Académique Documents
Professionnel Documents
Culture Documents
Closed-Book Practice-Ch 02 (2017!06!19)
Transféré par
JuanTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Closed-Book Practice-Ch 02 (2017!06!19)
Transféré par
JuanDroits d'auteur :
Formats disponibles
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 1/9
CLOSED-BOOK PRACTICE
CHAPTER 2: ATOMIC STRUCTURE AND INTERATOMIC BONDING
CONCEPT CHECK
1. Why are the atomic weights of the elements generally not integers? Cite two reasons.
Ans:
The atomic weights of the elements ordinarily are not integers because:
(1) the atomic masses of the atoms normally are not integers (except for 12C), and
(2) the atomic weight is taken as the weighted average of the atomic masses of an atoms naturally
occurring isotopes.
2. Give electron configurations for the Fe3 and S2 ions.
Ans:
The Fe3 ion is an iron atom that has lost three electrons. Since the electron configuration of iron (Fe, atomic
number: 26) is 1s22s22p63s23p63d64s2 (Table 2.2), the configuration for Fe3 is 1s22s22p63s23p63d5.
The S2 ion is a sulfur atom that has gained two electrons. Since the electron configuration of sulfur (S,
atomic number: 16) is 1s22s22p63s23p4 (Table 2.2), the configuration for S2 is 1s22s22p63s23p6.
3. Explain why covalently bonded materials are generally less dense than ionically or metallically bonded
ones.
Ans:
Covalently bonded materials are less dense than metallic or ionically bonded ones because covalent bonds
are directional in nature whereas metallic and ionic are not. When bonds are directional, the atoms cannot
pack together in as dense, yielding a lower mass density.
QUESTIONS & PROBLEMS
Fundamental Concepts/Electrons in Atoms
Cite the difference between atomic mass and atomic weight.
Ans:
Atomic mass is the mass of an individual atom, whereas atomic weight is the average (weighted) of the
atomic masses of an atoms naturally occurring isotopes.
How many grams are there in one amu of a material? Note: the Avogadros number = 6.0221023 atoms/mol
Ans:
In order to determine the number of grams in one amu of material, appropriate manipulation of the
amu/atom, g/mol, and atom/mol relationships is all that is necessary, as:
1 mol 1 g mol 24
#g amu 1.66 10 g amu
6.022 10 atoms 1 amu atom
23
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 2/9
The Periodic Table
From the periodic table of the chemical elements (Fig. 2.8, p. 28), the atomic mass of Fe 55.845 g/mol .
Calculate the number of atoms in 1 kg of pure iron.
Ans:
# of atoms in 1 kg Fe 1,000 g 6.022 1023 atoms mol 55.845 g mol 1.078 1025 atoms
Bonding Forces and Energies
The net potential energy between two adjacent ions EN may be represented by the sum of Eqs. (2.9) and
(2.11); that is:
A B
EN (2.17)
r rn
Calculate the bonding energy E0 in terms of the parameters A, B and n using the following procedure:
(a) Differentiate EN with respect to r, and then set the resulting expression equal to zero, since the curve of
EN versus r is a minimum at E0 .
(b) Solve for r in terms of A, B and n, which yields r0 , the equilibrium interionic spacing.
(c) Determine the expression for E0 by substitution of r0 into Eq. (2.17).
Ans:
dEN r d A B A nB
(a) Differentiation of Eq. (2.17) yields: n 2 n1
dr dr r r r r
dE N r A nB
(b) @ equilibrium interionic spacing r0 , we have 0 ; thus, 2 n1 0
dr r r r0 r0
0
n 1 nB
r0
1 1
A nB n 1 A nB n 1 A 1n
2 n 1 Ar0 nBr0
2
r0
r0 r0 r01n A A nB
nB
(c) Substitution for r0 into Eq. (2.17) and solving for E0 EN r0 , we obtain:
A B A B A B
E0 n E0 1
n
1
n
r0 r0 nB n 1 nB n 1 A 1n A 1n
A A nB nB
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 3/9
The attractive and repulsive forces, FA and FR , between two adjacent ions may be represented as:
A
FA r r 2
, where A, B and n are constants and n 1 . Express the equilibrium spacing r0 of the two
F r nB
R r n 1
adjacent ions in terms of the parameters A, B and n.
Ans:
A nB
@ equilibrium: net force FN r r r FA r r r FR r r r 0 2 n 1 0
0 0 0
r r r r0
n 1 nB
r0 A
1 1
A nB A nB n 1 nB n 1 A 1n
2 n 1 0 2 n 1 Ar0 nBr0 2
r0
r0 r0 r0 r0 r01n A A nB
nB
which is the same as the exercise above based on the Energy Well approach.
C r
The net potential energy between two adjacent ions EN may be represented as: EN D exp ,
r
where C, D and are constants. Prove that the equilibrium spacing r0 of the two adjacent ions can be
C D r
expressed as: exp 0 0 .
r0
2
Ans:
dEN r d C r C D r
Differentiation yields: D exp 2 exp
dr dr r r
dE N r C D r
@ equilibrium interionic spacing r0 , we have 0 ; thus, 2 exp 0 0
dr r r r0
0
The attractive and repulsive forces, FA and FR , between two adjacent ions may be represented as:
C
FA r r 2
, where C, D and are constants. Prove that the equilibrium spacing r0 of the two
D r
FR r exp
C D r
adjacent ions can be expressed as: exp 0 0 .
r0
2
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 4/9
Ans:
C D r
@ equilibrium: net force FN r r r FA r r r FR r r r 0 2 exp 0
0 0 0
r r r0
C D r
exp 0 0
r0
2
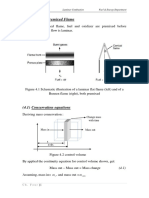
The figure on the right shows the bond energy distributions U of
Materials 1 and 2. Here r represents the interatomic separation of two
isolated atoms of a material.
(a) Determine which material has a higher melting temperature Tm .
(b) Determine which material has a higher coefficient of linear
thermal expansion .
(c) The well-known Hookes law: E correlates stress and
L
strain , which represents the unit elongation of a solid
L
element in a uniaxial stress state. The material constant E is called
Youngs modulus (or modulus of elasticity), which represents
the stiffness property of a material. Determine which material has a higher Youngs modulus E.
V
(d) Another elasticity law: p Ke correlates hyrdrostatic pressure p and dilatation e , which
V
represents the unit volume change of a solid element in a triaxial stress state. The material constant K is
called bulk modulus of elasticity. Determine which material has a higher bulk modulus K.
Ans:
(a) Upon melting (i.e., transforming from a solid to a liquid upon heating), most substances experience an
increase in volume. That is, correspondingly, an increase in r, the interatomic separation of two isolated
atoms of a material. To melt, the change in interatomic separation r r r0 is the same for both
materials. Since Material 2 has a lower energy well; i.e., U 0,2 U 0,1 . To reach the same r r r0
, Material 2 will need to absorb more energy, which implies more heating or higher temperature change.
Hence Material 2 has a higher melting temperature Tm .
(b) If the change in temperature: T T2 T1 is the same, the change in energy of the two materials will
also be the same. Since Material 1 has a higher energy well; i.e., U 0,1 U 0,2 the change in
interatomic separation r r r0 for Material 1 will be larger under the same temperature change:
L
T. Thus, the change in length L will also be greater. Knowing that: T , hence Material 1 has
L
a higher .
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 5/9
(c) In order to produce the same change in interatomic separation r r r0 for the same strain , since
Material 1 has a higher energy well; i.e., U 0,1 U 0,2 , we have: U1 U 2 Thus, 1 2
1 2
E1 E2 . Hence Material 2 has a higher Youngs modulus E.
(d) Similarly, in order to produce the same change in interatomic separation r r r0 for the same
dilatation e, since Material 1 has a higher energy well; i.e., U 0,1 U 0,2 , we have: U1 U 2 Thus,
p1 p
p1 p2 2 K1 K 2 . Hence Material 2 has a higher bulk modulus K.
e e
Interatomic Bonding
Cite the main differences among the mechanisms of forming the ionic, covalent, and metallic bonding. That
is, the type(s) of elements forming the bonding and the resultant configuration of the valence electrons for
that bonding. Discuss also the directionality of each bond.
Ans:
The main differences among the various forms of primary bonding are:
Ionic bonding is formed when an atom of a metallic element gives up its valence electron(s) to an atom of a
non-metallic element. The ionized particles, thus, become a cation and an anion, respectively. There is
electrostatic attraction between the two oppositely charged ions. The bonding is non-directional.
Covalent bonding is formed when valence electrons are shared between two adjacent non-metallic atoms
such that each atom assumes a stable electron configuration, i.e., no more free valence electrons for the
chemical compound as a whole. The bonding is directional.
Metallic bonding is formed when the positively charged ion cores of a metallic element are shielded from
one another and also glued together by the sea of valence electrons from the atoms of the re metallic
element. The bonding is non-directional.
If all other conditions are the same, will a covalently bonded material be generally (a) more dense than,
(b) about the same as, or (c) less dense than a metallically bonded one. Explain why.
Ans:
If all other conditions are the same, a covalently bonded material will in general be (c) less dense than a
metallically bonded one because covalent bonds are directional in nature whereas metallic bonds are not.
When bonds are directional, the atoms cannot pack in together as dense as possible, yielding a lower mass
density.
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 6/9
The atomic number, weight and radius of fluorine (F) are 9, 18.998 g/mol and 0.42, respectively; whereas
the atomic number, weight and radius of chlorine (Cl) are 17, 35.453 g/mol and 0.79, respectively. Based
on your knowledge of chemical bonds, explain why the chemical compound of hydrogen fluoride (HF) has
a higher boiling temperature than the chemical compound of hydrogen chloride (HCl) (19.4 vs. 85C),
even though HF has a lower molecular weight.
Ans:
The Coulombic force in the dipole bond between the fluorine particle of an HF molecule and the hydrogen
9
particle of a neighboring HF molecule is proportional to 51.02 , which is larger than its
0.42
2
17
counterpart of the HCl case: 27.24 . Hence it requires more energy to change HF molecules from
0.79
2
liquid to gas. That is why HF has a higher boiling temperature.
Another explanation is based on the fact that the intermolecular bonding for HF is hydrogen, whereas for
HCl, the intermolecular bonding is van der Waals. Since the hydrogen bond is stronger than van der Waals,
HF will have a higher boiling temperature.
Use your knowledge of hydrogen bond to explain why a can containing
water may rupture as shown in the figure at right if it is left outside
overnight during cold winter.
Ans:
Water exhibits an anomalous and familiar volume expansion upon freezing
into ice. When a water-containing can was left outside overnight during cold
winter, the expanded volume (approximately 9%) from water to ice resulted
in tensile stresses in the metallic can and caused rupture if the can was
overstressed. The behavior of this anomalous yet familiar volume expansion
can be explained on the basis of hydrogen bonding. As explained in
MATERIALS OF IMPORTANCE: Water (Its Volume Expansion Upon
Freezing), p. 42, each H 2O has two hydrogen atoms that can bond to oxygen atoms; in addition, its single
O atom can bond to two hydrogen atoms of other H 2O molecules. Thus, for solid ice, each water molecule
participates in four hydrogen bonds, as shown in the three-dimensional schematic of Fig. 2.24a, p. 42. This
is a relatively open structure. That is, the molecules are not closely packed together and as a result, the
density of solid ice is comparatively low.
Use your knowledge of hydrogen bond to explain why the freeze-thaw cycles during cold winter in the
metropolitan New York area tend to break up the pavement in streets and cause potholes to form.
Ans:
During the relatively warmer daytime, ice/snow will thaw to become water, which seeps through the
pavement. The under-pavement water will freeze into ice during the cold night time. Following the
explanation presented in MATERIALS OF IMPORTANCE: Water (Its Volume Expansion Upon Freezing),
p. 42, the volume of ice is larger than the volume of water of the same mass. Hence, the volume expansion
upon freezing will tend to push the pavement upward, break it up, and form a pothole.
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 7/9
FUNDAMENTALS OF ENGINEERING
The chemical composition of the repeat unit for nylon 6,6 is given by the formula C 12H22N2O2. Atomic
weights for the constituent elements are AC 12 , AH 1 , AN 14 and AO 16 . According to this chemical
formula for nylon 6,6, the percent (by weight) of carbon in nylon 6,6 is most nearly:
(A) 31.6%
(B) 4.3%
(C) 14.2%
(D) 63.7%
Ans: D. The total atomic weight, Atotal , of one repeat unit of nylon 6,6 is calculated as:
Atotal 12 atoms AC 22 atoms AH 2 atoms AN 2 atoms AO
12 12 g mol 22 1 g mol 2 14 g mol 2 16 g mol 226 g mol
Therefore the percent by weight of carbon is calculated as:
12 atoms AC 12 atoms 12 g mol
C wt% 100% 100% 63.7%
Atotal 226 g mol
which is answer D.
Which of the following electron configurations is for an inert gas?
(A) 1s22s22p63s23p6
(B) 1s22s22p63s2
(C) 1s22s22p63s23p64s1
(D) 1s22s22p63s23p63d24s2
Ans: A. The 1s22s22p63s23p6 electron configuration is that of an inert gas because of filled 3s and 3p
subshells. Indeed, the inert gas is argon (Ar, atomic number: 18).
What type(s) of bonding would be expected for bronze (a copper-tin alloy)?
(A) Ionic bonding
(B) Metallic bonding
(C) Covalent bonding with some van der Waals bonding
(D) van der Waals bonding
Ans: B. For bronze, the bonding is metallic because it is a metallic alloy.
What type(s) of bonding would be expected for rubber?
(A) Ionic bonding
(B) Metallic bonding
(C) Covalent bonding with some van der Waals bonding
(D) van der Waals bonding
Ans: C. Because rubber is composed primarily of carbon and hydrogen atoms, the bonding is covalent with
some van der Waals bonding.
How many atoms or molecules are there in a mole of a substance?
Ans: In a mole of a substance there are 6.0231023 atoms or molecules.
The electrons that occupy the outermost filled shell are called __________ electrons.
Ans: The electrons that occupy the outermost filled shell are called valence electrons.
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 8/9
The nucleus of an atom contains:
(A) Protons
(B) Electrons
(C) Neutrons
(D) All of the above
(E) Both A and C
Ans: E. The nucleus of an atom contains protons and neutrons.
What type(s) of electron subshell(s) does an L shell contain?
(A) d
(B) p
(C) f
(D) s
(E) s and f
(F) s and p
(G) All of the above
Ans: F. An L shell contains both s and p electron subshells.
What is the maximum number of electrons that an M shell may contain?
(A) 32
(B) 18
(C) 8
(D) 2
Ans: B. An M shell consists of a 1s-type subshell, which may contain 2 electrons, 3 p-type subshells, which
may contain 2 electrons each, and 5 d-type subshells, which may contain a maximum of 2 electrons each.
Therefore, the maximum number of electrons possible for an M shell is 18.
How many electrons does an element with the electron configuration 1s22s22p63s23p6 have?
Ans: There are 2 electrons in each of the 1s, 2s and 3s subshells for a total of 6 electrons; both 2p and 3p
subshells each contain 6 electrons. Therefore, the total number of electrons is 18.
Match the electron structure with the element type it represents: 1s22s22p63s23p63d104s24p65s1.
(A) Inert gas
(B) Halogen
(C) Alkali metal
(D) Alkaline earth metal
(E) Transition metal
Ans: C. An alkali metal has a single s electron.
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
CHAPTER 2 ATOMIC STRUCTURE AND INTERATOMIC BONDING Page 9/9
Of those elements listed below, select the one that is one electron short of having its outer shell of electrons
completely filled.
(A) Sr (strontium)
(B) Li (lithium)
(C) Ar (argon)
(D) Cr (chromium)
(E) S (sulfur)
(F) I (iodine)
(G) N (nitrogen)
(H) Ce (cerium)
Ans: F. Iodine (I) is the element that is one electron short of having its outer shell of electrons completely
filled, since it is located in column VIIA (the halogen group) of the Periodic Table.
What is the predominant type of bonding for titanium (Ti)?
(A) Ionic
(B) Hydrogen
(C) Covalent
(D) van der Waals
(E) Metallic
Ans: E. The predominant type of bonding for titanium is metallic, inasmuch as Ti is a metallic element.
With the aid of the periodic table shown below, calculate the ionic character (in percent) of the interatomic
bonds for Fe2O3.
Ans: The percent ionic character (%IC) is a function of the electronegativities of the A and B ions, X A and
X B , according to Eq. (2.16):
%IC 1 exp 0.25 X A X B 100%
2
From the Periodic Table, for Fe2O3, X Fe 1.8 and X O 3.5 , therefore:
%IC 1 exp 0.25 X Fe X O 100% 1 exp 0.251.8 3.5 100% 51.45%
2
ME 46100: ENGINEERING MATERIALS CLOSED-BOOK PRACTICE
Vous aimerez peut-être aussi
- Question Paper Code:: Nitro PDF Software 100 Portable Document Lane WonderlandDocument3 pagesQuestion Paper Code:: Nitro PDF Software 100 Portable Document Lane WonderlandBIBIN CHIDAMBARANATHANPas encore d'évaluation
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsD'EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsPas encore d'évaluation
- PRE4122 Exercise No. 2 Atomic Structure and Interatomic BondingDocument10 pagesPRE4122 Exercise No. 2 Atomic Structure and Interatomic BondingعبداللهأحمدPas encore d'évaluation
- Tutorial 1Document2 pagesTutorial 1Syrus SylarPas encore d'évaluation
- X Coursera Assignment Solar Energy Basics Part-2Document2 pagesX Coursera Assignment Solar Energy Basics Part-2Kenan Horoz100% (1)
- B.tech. Engineering ExpDocument39 pagesB.tech. Engineering ExpMr. CuriousPas encore d'évaluation
- ChBE3210 Spring2007 Exam2Document6 pagesChBE3210 Spring2007 Exam2Abishek KasturiPas encore d'évaluation
- HMT Unit 1Document4 pagesHMT Unit 1Thulasi RamPas encore d'évaluation
- 7 Fluid Dynamics Tutorial SolutionDocument25 pages7 Fluid Dynamics Tutorial SolutionAldhaAhmadyaningratPas encore d'évaluation
- Concepts of ThermodynamicsDocument41 pagesConcepts of ThermodynamicsMichael ElliottPas encore d'évaluation
- PROBLEMSgaskell Cap 6Document5 pagesPROBLEMSgaskell Cap 6Jimmy Mar100% (1)
- 04combustion TechnologyDocument70 pages04combustion TechnologySheensky V. SalasaPas encore d'évaluation
- Sample Problems and Solution - 2Document6 pagesSample Problems and Solution - 2김동욱Pas encore d'évaluation
- 1 - Energy and Energy BalancesDocument135 pages1 - Energy and Energy BalancesHabib Al-Aziz100% (2)
- Thermodynamics Practice Problems 2012 05 07.odtDocument26 pagesThermodynamics Practice Problems 2012 05 07.odtWillina Marie Chong MablePas encore d'évaluation
- Ramadan Youssef Sakr Moustafa - Lecture 3-1st-2nd Laws On CombustionDocument46 pagesRamadan Youssef Sakr Moustafa - Lecture 3-1st-2nd Laws On CombustionAbhinash KumarPas encore d'évaluation
- Perpan DD 122017133 Irpan SopianDocument16 pagesPerpan DD 122017133 Irpan SopianErnanda Pratama100% (1)
- Models - Heat.vacuum FlaskDocument34 pagesModels - Heat.vacuum FlaskNa DiaPas encore d'évaluation
- Tutorial-3 CRE1 CLL122 PDFDocument4 pagesTutorial-3 CRE1 CLL122 PDFSunandita BorahPas encore d'évaluation
- 5 R DPBui QMF ZPUq Exc 6 JFDocument53 pages5 R DPBui QMF ZPUq Exc 6 JFBhfjsukPas encore d'évaluation
- Ch03 - The Structure of Crystalline SolidsDocument40 pagesCh03 - The Structure of Crystalline SolidsShafiq HafizullahPas encore d'évaluation
- 9781292035444Document7 pages9781292035444Bwn Jangyeswar KumarPas encore d'évaluation
- Thermo FinalDocument66 pagesThermo Finalsossydj75% (4)
- Applied Electricity CourseDocument4 pagesApplied Electricity CourseZani Bai0% (1)
- H.W 7Document9 pagesH.W 7Mirvat ShamseddinePas encore d'évaluation
- EMF-Assignment Questions 0Document6 pagesEMF-Assignment Questions 0Akula VeerrajuPas encore d'évaluation
- Nptel 1 PDFDocument9 pagesNptel 1 PDFShubham KumarPas encore d'évaluation
- (4.1) Laminar Premixed FlameDocument31 pages(4.1) Laminar Premixed Flameمصطفى العباديPas encore d'évaluation
- Finite Control Volume AnalysisDocument30 pagesFinite Control Volume Analysishari tubagusPas encore d'évaluation
- Fundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0017Document1 pageFundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0017Clady100% (1)
- Properties of Mixtures and CombustionDocument7 pagesProperties of Mixtures and CombustionKhairul NajmiPas encore d'évaluation
- Lattive EnergyDocument44 pagesLattive EnergyClarize Soo HooPas encore d'évaluation
- Process Equipment Design-06Document25 pagesProcess Equipment Design-06PARAG DAHARWAL 20CH10020Pas encore d'évaluation
- Phase Diagrams: Solubility LimitDocument163 pagesPhase Diagrams: Solubility LimitseaPas encore d'évaluation
- A Textbook of Electrical Technology Vol. 2 - Theraja-P1Document29 pagesA Textbook of Electrical Technology Vol. 2 - Theraja-P1Muhammad TaimoorPas encore d'évaluation
- Chemical ThermodynamicsDocument33 pagesChemical ThermodynamicsAkash Ghosh0% (1)
- C.R.E. - II (All Units)Document88 pagesC.R.E. - II (All Units)Mohamed Shahid100% (1)
- Fuels and CombustionDocument12 pagesFuels and CombustionAbhishek PrasadPas encore d'évaluation
- SAMPLEDocument3 pagesSAMPLEAlexis JaraPas encore d'évaluation
- Thermal Engineering - I Jntua Question PapersDocument15 pagesThermal Engineering - I Jntua Question PapersHimadhar SaduPas encore d'évaluation
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDocument68 pagesGas Power Cycles Study Guide in Powerpoint: To AccompanyexceptionalhighdeePas encore d'évaluation
- Chapter 13 - Chemical EquilibriumDocument52 pagesChapter 13 - Chemical EquilibriummukhlishPas encore d'évaluation
- Solution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDocument16 pagesSolution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDeepak SharmaPas encore d'évaluation
- Thermodynamics ProblemsDocument2 pagesThermodynamics ProblemsAlexander Salado IbrahimPas encore d'évaluation
- Electrochemical-Cells Kec PDFDocument10 pagesElectrochemical-Cells Kec PDFsachinPas encore d'évaluation
- Phys 253 Thermal PhysicsDocument1 019 pagesPhys 253 Thermal Physicsdavid_berardo6537Pas encore d'évaluation
- ME3112-1 Lab Vibration MeasurementDocument8 pagesME3112-1 Lab Vibration MeasurementLinShaodunPas encore d'évaluation
- STUCOR Aucr2017 PDFDocument74 pagesSTUCOR Aucr2017 PDFksbalajiautPas encore d'évaluation
- ChE 413N Problem Set 1 - Atomic Structure-Ionic Arrangements 4-20-16vstudDocument1 pageChE 413N Problem Set 1 - Atomic Structure-Ionic Arrangements 4-20-16vstudLouie G NavaltaPas encore d'évaluation
- Combustion in Premixed & Diffusion FlamesDocument16 pagesCombustion in Premixed & Diffusion FlamesRahul Singh TomarPas encore d'évaluation
- Transport Phenomena Exam, January 2011, With Model AnswersDocument11 pagesTransport Phenomena Exam, January 2011, With Model AnswersHansraj RahulPas encore d'évaluation
- Introduction Nuclear Power PlantDocument20 pagesIntroduction Nuclear Power PlantAmmar Ahmed100% (1)
- MIN-305 Heat & Mass Transfer Tutorial - 1Document2 pagesMIN-305 Heat & Mass Transfer Tutorial - 1Ayush JaiswalPas encore d'évaluation
- Applications of First Order Differential Equations Discussion Part I PDFDocument22 pagesApplications of First Order Differential Equations Discussion Part I PDFErika Dawn Luciano AmbrayPas encore d'évaluation
- Mass Transfer QuizzesDocument10 pagesMass Transfer QuizzesNate JamesPas encore d'évaluation
- CHAPTER 6 Flow in Pipes FinalDocument22 pagesCHAPTER 6 Flow in Pipes FinalSyaiful Akmal AzizPas encore d'évaluation
- Exercises Unit1and2 PropertiesOfMaterialsDocument3 pagesExercises Unit1and2 PropertiesOfMaterialsPatricio VillarrealPas encore d'évaluation
- Mechanism of Corrosion ProcessesDocument24 pagesMechanism of Corrosion Processeshoang_700100% (1)
- Closed-Book Practice-Ch 03 (2016!12!10)Document8 pagesClosed-Book Practice-Ch 03 (2016!12!10)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 15 (2015!03!28)Document21 pagesClosed-Book Practice-Ch 15 (2015!03!28)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 15 (2015!03!28)Document21 pagesClosed-Book Practice-Ch 15 (2015!03!28)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 07 (2016!12!24)Document8 pagesClosed-Book Practice-Ch 07 (2016!12!24)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 13 (2017!04!18)Document9 pagesClosed-Book Practice-Ch 13 (2017!04!18)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 11 (2015!03!16)Document17 pagesClosed-Book Practice-Ch 11 (2015!03!16)Juan100% (1)
- Closed-Book Practice-Ch 10 (2017!08!08)Document12 pagesClosed-Book Practice-Ch 10 (2017!08!08)Juan0% (1)
- Closed-Book Practice-Ch 09 (2017!08!07)Document9 pagesClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- Closed-Book Practice-Ch 07 (2016!12!24)Document8 pagesClosed-Book Practice-Ch 07 (2016!12!24)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 14 (2015!03!28)Document9 pagesClosed-Book Practice-Ch 14 (2015!03!28)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 03 (2016!12!10)Document8 pagesClosed-Book Practice-Ch 03 (2016!12!10)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 06 (2016!07!31)Document8 pagesClosed-Book Practice-Ch 06 (2016!07!31)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 12 (2015!03!18)Document7 pagesClosed-Book Practice-Ch 12 (2015!03!18)JuanPas encore d'évaluation
- Closed-Book Practice-Ch 16 (2017!06!01)Document11 pagesClosed-Book Practice-Ch 16 (2017!06!01)Juan100% (6)
- Introduction To Physical ElectronicsDocument369 pagesIntroduction To Physical ElectronicsAbhishek yadavPas encore d'évaluation
- Testing Dalton's Atomic Theory: SolutionDocument1 pageTesting Dalton's Atomic Theory: SolutionDennoh OlengoPas encore d'évaluation
- Fire-Fighting Guidance Notes - E-Feb15Document161 pagesFire-Fighting Guidance Notes - E-Feb15Moe LattPas encore d'évaluation
- AM Week 2 ECM NOTESDocument19 pagesAM Week 2 ECM NOTESa38659158Pas encore d'évaluation
- LPGDocument8 pagesLPGraritylimPas encore d'évaluation
- CH2254 CPC Unit 3 Solution PS3Document15 pagesCH2254 CPC Unit 3 Solution PS3Vignesh KPas encore d'évaluation
- Additives For CoatingsDocument42 pagesAdditives For Coatingsamarghumatkar_466819100% (1)
- J Scitotenv 2020 142108Document26 pagesJ Scitotenv 2020 142108SHERLY KIMBERLY RAMOS JESUSPas encore d'évaluation
- Acene CreamDocument4 pagesAcene CreamJai MurugeshPas encore d'évaluation
- 6 Photoelectron Spetroscopy - SDocument9 pages6 Photoelectron Spetroscopy - SJoyce HongPas encore d'évaluation
- Steamdat InfoDocument2 pagesSteamdat InfoAlmirFariaPas encore d'évaluation
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoPas encore d'évaluation
- Slides - Plug Flow Reactor (2018)Document36 pagesSlides - Plug Flow Reactor (2018)Meireza Ajeng Pratiwi100% (1)
- Logic PuzzleufhfgDocument1 pageLogic Puzzleufhfgkjj77600% (2)
- Helical Baffles in A Shell and Tube Heat ExchangerDocument74 pagesHelical Baffles in A Shell and Tube Heat ExchangerErich FouriePas encore d'évaluation
- PHYS 622 - Statistical Mechanics - Spring 2016: Chung-Sang - Ng@gi - Alaska.eduDocument7 pagesPHYS 622 - Statistical Mechanics - Spring 2016: Chung-Sang - Ng@gi - Alaska.eduAmina IbrahimPas encore d'évaluation
- Piping SolutionDocument41 pagesPiping SolutionSiddhi MhatrePas encore d'évaluation
- CHEM225 Organic Chemistry 1 Module Week 1-4Document17 pagesCHEM225 Organic Chemistry 1 Module Week 1-4Kezia CaratorPas encore d'évaluation
- TD1005 Free and Forced Convection DatasheetDocument3 pagesTD1005 Free and Forced Convection DatasheetMahmoudPas encore d'évaluation
- Nickel-Gallium-Catalyzed Electrochemical Reduction of CO2 To Highly Reduced Products at Low OverpotentialsDocument17 pagesNickel-Gallium-Catalyzed Electrochemical Reduction of CO2 To Highly Reduced Products at Low Overpotentialstunganh1110Pas encore d'évaluation
- Batch DistillationDocument3 pagesBatch DistillationNamratha NairPas encore d'évaluation
- Journal of Alloys and Compounds: Karandeep, H.C. Gupta, S. KumarDocument5 pagesJournal of Alloys and Compounds: Karandeep, H.C. Gupta, S. Kumarshivika gaurPas encore d'évaluation
- Corrosion Solved ProblemsDocument39 pagesCorrosion Solved Problemshanna1100% (4)
- Physical Chemistry 3th CastellanDocument1 038 pagesPhysical Chemistry 3th CastellanPablo Gallardo94% (18)
- Module 1.2: Parameters of ComfortDocument2 pagesModule 1.2: Parameters of ComfortkaushikaPas encore d'évaluation
- Defining WOG TBDocument2 pagesDefining WOG TBJavier Caamaño VillafañePas encore d'évaluation
- Science: Quarter 2 Types of Compounds Based On Their PropertiesDocument9 pagesScience: Quarter 2 Types of Compounds Based On Their PropertiesAriel Lomugdang PatricioPas encore d'évaluation
- PsychometryDocument31 pagesPsychometryRapheal EghianruwaPas encore d'évaluation
- Technical Report On SRV and Safety Fittings For TBI PDFDocument31 pagesTechnical Report On SRV and Safety Fittings For TBI PDFashraf ahmadPas encore d'évaluation
- Unit 1 Electromagnetic Radiation-1Document23 pagesUnit 1 Electromagnetic Radiation-1Saif YounusPas encore d'évaluation