Académique Documents
Professionnel Documents
Culture Documents
A Handbook of Laoratory Solution
Transféré par
Fickrhy Chamboshy0 évaluation0% ont trouvé ce document utile (0 vote)
28 vues1 pageTitre original
A HANDBOOK OF LAORATORY SOLUTION.docx
Copyright
© © All Rights Reserved
Formats disponibles
DOCX, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
28 vues1 pageA Handbook of Laoratory Solution
Transféré par
Fickrhy ChamboshyDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 1
A HANDBOOK OF LAORATORY SOLUTION
Standariaze with silver nitrate solution acidified with 20 mL of 2 N nitric acid per 25
mL usind ferric alum indicator.
Amonium thiocyanat As potassium thiocyanat using 8 g of crystals.
Sodium chloride. See primary standards. Use 5.845 g per litre.
Silver nitrat. See primary standards. Dry a grade crystals at 110oC for 2 hours. Use
16.988 g per litre. It is cheaper to use 17 g chloride soln, using potassium chromate
indicator or an adsorption indicator
MISCELLANEOUS TITRATION SOLUTION
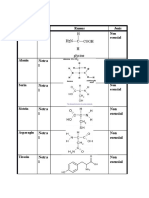
EDTA solution ( diamino ethane tetra - acetic acid disodium salt).
CH2N(CH2.COOH).CH2COONa]2.2H2O. F.W =372.25 Eq = 372.25 M=N. For 0,1M
soln disolve 37.3 g in 1 litre of water.standardize with standard calcium ion soln at pH
10 using special buffer soln and solochorme black T indicator in the presence of a
known concentration of magnesium ion.
Pottasium ferrocyanide. K4Fe(CN)6. 3H2O. F.W = 422.41 Eq= 422.41 is eqivalen to
98.07 of zinc. Dissolve 42.241 g in water add about 1 g of sodium carbonat and dilute
to 1 litre.
Soap solution. Dissolve 10 g of castile soap in 100 mL of ethanol by warming it in a
beaker of hot water. Add another 500 mL of ehtanol and dilute to 1 litre. Filter after
standing for a day or two. Standardize calcium ion soln.
Calcium ion solution. To 1 g of pure dry calcium carbonate add a little water and
enough 2N hydrochloric acid in a drops to this solve the chalk, boil, cool and make up
to 1 litre . 1 mL = 0.004 g of calcium ion
Zimmermen-Reinhardt solution. This is use for the titration of ferrous ion in the
presence of hydrochloric acid using permanganate solution. Dissolve 70 g of hydrated
manganous sulphate crystals in approx 500 mL of water add in succession, with
stirring 125 mL of cone sulphuric acid and 125 mL syrupy phosphoric acid (85%) and
dilute to 1 litre. Use 25 mL per titration with approx 0,1 N soln.
Clarks soap solution. Dissolve 100 g of Castile soap in 800mL 0f ethanol and 200
mL of water,leave it to stand for 24 hours, decant off the liquid and titrate against
standard calcium solution. Dilute with the same 80% ethanoluntil 1 mL = 1 mg of
chalk, including the lather factor, which is the volume of soap solution needed to
make a permanent lather with 50 mL of distilled water.
Boutron-Boudet soap solution. Dissolve 100 g of Castile soap in 2500 mL of 56%
ethanol (1400 mL ethanol + 1100 water), titrate against 40 mL of a solution of 0.59 g
of barium nitate perlitre. Dilute so that 2.4 mL of the soap of solution after dilution
gives a permanent lather with 40 mL of barium nitrate soln. 2.4 = 220 part per million
calcium carbonat = 22 french degrees of hardness.
Vous aimerez peut-être aussi
- Daftar Pustaka: and Primary Grades. Toronto: Allyn and BaconDocument1 pageDaftar Pustaka: and Primary Grades. Toronto: Allyn and BaconFickrhy ChamboshyPas encore d'évaluation
- Plan 8: Unit 5: Acid - BaseDocument9 pagesPlan 8: Unit 5: Acid - BaseFickrhy ChamboshyPas encore d'évaluation
- Atoms PDFDocument26 pagesAtoms PDFFickrhy Chamboshy100% (1)
- No Nama Siswa Nilai PG Nilai Essai Total NilaiDocument1 pageNo Nama Siswa Nilai PG Nilai Essai Total NilaiFickrhy ChamboshyPas encore d'évaluation
- Netra L: Asam Amino Nama Sifat Rumus Jenis Glisin Non EsensialDocument4 pagesNetra L: Asam Amino Nama Sifat Rumus Jenis Glisin Non EsensialFickrhy ChamboshyPas encore d'évaluation
- Presentation 1Document1 pagePresentation 1Fickrhy ChamboshyPas encore d'évaluation
- Desease Amino Acid: Penyakit Metabolisme Asam Amin0Document1 pageDesease Amino Acid: Penyakit Metabolisme Asam Amin0Fickrhy ChamboshyPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Chlorine and Its CompoundsDocument19 pagesChlorine and Its Compoundskakembo hakimPas encore d'évaluation
- HB EstimationDocument4 pagesHB EstimationSushmitaPas encore d'évaluation
- Pre-Lab Activity WorksheetDocument2 pagesPre-Lab Activity WorksheetJames AndrewsPas encore d'évaluation
- Easy Keto and Intermittent FastingDocument98 pagesEasy Keto and Intermittent FastingTony Multh100% (3)
- Polycarbonate Chemical Compatibility OverviewDocument2 pagesPolycarbonate Chemical Compatibility OverviewabasakPas encore d'évaluation
- IGCSE Chemistry Section 2 Lesson 1Document66 pagesIGCSE Chemistry Section 2 Lesson 1Antonia Putri Sri Nova YolandhaPas encore d'évaluation
- The Oxidation Potential of Postassium Ferrocyanide-Potassium FerricyanideDocument10 pagesThe Oxidation Potential of Postassium Ferrocyanide-Potassium FerricyanidescribedbioaPas encore d'évaluation
- Msds (nh4) 2so4Document6 pagesMsds (nh4) 2so4rodhiatul jurdillah0% (1)
- Chemistry Full v1.5Document52 pagesChemistry Full v1.5ZainBaloch100% (1)
- Astm B604Document10 pagesAstm B604caop217Pas encore d'évaluation
- Mba-Cet Sample Paper-1 PDFDocument30 pagesMba-Cet Sample Paper-1 PDFGanapati KattigePas encore d'évaluation
- InTech-Hydrogen Generation by Treatment of Aluminium Metal With Aqueous Solutions Procedures and UsesDocument22 pagesInTech-Hydrogen Generation by Treatment of Aluminium Metal With Aqueous Solutions Procedures and UsesDaniel Medina SánchezPas encore d'évaluation
- 1 - Eklund, Jon - British Eighteenth-Century Chemical Terms - Part 1 - 2 - 3 - The Best For Learning All Alchemical GlossaryDocument116 pages1 - Eklund, Jon - British Eighteenth-Century Chemical Terms - Part 1 - 2 - 3 - The Best For Learning All Alchemical GlossaryAB236Pas encore d'évaluation
- Postharvest Physiology 4th YearDocument54 pagesPostharvest Physiology 4th YearYobsan BushuraPas encore d'évaluation
- Vibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyDocument9 pagesVibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyMelin YohanaPas encore d'évaluation
- SG Removal in BiodieselDocument7 pagesSG Removal in BiodieselPe TerPas encore d'évaluation
- Whey and Whey Powders Production and UsesDocument10 pagesWhey and Whey Powders Production and UsesEmanuele DanielePas encore d'évaluation
- TDS Potasa - Inter-Chemie LimitedDocument1 pageTDS Potasa - Inter-Chemie Limitedxibs2009Pas encore d'évaluation
- FertilizersDocument84 pagesFertilizersnithansa100% (4)
- WS6 IG I Chemistry BEHAVIOUR OF METALSDocument4 pagesWS6 IG I Chemistry BEHAVIOUR OF METALSRaj MalkanPas encore d'évaluation
- Catalog Orlandos - Web PDFDocument18 pagesCatalog Orlandos - Web PDFGeorge PreoteasaPas encore d'évaluation
- 1 Integración de Desórdenes Electrolíticos y Ácido-Base RB IR Med BahamondeDocument11 pages1 Integración de Desórdenes Electrolíticos y Ácido-Base RB IR Med BahamondeMaría Jose BahamondePas encore d'évaluation
- Epsom Salt Production ProcessDocument17 pagesEpsom Salt Production ProcessThomy Vollmer0% (1)
- Cambridge IGCSE: CHEMISTRY 0620/32Document16 pagesCambridge IGCSE: CHEMISTRY 0620/32Tshegofatso SaliPas encore d'évaluation
- GCE O'Level 5070 Past Paper Questions (Salts)Document5 pagesGCE O'Level 5070 Past Paper Questions (Salts)Mohamed Saddam Hussain67% (3)
- Cambridge International Advanced Subsidiary and Advanced LevelDocument16 pagesCambridge International Advanced Subsidiary and Advanced LevelRosePas encore d'évaluation
- SILDRIL System Engineering GuidelinesDocument7 pagesSILDRIL System Engineering GuidelinesAhmer AkhlaquePas encore d'évaluation
- Red Phosphorus FactsDocument2 pagesRed Phosphorus FactsJames Weninger0% (1)
- Abbyshaygayle Flame TestDocument3 pagesAbbyshaygayle Flame TestAbby Shay GaylePas encore d'évaluation