No. 2 2013 www.chemijournal.com Page | 77
International Journal of Chemical Studies
30. Z. Amjad, Kinetics of crystal growth of calcium
sulfate dihydrate. The influence of polymer
composition, molecular weight, and solution
pH, Can. J. Chem. 66 (1988) 1529.
31. D. W. Griffiths, S. D. Roberts and S. T. Lru. Int.
Symp.Oilfield, Geothermal Chem. Society of
Petroleum Engineers (1979) 7861.
32. W. H. Leung and G. H. Nancollas, A kinetic study
of the seeded growth of barium sulfate in the
presence of additives, J. Inorg. Nucl. Chem. 40
(1978) 187.
Vol. 1 No. 2 2013 www.chemijournal.com Page | 78
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- ID Sintesis Dimetil Asetal Sitronelal DengaDocument8 pagesID Sintesis Dimetil Asetal Sitronelal DengaMaslia Olfah06Pas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Analisis JurnalDocument5 pagesAnalisis JurnalMaslia Olfah06Pas encore d'évaluation
- ZainabDocument13 pagesZainabMaslia Olfah06Pas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Titrasi Redoks PermanganatDocument0 pageTitrasi Redoks PermanganatNina Trisna NurmalasariPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- 58Document7 pages58Maslia Olfah06Pas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Gravimetri ADocument6 pagesGravimetri ALaurentia ViraliaPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Alternative Gravimetric Methodology For Isostatic Analyses. An Example For Bolivian AndesDocument12 pagesAlternative Gravimetric Methodology For Isostatic Analyses. An Example For Bolivian AndesMaslia Olfah06Pas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- CO2 Capture PaperDocument12 pagesCO2 Capture PaperTeresa VicentePas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- 1 Journal of Colloid Science and BiotechnologyDocument10 pages1 Journal of Colloid Science and BiotechnologyMaslia Olfah06Pas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- CO2 Capture PaperDocument12 pagesCO2 Capture PaperTeresa VicentePas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Tugas Pemurnian LogamDocument12 pagesTugas Pemurnian LogamMaslia Olfah06Pas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- 051-11 BoulayDocument10 pages051-11 BoulayMaslia Olfah06Pas encore d'évaluation
- 1 Journal of Colloid Science and BiotechnologyDocument10 pages1 Journal of Colloid Science and BiotechnologyMaslia Olfah06Pas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- 1 Journal of Colloid Science and BiotechnologyDocument10 pages1 Journal of Colloid Science and BiotechnologyMaslia Olfah06Pas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Location of End Point in Potentiometric Argentometric Titration Using Gran Plot and Titration ErrorsDocument8 pagesLocation of End Point in Potentiometric Argentometric Titration Using Gran Plot and Titration ErrorsNayalahurePas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- CO2 Capture PaperDocument12 pagesCO2 Capture PaperTeresa VicentePas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Gravimetri ADocument6 pagesGravimetri ALaurentia ViraliaPas encore d'évaluation
- 14Document9 pages14Maslia Olfah06Pas encore d'évaluation
- 1323 1331 1 PBDocument5 pages1323 1331 1 PBMaslia Olfah06Pas encore d'évaluation
- SolvenDocument2 pagesSolvenMaslia Olfah06Pas encore d'évaluation
- SolvenDocument2 pagesSolvenMaslia Olfah06Pas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- SolvenDocument2 pagesSolvenMaslia Olfah06Pas encore d'évaluation
- Biology BIOL 1003 CH 1-3 QuizDocument14 pagesBiology BIOL 1003 CH 1-3 QuizYummyWords100% (1)
- The Physical Features of Pakistan 2Document2 pagesThe Physical Features of Pakistan 2Tabeer Ahmed69% (26)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Section - 15985 Temperature Control Specifiers GuideDocument6 pagesSection - 15985 Temperature Control Specifiers GuideAntonio LebrunPas encore d'évaluation
- YORK Marine Screw Chiller Units: Type MCSH-SDocument2 pagesYORK Marine Screw Chiller Units: Type MCSH-SMike ErftmierPas encore d'évaluation
- Chilton CoburnDocument5 pagesChilton Coburnandreluisalberton100% (1)
- Pwps Mobilepac BrochureDocument6 pagesPwps Mobilepac BrochureMarcos Huata100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (120)
- Project ADocument21 pagesProject Amustafabeda06Pas encore d'évaluation
- RS3554 - O2 Generator General 2020 Rev 3 RSID 3554 enDocument12 pagesRS3554 - O2 Generator General 2020 Rev 3 RSID 3554 enimanhtglng2406Pas encore d'évaluation
- Bridge FoundationDocument24 pagesBridge FoundationR MathewPas encore d'évaluation
- Alok NandanDocument41 pagesAlok NandanSean Curnow100% (1)
- Corrosion of Steel in ConcreteDocument2 pagesCorrosion of Steel in ConcreteaaPas encore d'évaluation
- Ashrae 2002Document11 pagesAshrae 2002Luís SilvaPas encore d'évaluation
- Output - 3.1 - Greywater Reuse in NAWAMED CountriesDocument56 pagesOutput - 3.1 - Greywater Reuse in NAWAMED CountriesHussamPas encore d'évaluation
- 5.3.7 Kerabari FormationDocument47 pages5.3.7 Kerabari FormationPitambar Poudel100% (1)
- Literature Review Water Quality AnalysisDocument5 pagesLiterature Review Water Quality Analysisafmzmxkayjyoso100% (1)
- Sports Complex, Oman-GD-DR-SQ-01Document1 pageSports Complex, Oman-GD-DR-SQ-01bhupsjangirPas encore d'évaluation
- Chapter 11 - Dryland Strategies - A Permaculture Design Course HandbookDocument8 pagesChapter 11 - Dryland Strategies - A Permaculture Design Course Handbookikhlas65Pas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Heat Transfer Co-Efficient CEDocument17 pagesHeat Transfer Co-Efficient CEjaanabhenchodPas encore d'évaluation
- Principles of InfrastructureDocument47 pagesPrinciples of InfrastructureJosephine CepedaPas encore d'évaluation
- OvergrazingDocument6 pagesOvergrazingNoah AusejoPas encore d'évaluation
- EcosystemDocument3 pagesEcosystemTanya BurginPas encore d'évaluation
- PAPER 2222 With Mark AnswersDocument16 pagesPAPER 2222 With Mark AnswersAbhijeet GholapPas encore d'évaluation
- Introduction To SWAT: Léonard Bernard-JanninDocument24 pagesIntroduction To SWAT: Léonard Bernard-JanninAshraf E'daibatPas encore d'évaluation
- SNC Chart Listing-4Document63 pagesSNC Chart Listing-4Ali AskarogluPas encore d'évaluation
- Mobilgear 630 MSDS 영문Document6 pagesMobilgear 630 MSDS 영문thisbaboPas encore d'évaluation
- Urban Genius Living Solutions: Introduction LetterDocument1 pageUrban Genius Living Solutions: Introduction LetterShilpa GodbolePas encore d'évaluation
- Test PHDocument16 pagesTest PHMarielys DorantePas encore d'évaluation
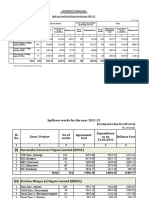
- Spill-Over and Fresh Works For The Year 2021-22: Rs. in CroresDocument4 pagesSpill-Over and Fresh Works For The Year 2021-22: Rs. in Croreshoney honeyPas encore d'évaluation
- Gas Pipe Sizing (Propane)Document6 pagesGas Pipe Sizing (Propane)Mike AdvinculaPas encore d'évaluation
- Nital Etch Procedure PDFDocument1 pageNital Etch Procedure PDFKazuoPas encore d'évaluation
- The End of Craving: Recovering the Lost Wisdom of Eating WellD'EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellÉvaluation : 4.5 sur 5 étoiles4.5/5 (80)
- Sully: The Untold Story Behind the Miracle on the HudsonD'EverandSully: The Untold Story Behind the Miracle on the HudsonÉvaluation : 4 sur 5 étoiles4/5 (103)
- Highest Duty: My Search for What Really MattersD'EverandHighest Duty: My Search for What Really MattersPas encore d'évaluation
- Hero Found: The Greatest POW Escape of the Vietnam WarD'EverandHero Found: The Greatest POW Escape of the Vietnam WarÉvaluation : 4 sur 5 étoiles4/5 (19)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaD'EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaPas encore d'évaluation