Académique Documents
Professionnel Documents
Culture Documents
Cell Disease Lab
Transféré par
api-340815123Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Cell Disease Lab
Transféré par
api-340815123Droits d'auteur :
Formats disponibles
Parkinsons Disease Special thanks to Medicinenet.com, nature.com,biomedical.closeupengineering.it www.ninds.nih.
gov
Central nervous system controlling movement is in disorder
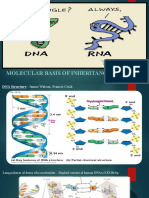
DNA: TAC-ATA-AAC-CAA-AGA-CGG-AGG
RNA: AUG-UAU-UUG-GUU-UCU-GCC-UCC
POL.PEP.: start-tyro-lev-val-ser-ala-ser A single molecule study in 2008 suggests alpha-synuclein exists as a
1.Transcription
mix of unstructured, alpha-helix, and beta-sheet-rich conformers in
a. The partial unwinding of the DNA molecule so that the portion of equilibrium. Mutations or buffer conditions known to improve
DNA that codes for the needed protein can be transcribed.
b.Then once the DNA molecule is unwound at the correct location, an aggregation strongly increase the population of the beta conformer,
enzyme called RNA polymerase helps line up nucleotides to create a
complementary strand of mRNA
thus suggesting this could be a conformation related to pathogenic
Symptoms: hand tremors, slow c.The new strand of RNA is made according to the rules of the base aggregation. One theory is that the majority of alpha-synuclein
movement, stiffness, and loss of pairing
balance i.DNA cytosine pairs with RNA guanine aggregates are located in the presynapse as smaller deposits which
ii.DNA guanine pairs with RNA cytosine
Protein affiliated: a-synuclein is the i.DNA thymine pairs with RNA adenine
causes synaptic dysfunction. Among the strategies for treating
primary component of Lewy bodies i. DNA adenine pairs with RNA uracil synucleinopathies are compounds that inhibit aggregation of
1.To mRNA
(abnormal aggregates of protein that a.The new RNA strand is released and the two unzipped DNA strands bind together again to form the alpha-synuclein. It has been shown that the small molecule
develop inside nerve cells in double helix.
cuminaldehyde inhibits fibrillation of alpha-synuclein.
i.The DNA template remains unchanged after transcription, it is possible to transcribe another
Parkinson's disease) identical molecule of RNA Protein synthesis relates to parkinson's because it is caused by
Women over 50 affected 1.A single gene on a DNA strand can produce enough RNA to make thousands of copies of the same
protein aggregation in the brain. Given that increased protein
protein, in a very short time.
Treatments balance out dopamine 1. Then is translated
aggregation may result not only from an increase in production, but
levels, but there are no cures a.mRNA is sent to the cytoplasm, where it bonds with ribosomes. There are 3 important
Interesting facts: Approximately 10 bonding sites; one for mRNA and two for tRNA. One is called P site and the other, A also from decreased protein clearance, it is imperative to investigate
site.
million people worldwide suffer from i.The ribosome slides down the mRNA, so that the tRNA in the A site moves over to both possibilities as potential PD culprits.
the P site, a new codon fills the A site. (the A site brings new amino acids to the Amino acid in the polypeptide chain is not right for that specific
Parkinsons disease. Around 127,000 growing polypeptide at the P site.
people in the UK have the condition. ii.The tRNA with the appropriate amino acid pairs bases with the new codon at the situation and is still used in the protein domain within the alpha helix
iii.A peptide bond forms between the two adjacent amino acids held by tRNA
1 in every 500 people have it. It has molecules. This is what forms the first two links of a chain.
and/or the B sheets to misfold.
costed the U.S. $25 billion so far. For iv.The tRNA that was in the P site goes to the cytoplasm and the ribosome sliding
medication, per patient, it costs process begins again.
v.This continues until a stop codon enters the A site. When a stop protein enters, the
$2,500. tRNA in the P site releases.
a-synuclein synthesize process: vi.Translation to the protein has completed.
1.The Protein is folded
https://www.ncbi.nlm.nih.gov/pmc/art a. The polypeptide chain is folded into alpha-helices and Beta-Sheets. (2 structure)
icles/PMC3631137/ i.The domains are formed by combining alpha-helices and Beta-Sheet (3 structure)
1.All domains combine to form functioning protein (4 structure)
Vous aimerez peut-être aussi
- Draft Assigment Bio 2Document2 pagesDraft Assigment Bio 2puteri hanisPas encore d'évaluation
- Huntingtons PresentationDocument3 pagesHuntingtons Presentationapi-341316130Pas encore d'évaluation
- DNA Kita Iiwan RNA CytoDocument3 pagesDNA Kita Iiwan RNA CytozairahPas encore d'évaluation
- Deoxyribonucleic AcidDocument17 pagesDeoxyribonucleic AcidJennifer YuPas encore d'évaluation
- Molecular Basis of Inheritance HalfDocument15 pagesMolecular Basis of Inheritance HalfNaY 7347Pas encore d'évaluation
- GenBIO REVIEWERDocument39 pagesGenBIO REVIEWERblismae genotivaPas encore d'évaluation
- NCERT Solutions For Class 12 Biology Chapter 6 Molecular Basis of InheritanceDocument10 pagesNCERT Solutions For Class 12 Biology Chapter 6 Molecular Basis of InheritanceSneha HipparkarPas encore d'évaluation
- Reviewer Biochem For NucleotidesDocument3 pagesReviewer Biochem For NucleotidesNino D. AtilanoPas encore d'évaluation
- Molecular Basis of InheritanceDocument5 pagesMolecular Basis of InheritanceJust MePas encore d'évaluation
- Answered Topic 6 Worksheet AsDocument28 pagesAnswered Topic 6 Worksheet Ashayatnazmi50Pas encore d'évaluation
- Molecular Basis of Inheritance - Short NotesDocument3 pagesMolecular Basis of Inheritance - Short Notesp11925885Pas encore d'évaluation
- Molecular Basis of Inheritance - Short Notes - Lakshya NEET 2025Document3 pagesMolecular Basis of Inheritance - Short Notes - Lakshya NEET 2025anantpounikar02Pas encore d'évaluation
- Mol Basis of InheritanceDocument74 pagesMol Basis of InheritanceNishita BharaliPas encore d'évaluation
- Protein Biosynthesis - 070214Document7 pagesProtein Biosynthesis - 070214LakshmipriyaPas encore d'évaluation
- Molecular Basis of InheritanceDocument4 pagesMolecular Basis of Inheritancereet.patidar18Pas encore d'évaluation
- Genetics: Unit III - Living Things and Its Environment - Heredity: Inheritance & VariationDocument4 pagesGenetics: Unit III - Living Things and Its Environment - Heredity: Inheritance & VariationKirito TobioPas encore d'évaluation
- Nucleic AcidsDocument3 pagesNucleic AcidsJovan SernaPas encore d'évaluation
- Nucleic Acids + Protein SynthesisDocument5 pagesNucleic Acids + Protein SynthesisNezza WidarkoPas encore d'évaluation
- Protein SynthesisDocument35 pagesProtein SynthesisDaryl GumacalPas encore d'évaluation
- THE CENTRAL DOGMA HandoutDocument6 pagesTHE CENTRAL DOGMA HandoutLeah AturoPas encore d'évaluation
- BIO-5dll 3rdDocument15 pagesBIO-5dll 3rdarlene dioknoPas encore d'évaluation
- Gene ExpressionDocument44 pagesGene Expression20.020 Cahya MaharaniPas encore d'évaluation
- Gene ExpressionDocument44 pagesGene ExpressionCahya MaharaniPas encore d'évaluation
- DNAto ProteinDocument41 pagesDNAto ProteinJimmy gogoPas encore d'évaluation
- COMPETANCE BASED QUESTION Chapter 6 Molecular Basis of InheritanceDocument9 pagesCOMPETANCE BASED QUESTION Chapter 6 Molecular Basis of InheritanceKarthika UmashankarPas encore d'évaluation
- What Is DNADocument5 pagesWhat Is DNA꧁༒૮αll ʍ૯ ૨αj༒꧂Pas encore d'évaluation
- GeneticsDocument20 pagesGeneticsMadaPas encore d'évaluation
- BIOTECHNOLOGYDocument4 pagesBIOTECHNOLOGYheyyouPas encore d'évaluation
- Self Assessment Answers 6 Asal Biology CBDocument2 pagesSelf Assessment Answers 6 Asal Biology CBsaeed.khedrizaPas encore d'évaluation
- Virology TableDocument10 pagesVirology TableFrances Ijeoma ObiakorPas encore d'évaluation
- Nucleic Acid: Genetic CodeDocument2 pagesNucleic Acid: Genetic CodeAngelica AycardoPas encore d'évaluation
- Basic Molecular BiologyDocument10 pagesBasic Molecular BiologyPaolaPas encore d'évaluation
- Biology Lesson 1.1Document16 pagesBiology Lesson 1.1Crystal Joy BondadPas encore d'évaluation
- CO3 TranscriptionDocument8 pagesCO3 Transcriptionpriya27suranaPas encore d'évaluation
- Sunshine23 Biochem-Ch21Document4 pagesSunshine23 Biochem-Ch21Alexa Nicole PamaPas encore d'évaluation
- Genitics Asignment MD Asif Roll-088Document11 pagesGenitics Asignment MD Asif Roll-088Asif SheikhPas encore d'évaluation
- Principles of Microbiology 1 (Summary)Document8 pagesPrinciples of Microbiology 1 (Summary)Mabelle DucusinPas encore d'évaluation
- Essentials of The Living World 5th Edition George Johnson Solutions ManualDocument5 pagesEssentials of The Living World 5th Edition George Johnson Solutions Manualjuliemcintyretzydiengcr100% (26)
- General Biology - DNA ReplicationDocument3 pagesGeneral Biology - DNA ReplicationChristine Marylou PalomoPas encore d'évaluation
- 2 7Document5 pages2 7totorobles92Pas encore d'évaluation
- MOLECULAR GENETICS AND EVOLUTION-activityDocument2 pagesMOLECULAR GENETICS AND EVOLUTION-activityIvy ManagPas encore d'évaluation
- DNA Structure and ReplicationDocument12 pagesDNA Structure and ReplicationJhune Dominique GalangPas encore d'évaluation
- CO3 TranslationDocument8 pagesCO3 Translationpriya27suranaPas encore d'évaluation
- Molecular BiologyDocument52 pagesMolecular Biologychandu SahaPas encore d'évaluation
- Ganong PhysiologyDocument64 pagesGanong PhysiologySaadia Riaz64% (14)
- AP Biology Unit 6 GuideDocument7 pagesAP Biology Unit 6 Guidelolo2212008Pas encore d'évaluation
- Translation in Prokaryotes: B.Sc. Biotechnology Molecular BiologyDocument32 pagesTranslation in Prokaryotes: B.Sc. Biotechnology Molecular Biologysanthosh Kumar c sPas encore d'évaluation
- Sathyanarayan Biochemistry 4epdfDocument35 pagesSathyanarayan Biochemistry 4epdfAbubakar SuleimanPas encore d'évaluation
- Binder Key 2010-DnaDocument15 pagesBinder Key 2010-Dnaapi-292000448Pas encore d'évaluation
- Science10 q3 - Mod4.1 Week4 Protein-Synthesis v4-1Document11 pagesScience10 q3 - Mod4.1 Week4 Protein-Synthesis v4-1Mhira MuliPas encore d'évaluation
- Miniature Protein BIologyDocument83 pagesMiniature Protein BIologyPREET KANOOGAPas encore d'évaluation
- Control of Eukaryotic Genes: AP BiologyDocument21 pagesControl of Eukaryotic Genes: AP BiologyGabe GallagherPas encore d'évaluation
- Biology Gene Expression Article Title: Expression of Biological InformationDocument6 pagesBiology Gene Expression Article Title: Expression of Biological InformationNUR FAQIHAH BINTI FARISH MoePas encore d'évaluation
- IB Biology Notes - 35 Transcription TranslationDocument2 pagesIB Biology Notes - 35 Transcription TranslationJohn Philip D. NapalPas encore d'évaluation
- A Cholesterol Connection in Rnai: News and ViewsDocument2 pagesA Cholesterol Connection in Rnai: News and ViewsJoseph Arimateias Diniz de CarvalhoPas encore d'évaluation
- Meridian Protein SynthesisDocument7 pagesMeridian Protein SynthesisjosewaltPas encore d'évaluation
- PCR in Infectious DiseasesDocument3 pagesPCR in Infectious Diseasesthị sô phiaPas encore d'évaluation
- Learner's Activity Sheet: Science (Quarter III - Week 4)Document10 pagesLearner's Activity Sheet: Science (Quarter III - Week 4)MARITESS COLLADOPas encore d'évaluation
- Lesson - Perpetu-Wps OfficeDocument6 pagesLesson - Perpetu-Wps OfficeAngelica Pardeño100% (1)
- Reverse Engineering Step by StepDocument3 pagesReverse Engineering Step by Stepapi-340815123Pas encore d'évaluation
- ReportDocument8 pagesReportapi-341234787Pas encore d'évaluation
- Science ProjectDocument9 pagesScience Projectapi-340815123Pas encore d'évaluation
- Poster - Holly Buck FinalDocument1 pagePoster - Holly Buck Finalapi-340815123Pas encore d'évaluation
- Straw FiltersDocument9 pagesStraw Filtersapi-340815123Pas encore d'évaluation
- Gantt Chart - Gantt Chart TemplateDocument1 pageGantt Chart - Gantt Chart Templateapi-340815123Pas encore d'évaluation
- Final ProjectDocument10 pagesFinal Projectapi-340815123Pas encore d'évaluation
- Proof of Efficacy - ChemDocument3 pagesProof of Efficacy - Chemapi-340815123Pas encore d'évaluation
- Reverse EngineeringDocument12 pagesReverse Engineeringapi-340815123Pas encore d'évaluation
- SubstancesDocument3 pagesSubstancesapi-340815123Pas encore d'évaluation
- ChemicaldominoesDocument2 pagesChemicaldominoesapi-338167819Pas encore d'évaluation
- CodeDocument3 pagesCodeapi-340815123Pas encore d'évaluation
- Case PresentationDocument12 pagesCase Presentationapi-352673659Pas encore d'évaluation
- SpermwhaleDocument9 pagesSpermwhaleapi-340815123Pas encore d'évaluation
- Sea Turtle 2 0Document9 pagesSea Turtle 2 0api-340815123Pas encore d'évaluation
- Gannt Chart - Sheet1Document1 pageGannt Chart - Sheet1api-340815123Pas encore d'évaluation
- Heart Rate HomeostasisDocument2 pagesHeart Rate Homeostasisapi-332478778Pas encore d'évaluation
- Cheese Lab - HollyDocument15 pagesCheese Lab - Hollyapi-340815123Pas encore d'évaluation
- Holly Jossart PCR LabDocument4 pagesHolly Jossart PCR Labapi-340815123Pas encore d'évaluation
- Heart Rate ArticleDocument4 pagesHeart Rate Articleapi-332478778Pas encore d'évaluation
- BuildabandDocument5 pagesBuildabandapi-340815123Pas encore d'évaluation
- Cladogram Story ImprovedDocument20 pagesCladogram Story Improvedapi-340815123Pas encore d'évaluation
- Cell Structure and FunctionADMModule - Grade12 - Quarter1STEM - BIO12 Ia C 2Document14 pagesCell Structure and FunctionADMModule - Grade12 - Quarter1STEM - BIO12 Ia C 2Lyka Mae BenitoPas encore d'évaluation
- Biochemistry Lec 1st ShiftDocument12 pagesBiochemistry Lec 1st ShiftNicole RomeroPas encore d'évaluation
- 3 Molecular Evidence - BioNinjaDocument4 pages3 Molecular Evidence - BioNinjaRosie SunPas encore d'évaluation
- Research PaperDocument13 pagesResearch PaperJohnny AnPas encore d'évaluation
- 2021-Review-CRISPR Technologies and PAM-free NucleaseDocument12 pages2021-Review-CRISPR Technologies and PAM-free NucleaseCristian Felipe Sandoval QuiñonezPas encore d'évaluation
- Proteins MetabolismDocument27 pagesProteins MetabolismFouzia GillPas encore d'évaluation
- Protein SynthesisDocument2 pagesProtein SynthesisAbigailPas encore d'évaluation
- Lab 3 - Biology 1003A (Joseph)Document8 pagesLab 3 - Biology 1003A (Joseph)JosephDionPas encore d'évaluation
- 2022.05.27 Cruz PadillaDocument46 pages2022.05.27 Cruz Padillaarooj sheikhPas encore d'évaluation
- Site Directed MutagenesisDocument77 pagesSite Directed MutagenesisDanny Sebastian Thomas100% (1)
- Protein Requirements and Supplementation in Strength Sports: Nutrition July 2004Document8 pagesProtein Requirements and Supplementation in Strength Sports: Nutrition July 2004Andrei ChirilaPas encore d'évaluation
- Chem 2221 BSPsych Syllabus OBE - RRRDocument16 pagesChem 2221 BSPsych Syllabus OBE - RRRFrance Jan First SaplacoPas encore d'évaluation
- L2 - Q2 - Introduction To Life ScienceDocument41 pagesL2 - Q2 - Introduction To Life ScienceMJ CaparidaPas encore d'évaluation
- The Structural Components of The Cell Membrane and Its Functions, With Transport Mechanisms.Document17 pagesThe Structural Components of The Cell Membrane and Its Functions, With Transport Mechanisms.Vieyah Angela VicentePas encore d'évaluation
- Organic Molecules LabDocument10 pagesOrganic Molecules Labbassoon11Pas encore d'évaluation
- Life Sciences Study GuideDocument246 pagesLife Sciences Study GuideAphane Ka Sebele100% (2)
- LEC - 5 - Complexation and Protein BindingDocument50 pagesLEC - 5 - Complexation and Protein BindingGRACE MAR CABAHUGPas encore d'évaluation
- AP Biology Summer Packet 2020-2021Document14 pagesAP Biology Summer Packet 2020-2021Ferdous Al-ShimaryPas encore d'évaluation
- Peptide BondDocument2 pagesPeptide BondA PutoyPas encore d'évaluation
- 3.2 Cell Transport PDFDocument146 pages3.2 Cell Transport PDFChryssa EconomouPas encore d'évaluation
- From Gene To Protein: Lecture OutlineDocument15 pagesFrom Gene To Protein: Lecture OutlineEiann Jasper LongcayanaPas encore d'évaluation
- Biochem Lab Reviewer Midterms With EditsDocument8 pagesBiochem Lab Reviewer Midterms With EditsDyosAra100% (1)
- BM 501 Essentials of BiophysicsDocument2 pagesBM 501 Essentials of BiophysicssampotPas encore d'évaluation
- NH CH CO H: 6.11 Amino Acids, Proteins and DNADocument14 pagesNH CH CO H: 6.11 Amino Acids, Proteins and DNAPedro Moreno de SouzaPas encore d'évaluation
- 2021 Biochem and Genetics Honours ProjectsDocument56 pages2021 Biochem and Genetics Honours ProjectsJuanPas encore d'évaluation
- SCIENCE 10 DEMO 4th QUARTERDocument3 pagesSCIENCE 10 DEMO 4th QUARTERJane Elam MontesPas encore d'évaluation
- Getting Started With CalisthenicsDocument178 pagesGetting Started With Calisthenicshierophant2411100% (6)
- Module 2 EdditedDocument22 pagesModule 2 EdditedMARIE ANN DIAMAPas encore d'évaluation
- Unit 1: Chemistry of LifeDocument44 pagesUnit 1: Chemistry of Life(25) Yahya Kagan AksunPas encore d'évaluation
- Monalisa ManurungDocument13 pagesMonalisa ManurungPradinta BayuPas encore d'évaluation