Académique Documents
Professionnel Documents
Culture Documents
Intraoperative High-Dose Dexamethasone
Transféré par
inoesienaCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Intraoperative High-Dose Dexamethasone
Transféré par
inoesienaDroits d'auteur :
Formats disponibles
ORIGINAL CONTRIBUTION
Intraoperative High-Dose Dexamethasone
for Cardiac Surgery
A Randomized Controlled Trial
Jan M. Dieleman, MD Context Prophylactic corticosteroids are often administered during cardiac surgery to at-
Arno P. Nierich, MD tenuate the inflammatory response to cardiopulmonary bypass and surgical trauma; how-
Peter M. Rosseel, MD ever, evidence that routine corticosteroid use can prevent major adverse events is lacking.
Joost M. van der Maaten, MD Objective To quantify the effect of intraoperative high-dose dexamethasone on the
incidence of major adverse events in patients undergoing cardiac surgery.
Jan Hofland, MD
Design, Setting, and Participants A multicenter, randomized, double-blind, placebo-
Jan C. Diephuis, MD controlled trial of 4494 patients aged 18 years or older undergoing cardiac surgery
Ronald M. Schepp, MD with cardiopulmonary bypass at 8 cardiac surgical centers in the Netherlands enrolled
between April 13, 2006, and November 23, 2011.
Christa Boer, PhD
Intervention Patients were randomly assigned to receive a single intraoperative dose
Karel G. Moons, PhD of 1 mg/kg dexamethasone (n=2239) or placebo (n=2255).
Lex A. van Herwerden, MD Main Outcome Measures A composite of death, myocardial infarction, stroke,
Jan G. Tijssen, MD renal failure, or respiratory failure, within 30 days of randomization.
Sandra C. Numan, MSc Results Of the 4494 patients who underwent randomization, 4482 (99.7%) could
be evaluated for the primary outcome. A total of 157 patients (7.0%) in the dexa-
Cor J. Kalkman, MD
methasone group and 191 patients (8.5%) in the placebo group reached the primary
Diederik van Dijk, MD study end point (relative risk, 0.83; 95% CI, 0.67-1.01; absolute risk reduction, 1.5%;
for the Dexamethasone for Cardiac 95% CI, 3.0% to 0.1%; P=.07). Dexamethasone was associated with reductions in
Surgery (DECS) Study Group postoperative infection, duration of postoperative mechanical ventilation, and lengths
of intensive care unit and hospital stays. In contrast, dexamethasone was associated
with higher postoperative glucose levels.
C
ARDIAC SURGERY IS AMONG THE Conclusion In our trial of adults undergoing cardiac surgery, the use of intraopera-
most commonly performed tive dexamethasone did not reduce the 30-day incidence of major adverse events com-
surgical procedures.1 Despite pared with placebo.
important improvements in Trial Registration clinicaltrials.gov Identifier: NCT00293592
surgical technique, anesthesia manage- JAMA. 2012;308(17):1761-1767 www.jama.com
ment, and postoperative care, cardiac sur-
gery is still associated with a substantial tisystem organ failure. It therefore dose of intravenous methylpredniso-
risk of major adverse events.1-3 seems reasonable to try to attenuate the lone or dexamethasone during the sur-
Cardiopulmonary bypass (CPB) may inflammatory response, which is part gery. These drugs are low-cost, potent
play a role in the development of many of routine care in many cardiac surgi- anti-inflammatory agents and there-
of these adverse outcomes.4,5 Cardio- cal centers. Often, this is accom- fore represent an appealing treatment
pulmonary bypass induces a complex plished with long-acting corticoste- option in this scenario.6,7 However, con-
acute phase response, characterized by roids, typically by administering a high cerns about potential adverse effects, in-
both cell and protein activation, which
may further be intensified by the sur- Author Affiliations: Division of Anesthesiology, In- Center, Amsterdam (Dr Boer); and Academic Medi-
tensive Care, and Emergency Medicine, University cal Center, University of Amsterdam, Amsterdam (Dr
gical trauma, ischemia-reperfusion in- Medical Center, Utrecht (Drs Dieleman, Moons, van Tijssen), the Netherlands.
jury, and endotoxemia. In a signifi- Herwerden, Kalkman, and van Dijk and Ms Numan); Members of the Dexamethasone for Cardiac Surgery
Isala Klinieken, Zwolle (Dr Nierich); Amphia Zieken- (DECS) Study Group are listed at the end of this article.
cant number of patients, a postoperative huis, Breda (Dr Rosseel); University Medical Center, Corresponding Author: Jan M. Dieleman, MD, Divi-
systemic inflammatory response syn- Groningen (Dr van der Maaten); Erasmus Medical Cen- sion of Anesthesiology, Intensive Care, and Emer-
ter, Rotterdam (Dr Hofland); Medisch Spectrum gency Medicine, University Medical Center Utrecht,
drome develops, characterized by fe- Twente, Enschede (Dr Diephuis); Medical Center, PO Box 85500, 3508 GA, Utrecht, the Netherlands
ver, organ dysfunction, and even mul- Leeuwarden (Dr Schepp); Vrije Universiteit Medical (s.dieleman@umcutrecht.nl.).
2012 American Medical Association. All rights reserved. JAMA, November 7, 2012Vol 308, No. 17 1761
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
cluding inadequate serum glucose con- Group. The study was conducted in ac- tropic support, antifibrinolytic therapy,
trol, infectious complications, poor cordance with Good Clinical Practice and cell saving techniques, were left at the
wound healing, and gastrointestinal principles and applicable national regu- discretion of the attending team. Use of
bleeding, have precluded their wide- lations. The research ethics committee corticosteroid-containing solutions for
spread adoption in cardiac surgical at each participating center approved the cardioplegia or bypass circuit prime was
practice.8,9 In contrast with several Eu- protocol. All patients were required to notallowed.Whenindicated,patientstak-
ropean countries, corticosteroids are not provide written informed consent be- ing preoperative systemic corticosteroids
routinely used during cardiac surgery fore randomization. received a perioperative corticosteroid
in most centers in the United States.10 stress regimen.
Previous studies have shown that cor- Randomization and Masking Aftersurgery,patientsweretransferred
ticosteroids attenuate the increase of se- Afterprovidingwritteninformedconsent, to the intensive care unit (ICU) and
rum inflammatory markers and may im- patientswererandomizedtoreceiveeither weanedfrommechanicalventilationwhen
provepulmonarygasexchangeandreduce dexamethasone or placebo treatment. therewasnoexcessiveongoingbloodloss
the need for postoperative inotropic sup- Dexamethasone(1mg/kgofbodyweight, and patients were cooperative and hemo-
port.7 However, appropriately sized stud- with a 100 mg maximum) or placebo was dynamically stable. Perioperative serum
ies on important clinical outcomes are administered as a single intravenous in- glucose was regulated according to local
lacking.Also,recentmeta-analysesdidnot jection after induction of anesthesia, but sliding scale protocols.
generate sufficient statistical power to al- before initiation of CPB. The study drug
low conclusions on the effectiveness of was supplied in packaged ampoules, each Statistical Analysis
corticosteroids in the reduction of major assigned to a unique study number. Pack- The primary study end point of major ad-
adverse events.11-13 As a result, corticoste- agesandampoulesofdexamethasoneand verse events was a composite of death,
roid administration during cardiac sur- placebo were identical and contained an myocardial infarction (MI), stroke, renal
gery is still controversialin many Eu- equalvolume(5mL)ofa20mg/mLdexa- failure, or respiratory failure, occurring
ropean hospitals it is part of routine care, methasone solution or normal saline, within 30 days of randomization. Peri-
whereas this is not the case in most North respectively. An independent statistician operative MI was defined as the presence
American cardiac surgical centers.14 created a computer-generated 1:1 ran- of new Q waves or a new left bundle
We conducted a large randomized domization scheme, which was stratified branch block on the electrocardiogram,
clinical trial to quantify the effect of a toparticipatingcenterandinblocksof40. combined with a biomarker (creatine
single intraoperative dose of dexameth- The research pharmacist of the Univer- kinaseMBortroponin)elevationofmore
asone on the incidence of major ad- sity Medical Center Utrecht, Utrecht, the than 5 times the upper reference limit.
verse events in patients undergoing car- Netherlands, prepared and delivered Data from routine cardiac biomarker
diac surgery. batches of 40 ampoules to each center. surveillance were used to detect possible
When a consenting patient arrived in the perioperative MI. The specific type of bio-
METHODS operating department, a packaged am- markerusedwasdictatedbythelocalpro-
Study Design and Participants poulewastakenfromthebatch.Whenthe tocol in each center, rather than by the
The Dexamethasone for Cardiac Sur- ampoule had been opened and the study study protocol. Postdischarge MI was de-
gery (DECS) study is a multicenter, drug was administered, the patient was fined according to the criteria of the Uni-
randomized, double-blind, placebo- considered randomized and the corre- versal Definition of Myocardial Infarc-
controlled study comparing high- sponding study number was assigned to tion.15 Stroke was defined as a neurologic
dose intravenous dexamethasone with that patient. Patients, caregivers, and re- deficit lasting more than 24 hours, with
placebo treatment in patients under- searchers were unaware of study group increased invalidity (increase on Rankin
going cardiac surgery. Between April 13, assignment. scale16 of 1 point) and signs of a new
2006, and November 23, 2011, we re- ischemic cerebral infarction on com-
cruited patients in 8 cardiac surgical Procedures puted tomography or magnetic resonance
centers in the Netherlands. Patients Anesthesia and surgical treatment were imaging scan. Renal failure in patients not
aged 18 years or older who were sched- performed according to the standard pro- previously receiving dialysis was defined
uled for any type of elective or urgent cedures of each participating center. Sur- according to the RIFLE criteria as an in-
cardiac surgical procedure requiring gical access to the heart was achieved via crease in postoperative serum creatinine
CPB were considered eligible. Exclu- midline sternotomy. The anesthetic tech- of at least 3 times the preoperative value,
sion criteria included an emergent or nique was based on either total intrave- or a serum creatinine level of more than
off-pump procedure and a life expec- nous anesthesia or a combination of in- 4 mg/dL (to convert to micromoles per
tancy of less than 6 months. travenous opioids and muscle relaxants liter, multiply by 88.4) associated with an
The study protocol was designed by in combination with volatile anesthetics. acute increase of serum creatinine of at
the academic authors, in collaboration Techniques for cardioplegia, myocardial least 0.5 mg/dL.17 Respiratory failure was
with the members of the DECS Study protection, and CPB, as well as use of ino- defined as postoperative mechanical ven-
1762 JAMA, November 7, 2012Vol 308, No. 17 2012 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
tilation or reinstitution of mechanical re-
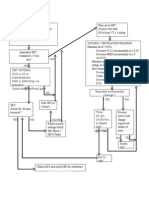
Figure 1. Enrollment Flowchart
spiratory support via an orotracheal tube
or tracheostomy for an uninterrupted pe- 25 085 Patients assessed for eligibility
riod of at least 48 hours.
An exploratory analysis of prospec- 3504 Not eligible for inclusion because of planned
off-pump surgery
tively defined secondary outcomes in-
cluded each separate component of the 21 581 Eligible for inclusion
primary end point (ie, death, MI, stroke,
renal failure, or respiratory failure, within 17 087 Excluded
the first 30 days); postoperative infec- 16 754 Not invited or declined participation
333 Provided informed consent but not
tions; postoperative atrial fibrillation; randomized
highest serum glucose concentration in 14 Informed consent withdrawn
25 Corticosteroids considered indicated
the ICU; highest body temperature in the or contraindicated a
65 Not randomized for logistic reasons
ICU; postoperative delirium (defined as 114 Intervention canceled
the postoperative indication for treat- 115 Other reasons
ment with neuroleptic drugs); time to
weaning from postoperative mechani- 4494 Randomized
cal ventilation; and time to discharge
from the ICU and from the hospital. 2239 Randomized to receive dexamethasone 2255 Randomized to receive placebo
An independent, blinded critical event 2239 Received dexamethasone as 2255 Received placebo as randomized
randomized
adjudicationcommitteereviewedallcases
of death, possible MI, and possible stroke. 4 No follow-up for primary end point 8 No follow-up for primary end point
Cases of possible MI or stroke were either 2 Withdrew informed consent 5 Withdrew informed consent
2 Patients could not be traced 3 Patients could not be traced
confirmed or revoked according to the
study definitions of these events.
2235 Included in primary analysis 2247 Included in primary analysis
We hypothesized that dexamethasone 4 Excluded due to no follow-up 8 Excluded due to no follow-up
administration would reduce the inci-
Data on the number of patients that were not invited to participate, or that declined participation, were not con-
denceoftheprimarystudyoutcome.Based sistently logged in all centers and are therefore not sufficiently accurate to be reported in detail. aIndication or
on the Society of Thoracic Surgeons da- contraindication dictated by either the treating medical team or the clinical situation during the start of the pro-
cedure in the operating department.
tabase,18 the incidence of the primary out-
come in the placebo group was estimated
to be 6%. To detect an absolute difference Patient follow-up for secondary out- ondary outcomes, we used the 2 test.
of 2% (from 6% to 4%) with a power of comes was until 1 year from randomiza- Absolute risk reduction or relative risk
80% at a 2-sided .05 significance level, tion by study protocol. Herein, we report (RR) with 95% CIs was calculated for
1962 patients would be required in each theprimarystudyendpointtogetherwith each dichotomous outcome measure.
study group. To compensate for possible exploratory analyses of other outcomes Logistic regression was used for assess-
lossestofollow-up,weplannedtoinclude in the first 30 days after randomization. ing heterogeneity in the subgroup
2250 patients per study group. Analyses were conducted according to analyses, with a .10 threshold for sig-
During the study, 3 preplanned in- randomization (intention-to-treat analy- nificance. For comparison of mean and
terim analyses on the primary study end ses).Baselinecharacteristicsinthe2study median values of the continuous sec-
point were performed when 1000, 2000, groups were evaluated using frequency ondary outcome measures, we used Stu-
and 3250 patients, respectively, had been distributions. dent t test or Mann-Whitney U test, as
enrolled. Interim analyses were per- We also performed preplanned sub- appropriate. IBM SPSS version 19 (SPSS
formed by the independent data and group analyses for the primary out- Inc) was used for all analyses.
safety monitoring board, which con- come and its separate components,
sisted of an epidemiologist, a cardiac sur- which included 4 age groups (65, 65- RESULTS
geon, and a cardiac anesthesiologist not 74, 75-79, and 80 years), sex, diabe- Study Population
involved in the study. These blinded tes, chronic obstructive pulmonary dis- An estimated 25 085 patients sched-
analyses were adjusted according to an ease, higher (5) vs low (4) EuroScore uled to undergo elective or urgent car-
OBrien and Fleming type I error spend- preoperative risk estimate20 (cutoff value diac surgery were screened, of whom
ing function,19 using an overall .05 sig- based on the median EuroScore of the 21 581 were eligible for inclusion. Of the
nificance level. As a result of these in- study population), and prolonged CPB 4827 patients who provided written in-
terim analyses, the threshold for duration (defined as 150 minutes). formed consent, 4494 eventually under-
significance of the primary study end For the comparison of the propor- went randomization (FIGURE 1). Two
point in the final analysis was .044. tions of patients with primary and sec- patients who were unintentionally
2012 American Medical Association. All rights reserved. JAMA, November 7, 2012Vol 308, No. 17 1763
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
randomized without having provided the dexamethasone group and 5 sone group and 3 patients (0.1%) in
informed consent were excluded patients (0.2%) in the placebo group the placebo group after hospital dis-
from the analysis. Of the 4494 ran- withdrew consent; therefore, their charge. Thus, the analyzed popula-
domized patients, 2239 (49.8%) were primary outcome could not be tion consisted of 4482 patients
randomized to dexamethasone treat- assessed. We were unable to obtain (99.7%). Patients in the study groups
ment and 2255 (50.2%) to placebo 30-day outcome information in 2 were similar with respect to baseline
treatment. Two patients (0.1%) in patients (0.1%) in the dexametha- demographic, clinical, and surgical
characteristics (TABLE 1).
Table 1. Demographic, Clinical, and Surgical Characteristics of the Dexamethasone and Primary Study End Point
Placebo Groups a
Dexamethasone Placebo In total, 348 patients (7.8%) reached the
Characteristics (n = 2235) (n = 2247) composite primary study end point of
Demographics death, MI, stroke, renal failure, or re-
Age, mean (SD), y 66.2 (11.0) 66.1 (10.7) spiratory failure, within 30 days after
Male sex 1622 (72.6) 1628 (72.5)
randomization (TABLE 2). The pri-
Weight, mean (SD), kg 82.4 (14.3) 82.0 (14.4)
mary study end point occurred in 157
Height, mean (SD), cm 174 (9.1) 173 (9.2)
of the 2235 patients (7.0%) random-
Coexisting medical conditions
Hypertension 1179 (54.7) 1180 (54.8) ized to dexamethasone and in 191 of
Diabetes mellitus the 2247 patients (8.5%) randomized
Insulin dependent 106 (4.8) 125 (5.8) to placebo (RR, 0.83; 95% CI, 0.67-
Noninsulin dependent 309 (13.9) 311 (13.9) 1.01; absolute risk reduction, 1.5%;
Treatment for pulmonary disease 243 (10.9) 266 (11.9) 95% CI, 3.0% to 0.1%; P =.07).
Previous cerebrovascular event
Stroke 86 (3.9) 78 (3.5) Exploratory Analysis of the
Transient ischemic attack 107 (4.8) 103 (4.6) Combined End Point Components
Peripheral vascular disease 191 (8.6) 192 (8.6)
The rate of death, MI, stroke, and re-
Preoperative creatinine, mean (SD), mg/dL 1.04 (0.38) 1.07 (0.57)
nal failure was similar in both groups.
Chronic renal dysfunction 17 (0.8) 29 (1.3)
In the dexamethasone group, 67 pa-
Cardiac status
Recent myocardial infarction (90 d) 195 (8.7) 176 (7.8) tients (3.0%) experienced respiratory
Left ventricular function b failure compared with 97 patients
Moderate 503 (22.6) 534 (23.9) (4.3%) in the placebo group (RR, 0.69;
Poor 103 (4.6) 117 (5.2) 95% CI, 0.51-0.94; P =.02) (Table 2).
EuroScore, median (IQR) c 5 (3-7) 5 (3-7) The RR for a composite end point with-
Preoperative medication out the respiratory failure compo-
-Blocker 1485 (68.4) 1479 (68.2)
nent, consisting of only mortality, MI,
Statin 836 (58.0) 771 (53.8)
stroke, and renal failure, was 0.84 (95%
Corticosteroid 130 (7.2) 98 (5.4)
Type of surgery
CI, 0.66-1.08; P=.18).
Isolated CABG 873 (39.1) 895 (39.8)
CABG plus valve 360 (16.1) 388 (17.3) Secondary End Points
Single valve 575 (25.7) 558 (24.8) The median (interquartile range [IQR])
Surgery on multiple valves 82 (3.7) 99 (4.4) time to weaning from mechanical ven-
Other procedures 336 (15.0) 295 (13.1) tilation was 7.0 (4.7-10.0) hours (mean,
Repeat surgery 140 (6.3) 147 (6.6) 11.0 hours) in the dexamethasone group
Duration of procedure, mean (SD), min 244 (102) 242 (93) and 7.0 (5.0-11.0) hours (mean, 14.3
Duration of extracorporeal circulation, mean (SD), min 125 (68) 124 (64) hours) in the placebo group (P.001)
Duration of aortic cross-clamping, mean (SD), min 87 (47) 85 (44) (TABLE 3). The median (IQR) time to dis-
Deep hypothermic circulatory arrest 15 (0.7) 23 (1.0) charge from the ICU was 22.0 (19.0-
Use of cell-saving device 1151 (51.8) 1104 (49.4) 24.0) hours (mean, 34.2 hours) in the
Use of antifibrinolytic drug dexamethasone group and 22.0 (19.0-
Tranexamic acid 1834 (82.4) 1835 (81.8)
25.0) hours (mean, 43.6 hours) in the
Other antifibrinolytic drug 10 (0.4) 18 (0.8)
placebo group (P.001). The low P val-
Abbreviations: CABG, coronary artery bypass graft; IQR, interquartile range.
SI conversion: To convert creatinine to mol/L, multiply by 88.4. ues for both comparisons despite simi-
a Data are shown as No. (%) unless otherwise indicated.
b Definition of left ventricular function classes20: moderate, ejection fraction of 30% to 50%; and poor, ejection fraction lar median values are the result of a
of less than 30%.
c Higher EuroScores present increased risk of perioperative mortality.20
higher proportion of patients requiring
prolongedventilationtimesandprolonged
1764 JAMA, November 7, 2012Vol 308, No. 17 2012 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
ICU stay in the placebo group. For the mortality component of the pri- incidence of the composite primary
example, the proportion of patients mary study end point, which showed study end point of major adverse
requiring more than 24 hours of post- significant heterogeneity (P=.05). In pa- events (P = .07). In an exploratory
operative mechanical ventilation was tients younger than 65 years, the RR analysis of secondary end points, a
3.4% in the dexamethasone group for mortality was 0.42 (95% CI, 0.13- reduced incidence of respiratory fail-
compared with 4.9% in the placebo 1.34; P = .13), but it gradually in- ure was found, which was accompa-
group. Similarly, the proportion of creased with age to 3.87 (95% CI, 1.10- nied by an overall reduced time to
patients requiring more than 48 hours 13.6; P =.02) in patients aged 80 years weaning from mechanical ventila-
of ICU stay was lower in the dexa- or older. tion, a lower risk of pneumonia, and
methasone group than in the placebo a reduction in ICU and hospital stay.
group (10.2% vs 14.0%, respectively). COMMENT The DECS trial is the first large ran-
The median (IQR) time to discharge Our randomized study of 4494 domized controlled trial to our knowl-
from the hospital in the dexametha- patients undergoing cardiac surgery edge on the controversial topic of rou-
sone group was 8 (7-13) days (mean, failed to show a statistically signifi- tine corticosteroid use during cardiac
11.3 days) vs 9 (7-13) days (mean, cant benefit of intraoperative admin- surgery in adults. The numerous small
11.7 days) in the placebo group istration of dexamethasone on the randomized studies published in the last
(P=.009).
The risk of developing a postopera-
Table 2. Primary Study End Point and Components of the Primary Study End Point in the
tive infection was lower in the dexa- Dexamethasone and Placebo Groups
methasone group than in the placebo No. (%) of Patients
group (9.5% vs 14.8%, respectively; RR,
0.64; 95% CI, 0.54-0.75; P .001) Dexamethasone Placebo Relative Risk
(n = 2235) (n = 2247) (95% CI)
(Table 3). This protective effect was pri- Primary study end point a 157 (7.0) 191 (8.5) 0.83 (0.67-1.01)
marily related to a decreased inci- Components of the primary study end point
dence of pneumonia in the dexameth- Death 31 (1.4) 34 (1.5) 0.92 (0.57-1.49)
asone group (6.0% vs 10.6% in the Myocardial infarction 35 (1.6) 39 (1.7) 0.90 (0.57-1.42)
placebo group; RR, 0.56; 95% CI, 0.46- Stroke 29 (1.3) 32 (1.4) 0.91 (0.55-1.50)
0.69; P.001). In contrast, the mean Renal failure 28 (1.3) 40 (1.8) 0.70 (0.44-1.14)
highest serum glucose concentration Respiratory failure 67 (3.0) 97 (4.3) 0.69 (0.51-0.94)
was higher in the dexamethasone group a Primary study end point was a composite of death, myocardial infarction, stroke, renal failure, or respiratory failure,
within 30 days after surgery.
and the incidence of postoperative fe-
ver was higher in the placebo group
(Table 3).
Table 3. Secondary End Points in the Dexamethasone and Placebo Groups
Subgroup Analysis Dexamethasone Placebo Relative Risk P
Secondary End Points (n = 2235) (n = 2247) (95% CI) Value a
The preplanned subgroup analyses Median (IQR)
suggested an age-dependent effect of Duration of postoperative mechanical 7.0 (4.7-10.0) 7.0 (5.0-11.0) NA .001
ventilation, h
dexamethasone on the primary study
Length of stay in the ICU, h 22.0 (19.0-24.0) 22.0 (19.0-25.0) NA .001
end point (P for heterogeneity = .08)
Length of hospital stay, d 8 (7-13) 9 (7-13) NA .009
(FIGURE 2). In patients younger than
Mean (SD)
65 years, dexamethasone was associ- Highest serum glucose concentration 195 (50) 177 (59) NA .001
ated with lower likelihood for the in the ICU, mg/dL
primary end point (RR, 0.65; 95% No. (%)
Body temperature in the ICU of 38C 132 (5.9) 564 (25.2) 0.23 (0.20-0.27) .001
CI, 0.44-0.96; P = .03), whereas in
Atrial fibrillation 739 (33.1) 790 (35.2) 0.94 (0.87-1.02) .14
patients aged 80 years or older, the Delirium 205 (9.2) 262 (11.7) 0.79 (0.66-0.94) .006
RR was 1.69 (95% CI, 0.92-3.10; Gastrointestinal bleeding 13 (0.6) 11 (0.5) 1.19 (0.53-2.65) .67
P = .09). There was no differential Any postoperative infection 212 (9.5) 333 (14.8) 0.64 (0.54-0.75) .001
treatment effect in the subgroup Pneumonia 133 (6.0) 238 (10.6) 0.56 (0.46-0.69) .001
analyses on sex, diabetes, chronic Urinary tract infection 50 (2.2) 60 (2.7) 0.84 (0.58-1.21) .35
obstructive pulmonary disease, Wound infection 34 (1.5) 32 (1.4) 1.07 (0.66-1.72) .79
EuroScore, or prolonged CPB dur- Catheter-related infection 6 (0.3) 21 (0.9) 0.29 (0.12-0.71) .004
ation. Sepsis 18 (0.8) 26 (1.2) 0.70 (0.38-1.27) .23
The bidirectional effect of dexameth- Abbreviations: ICU, intenstive care unit; IQR, interquartile range; NA, not applicable.
asone across the 4 age groups ap- SI conversion: To convert glucose to mmol/L, multiply by 0.0555.
a For parametric and nonparametric comparisons of continuous data.
peared to be predominantly caused by
2012 American Medical Association. All rights reserved. JAMA, November 7, 2012Vol 308, No. 17 1765
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
Figure 2. Forest Plot of Subgroup Analyses
No. of Patients No. of Events
Relative Risk Dexamethasone Placebo P for
Subgroup Dexamethasone Placebo Dexamethasone Placebo (95% CI) Better Better Trend
Sex
Male 1622 1628 112 130 0.86 (0.68-1.10)
.50
Female 613 619 45 61 0.74 (0.52-1.08)
Age, y
<65 913 962 38 62 0.65 (0.44-0.96)
65-74 818 781 57 70 0.78 (0.56-1.09)
75-79 344 336 38 44 0.84 (0.56-1.27) .08
80 159 168 24 15 1.69 (0.92-3.10)
EuroScore
0-4 1025 1060 43 46 0.97 (0.64-1.45)
.32
5 1180 1147 113 142 0.77 (0.61-0.98)
Diabetes
No 1816 1805 115 142 0.81 (0.64-1.02)
.73
Yes 415 436 41 49 0.88 (0.59-1.30)
COPD
No 1987 1978 133 153 0.87 (0.69-1.08)
.39
Yes 243 266 24 38 0.69 (0.43-1.12)
Prolonged CPB
No 1674 1659 85 92 0.92 (0.69-1.22)
.37
Yes 536 563 71 96 0.78 (0.59-1.03)
Total 2235 2247 157 191 0.83 (0.67-1.01)
0.2 1.0 5.0
Relative Risk (95% CI)
COPD indicates chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass. The effect estimates for the primary study end point in the subgroup analyses
are shown. The size of each data marker correlates with the total number of patients in that subgroup.
decades had conflicting results13 and terrelated and explained by attenua- fections, in the dexamethasone group
provided only limited guidance for se- tion of the perioperative systemic was unexpected, and contrary to exist-
lection of components for the compos- inflammatory response syndrome, ing knowledge that corticosteroids in-
ite primary end point of our study. No which is the pathophysiologic goal of crease the risk of infections.7 How-
significant benefit from dexametha- the single high-dose corticosteroid ever, this adverse effect is mainly related
sone treatment was observed on the treatment.11-14 Thus, although the ef- to chronic corticosteroid use, rather
composite primary end point, which fect of dexamethasone on the primary than to a single prophylactic pulse dose
was largely cardiovascular. However, end point was negative, there is the pos- in circumstances wherein the activa-
further exploration of the study data sibility that a clinically significant ef- tion of the immune system could be det-
suggested a consistent pattern of im- fect was missed. Therefore, a new pro- rimental.11-14 The reduced infection risk
proved pulmonary condition, mani- spective study focusing on pulmonary persisted when patients with respira-
fested as a lower risk of postoperative outcomes seems a logical next step to tory failure were excluded from the
respiratory failure, shorter times to further explore the secondary find- analysis, suggesting that the observa-
weaning from the ventilator, and re- ings of our trial. Such a study should tion is not the result of shorter expo-
duced risk of pneumonia during post- also consider patient selection for the sure to mechanical ventilation.
operative hospitalization in the dexa- therapy as we found a larger beneficial A pooled analysis of this study with
methasone group. This improved effect in younger patients and no ap- the results of our previous meta-
respiratory condition was accompa- parent benefit in those aged 80 years or analysis of 56 studies on prophylactic
nied by earlier discharge from both the older. corticosteroids in cardiac surgery,
ICU and the hospital. It might be ar- Our study failed to confirm that cor- which was recently published,13 showed
gued that the beneficial effect of dexa- ticosteroids reduce the incidence of no effect of corticosteroids for mortal-
methasone on these multiple second- postoperative atrial fibrillation, as dem- ity or cardiac complications. How-
ary outcomes was only a coincident onstrated in a previous study.21 The 241 ever, corticosteroid treatment re-
finding. However, also at a more con- patients in that particular trial re- duced respiratory complications (Peto
servative cutoff value for statistical sig- ceived moderate-dose hydrocortisone odds ratio, 0.59; 95% CI, 0.49-0.71;
nificance of .0025to correct for test- 3 days postoperatively, instead of the P.001) (eMethods and eFigure, avail-
ing a total of 19 secondary outcomes single intraoperative high dose of dexa- able at http://www.jama.com).
using a Bonferroni approachmost of methasone in our study. A limitation of our trial is that we
the effects found remained signifi- The reduced risk of postoperative in- studied a high-dose dexamethasone
cant. These effects may actually be in- fections, in particular pulmonary in- regimen, which is often used during
1766 JAMA, November 7, 2012Vol 308, No. 17 2012 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
INTRAOPERATIVE HIGH-DOSE DEXAMETHASONE FOR CARDIAC SURGERY
cardiac surgery in several European Author Contributions: Drs Dieleman and van Dijk had Role of the Sponsors: The sponsors had no role in the
full access to all of the data in the study and take design and conduct of the study; in the collection,
countries. In North America, however, responsibility for the integrity of the data and the analysis, and interpretation of the data; or in the prepa-
methylprednisolone is usually pre- accuracy of the data analysis. ration, review, or approval of the manuscript.
Study concept and design: Dieleman, Nierich, Rosseel, Members of the DECS Study Group (the Nether-
ferred when corticosteroids are admin- van der Maaten, Hofland, Diephuis, Moons, lands): University Medical Center, Utrecht: Jaap J. Bre-
istered during cardiac surgery. The ef- van Herwerden, Tijssen, Kalkman, van Dijk. dee, MD, Wolfgang F. Buhre, MD, Jan M. Dieleman,
fect of high-dose methylprednisolone for Acquisition of data: Dieleman, Nierich, Rosseel, MD, Diederik van Dijk, MD, Lex A. van Herwerden,
van der Maaten, Hofland, Diephuis, Schepp, Boer, MD, Cor J. Kalkman, MD, Jan van Klarenbosch, MD,
cardiac surgery is being studied in an on- van Herwerden, Numan. Karel G. Moons, PhD, Hendrik M. Nathoe, MD, San-
going large study (Steroids In cardiac Analysis and interpretation of data: Dieleman, dra C. Numan, MSc, Thomas H. Ottens, MD, Kit C.
van der Maaten, Hofland, Schepp, Moons, Tijssen, Roes, PhD, Anne-Mette C. Sauer, MD, Arjen J. Slooter,
Surgery [SIRS] trial, NCT00427388). A Kalkman, van Dijk. MD; Isala Klinieken, Zwolle: Arno P. Nierich, MD, Ja-
strength of our study is that blinding for Drafting of the manuscript: Dieleman, Moons, Tijssen, cob J. Ennema, MD; Amphia Ziekenhuis, Breda: Pe-
Kalkman, van Dijk. ter M. Rosseel, MD, Nardo J. van der Meer, MD; Uni-
treatment was well maintained during Critical revision of the manuscript for important in- versity Medical Center, Groningen: Joost M. van der
the perioperative period. The small dif- tellectual content: Dieleman, Nierich, Rosseel, Maaten, MD, Vlado Cernak, MD; Erasmus Medical
van der Maaten, Hofland, Diephuis, Schepp, Boer, Center, Rotterdam: Jan Hofland, MD, Robert J. van
ferences in serum glucose and postop- Moons, van Herwerden, Tijssen, Numan, Kalkman, Thiel, MD; Medisch Spectrum Twente, Enschede: Jan
erative body temperature are unlikely to van Dijk. C. Diephuis, MD; Medical Center, Leeuwarden: Ron-
have caused awareness of randomiza- Statistical analysis: Dieleman, Moons, Tijssen, Kalkman, ald M. Schepp, MD, Jo Haenen, MD, Fellery de Lange,
van Dijk. MD; Vrije Universiteit Medical Center, Amsterdam:
tion. It is thus unlikely that such aware- Obtained funding: Dieleman, Moons, Kalkman, Christa Boer, PhD, Jan R. de Jong, MD; Academic
ness, if any, could have influenced clini- van Dijk. Medical Center, Amsterdam: Jan G. Tijssen, MD.
Administrative, technical, or material support: Steering Committee: Jan M. Dieleman, MD, Jan C.
cal management to a degree that could Dieleman, Nierich, Numan. Diephuis, MD, Diederik van Dijk, MD, Lex A. van Her-
have produced the present effects on pul- Study supervision: Dieleman, Moons, Kalkman, werden, MD, Jan Hofland, MD, Jan R. de Jong, MD,
van Dijk. Cor J. Kalkman, MD, Jan van Klarenbosch, MD, Joost
monary outcome and duration of ICU Conflict of Interest Disclosures: All authors have com- M. van der Maaten, MD, Karel G. Moons, PhD, Arno
and hospital stay. pleted and submitted the ICMJE Form for Disclosure P. Nierich, MD, Peter M. Rosseel, MD, Ronald M.
In conclusion, in our trial of adults un- of Potential Conflicts of Interest and none were re- Schepp, MD.
ported. Data and Safety Monitoring Board: Peter Bruins, MD,
dergoing cardiac surgery, the use of in- Funding/Support: This work was supported by Bas A. de Mol, MD, Jan G. Tijssen, MD.
traoperative dexamethasone did not re- grants 80-82310-98-08607 from the Netherlands Critical Event Adjudication Committee: Jaap J. Bredee,
Organization for Health Research and Development MD, Hendrik M. Nathoe, MD, Arjen J. Slooter, MD.
duce the 30-day incidence of major (ZonMw) and 2007B125 from the Dutch Heart Online-Only Material: eMethods and eFigure are avail-
adverse events compared with placebo. Foundation. able at http://www.jama.com.
REFERENCES
1. Roger VL, Go AS, Lloyd-Jones DM, et al; Ameri- cose control after coronary artery bypass surgery. Car- Myocardial Infarction. Universal definition of myo-
can Heart Association Statistics Committee and Stroke diovasc Diabetol. 2007;6(1):39. cardial infarction. Eur Heart J. 2007;28(20):2525-
Statistics Subcommittee. Heart disease and stroke sta- 9. Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, 2538.
tistics--2011 update: a report from the American Heart Soltesz E, Koch CG. Role of intraoperative and post- 16. van Swieten JC, Koudstaal PJ, Visser MC, Schouten
Association. Circulation. 2011;123(4):e18-e209. operative blood glucose concentrations in predicting HJ, van Gijn J. Interobserver agreement for the as-
2. Newman MF, Mathew JP, Grocott HP, et al. Cen- outcomes after cardiac surgery. Anesthesiology. 2010; sessment of handicap in stroke patients. Stroke. 1988;
tral nervous system injury associated with cardiac 112(4):860-871. 19(5):604-607.
surgery. Lancet. 2006;368(9536):694-703. 10. Sulzer CF, Mackensen GB, Grocott HP. Con: Meth- 17. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky
3. Mariscalco G, Lorusso R, Dominici C, Renzulli A, ylprednisolone is not indicated for patients during car- P; Acute Dialysis Quality Initiative workgroup. Acute
Sala A. Acute kidney injury: a relevant complication diopulmonary bypass. J Cardiothorac Vasc Anesth. renal failure: definition, outcome measures, animal
after cardiac surgery. Ann Thorac Surg. 2011;92 2005;19(2):255-258. models, fluid therapy and information technology
(4):1539-1547. 11. Whitlock RP, Chan S, Devereaux PJ, et al. Clini- needs: the Second International Consensus Confer-
4. Moat NE, Shore DF, Evans TW. Organ dysfunc- cal benefit of steroid use in patients undergoing car- ence of the Acute Dialysis Quality Initiative (ADQI)
tion and cardiopulmonary bypass: the role of comple- diopulmonary bypass: a meta-analysis of random- Group. Crit Care. 2004;8(4):R204-R212.
ment and complement regulatory proteins. Eur J Car- ized trials. Eur Heart J. 2008;29(21):2592-2600. 18. The Society of Thoracic Surgeons National
diothorac Surg. 1993;7(11):563-573. 12. Ho KM, Tan JA. Benefits and risks of corticoste- Database. http://www.ctsnet.org/section
5. Asimakopoulos G. Systemic inflammation and car- roid prophylaxis in adult cardiac surgery: a dose- /stsdatabase. Accessed October 1, 2012.
diac surgery: an update. Perfusion. 2001;16(5): response meta-analysis. Circulation. 2009;119 19. OBrien PC, Fleming TR. A multiple testing pro-
353-360. (14):1853-1866. cedure for clinical trials. Biometrics. 1979;35(3):
6. Wan S, LeClerc JL, Vincent JL. Inflammatory re- 13. Dieleman JM, van Paassen J, van Dijk D, et al. Pro- 549-556.
sponse to cardiopulmonary bypass: mechanisms in- phylactic corticosteroids for cardiopulmonary bypass 20. Nashef SA, Roques F, Michel P, Gauducheau E,
volved and possible therapeutic strategies. Chest. 1997; in adults. Cochrane Database Syst Rev. 2011;(5): Lemeshow S, Salamon R. European system for car-
112(3):676-692. CD005566. diac operative risk evaluation (EuroSCORE). Eur J Car-
7. Chaney MA. Corticosteroids and cardiopulmo- 14. Whitlock RP, Rubens FD, Young E, Teoh KH. Pro: diothorac Surg. 1999;16(1):9-13.
nary bypass : a review of clinical investigations. Chest. Steroids should be used for cardiopulmonary bypass. 21. Halonen J, Halonen P, Jarvinen O, et al. Cortico-
2002;121(3):921-931. J Cardiothorac Vasc Anesth. 2005;19(2):250-254. steroids for the prevention of atrial fibrillation after car-
8. Vogelzang M, Hoekstra M, Drost JT, et al. The im- 15. Thygesen K, Alpert JS, White HD; Joint ESC/ diac surgery: a randomized controlled trial. JAMA.
pact of a reduced dose of dexamethasone on glu- ACCF/AHA/WHF Task Force for the Redefinition of 2007;297(14):1562-1567.
2012 American Medical Association. All rights reserved. JAMA, November 7, 2012Vol 308, No. 17 1767
Downloaded From: http://jama.jamanetwork.com/ by a Florida State University User on 09/09/2015
Vous aimerez peut-être aussi
- Role SteroidDocument7 pagesRole SteroidinoesienaPas encore d'évaluation
- 5th Annual Meeting IsicmDocument26 pages5th Annual Meeting IsicmFitri 'Avicena'Pas encore d'évaluation
- Lekosit FilterDocument17 pagesLekosit FilterinoesienaPas encore d'évaluation
- Br. J. Anaesth. 2008 Castillo 618 26Document9 pagesBr. J. Anaesth. 2008 Castillo 618 26inoesienaPas encore d'évaluation
- Aspen Vs EspenDocument3 pagesAspen Vs EspeninoesienaPas encore d'évaluation
- J. Biol. Chem.-2005-Bradbury-29993-30000Document9 pagesJ. Biol. Chem.-2005-Bradbury-29993-30000inoesienaPas encore d'évaluation
- Br. J. Anaesth. 2002 Bruemmer - Smith 489 95Document7 pagesBr. J. Anaesth. 2002 Bruemmer - Smith 489 95inoesienaPas encore d'évaluation
- International Journal of SurgeryDocument5 pagesInternational Journal of SurgeryinoesienaPas encore d'évaluation
- CynaDocument7 pagesCynainoesienaPas encore d'évaluation
- Comparation Between Ketamin and Tramadol in P (Eritonsilar InfiltrationDocument7 pagesComparation Between Ketamin and Tramadol in P (Eritonsilar InfiltrationinoesienaPas encore d'évaluation
- CMJ Peritonsil Blok2Document3 pagesCMJ Peritonsil Blok2inoesienaPas encore d'évaluation
- Anaesthesia 2004-59-166 HarmsDocument8 pagesAnaesthesia 2004-59-166 HarmsinoesienaPas encore d'évaluation
- Cover Pak DamDocument1 pageCover Pak DaminoesienaPas encore d'évaluation
- 602Document3 pages602Shailav SahPas encore d'évaluation
- Anestesi Geriatri File Ujian Dr. VillyDocument5 pagesAnestesi Geriatri File Ujian Dr. VillyinoesienaPas encore d'évaluation
- Rapid Wean From VentilationDocument3 pagesRapid Wean From VentilationinoesienaPas encore d'évaluation
- SSC GuidelinesDocument61 pagesSSC GuidelinesZfgZ*Pas encore d'évaluation
- Cover Pak DamDocument1 pageCover Pak DaminoesienaPas encore d'évaluation
- Rapid Wean From VentilationDocument3 pagesRapid Wean From VentilationinoesienaPas encore d'évaluation
- Effect of Palonosetron On The QTC Interval inDocument12 pagesEffect of Palonosetron On The QTC Interval ininoesienaPas encore d'évaluation
- IcuDocument2 pagesIcuinoesienaPas encore d'évaluation
- Effect of Palonosetron On The QTC Interval inDocument12 pagesEffect of Palonosetron On The QTC Interval ininoesienaPas encore d'évaluation
- Effect of Palonosetron On The QTC Interval inDocument12 pagesEffect of Palonosetron On The QTC Interval ininoesienaPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Pilates PaperDocument24 pagesPilates PaperSamantha SouzaPas encore d'évaluation
- 123Document10 pages123Aryan SanezPas encore d'évaluation
- Learning Agility WorksheetsDocument5 pagesLearning Agility WorksheetsThiago RaydanPas encore d'évaluation
- Good Study Guide For RRT ExamDocument2 pagesGood Study Guide For RRT ExamStevenPas encore d'évaluation
- Leave and Joining Time Rules: National Seeds Corporation Limited (A Government of India Undertaking)Document26 pagesLeave and Joining Time Rules: National Seeds Corporation Limited (A Government of India Undertaking)Rojan MathewPas encore d'évaluation
- Uji Fagositosis PDFDocument11 pagesUji Fagositosis PDFEno PauzahPas encore d'évaluation
- Paediatric Dosage of Some Drugs-1Document45 pagesPaediatric Dosage of Some Drugs-1JanePas encore d'évaluation
- DR - Nivedita KhareDocument3 pagesDR - Nivedita KhareBrijesh ChaurasiyaPas encore d'évaluation
- (Metals and Related Substances in Drinking Water Research Rep) M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic-Health Effects of Metals and Related Substances in Drinking Water-IWA Publishing (201Document150 pages(Metals and Related Substances in Drinking Water Research Rep) M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic-Health Effects of Metals and Related Substances in Drinking Water-IWA Publishing (201franklinPas encore d'évaluation
- Bruxism and Prostho TreatmentDocument10 pagesBruxism and Prostho Treatmentdorasani99Pas encore d'évaluation
- Ayurveda MD Panchakarma Academics DissertationDocument38 pagesAyurveda MD Panchakarma Academics DissertationManish KubawatPas encore d'évaluation
- The Teaching HospitalDocument5 pagesThe Teaching Hospitalspurtbd33% (3)
- Renal Physiology - ch25Document19 pagesRenal Physiology - ch25Jann Zaniel Allayne RiPas encore d'évaluation
- 0.9% Sodium Chloride Injection, USP: Prescribing InformationDocument8 pages0.9% Sodium Chloride Injection, USP: Prescribing InformationarinPas encore d'évaluation
- Deleuze - Critical and ClinicalDocument139 pagesDeleuze - Critical and Clinicalbornon8thofjulyPas encore d'évaluation
- Pta 1010 Observation Hours PaperDocument3 pagesPta 1010 Observation Hours Paperapi-269101384Pas encore d'évaluation
- WJCCM 11 33Document8 pagesWJCCM 11 33medicshinobiPas encore d'évaluation
- AutismScreen AssessDocument42 pagesAutismScreen AssessMelindaDuciagPas encore d'évaluation
- TechPatient CARDIO V3 BrochureDocument4 pagesTechPatient CARDIO V3 BrochureMaría Beatríz LPas encore d'évaluation
- NCP Inffective Tissue PerfusionDocument3 pagesNCP Inffective Tissue PerfusionPaul Cubacub0% (1)
- Crossmatching, Types, Principle, Procedure and InterpretationDocument5 pagesCrossmatching, Types, Principle, Procedure and InterpretationMerhan FoudaPas encore d'évaluation
- Pulse Oximeters Working PrincipleDocument2 pagesPulse Oximeters Working PrinciplealbertpatelusaPas encore d'évaluation
- BONUS - 7 Day Ab Targeted Cardio and Intervals PDFDocument42 pagesBONUS - 7 Day Ab Targeted Cardio and Intervals PDFScott Levine100% (6)
- ADHD: Clinical Practice Guideline For The Diagnosis, Evaluation, and TreatmentDocument18 pagesADHD: Clinical Practice Guideline For The Diagnosis, Evaluation, and TreatmentBen CulpepperPas encore d'évaluation
- RabiesDocument32 pagesRabiesKareen Mae Porras BienePas encore d'évaluation
- Tugas MedicineDocument2 pagesTugas MedicineRiza Ikhsan MuliaPas encore d'évaluation
- Ability Last enDocument12 pagesAbility Last enzelitePas encore d'évaluation
- Study Data Reviewer's Guide: LDCP, Inc. Study LDCP-0242-005Document16 pagesStudy Data Reviewer's Guide: LDCP, Inc. Study LDCP-0242-005anon_181306460Pas encore d'évaluation
- Evaluation of Wound Healing Activity of Leaves of Crinum AsiaticumDocument5 pagesEvaluation of Wound Healing Activity of Leaves of Crinum AsiaticumxiuhtlaltzinPas encore d'évaluation
- Yati Soenarto - RACP AucklandDocument34 pagesYati Soenarto - RACP AucklandIntan HartandyPas encore d'évaluation