Académique Documents
Professionnel Documents
Culture Documents
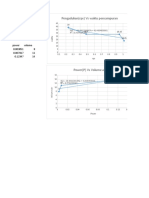
Charles' Law V Vs T Graph: 18 20 F (X) 0.0937375283x + 11.9128641745 R 0.8971111152
Transféré par
Erwin CabangalTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Charles' Law V Vs T Graph: 18 20 F (X) 0.0937375283x + 11.9128641745 R 0.8971111152
Transféré par
Erwin CabangalDroits d'auteur :
Formats disponibles
Group 5 3ChE C
x7g53CT
Cabangal, Erwin Cayl P.
Cabungcal, Cesarah Justine
EXPERIMENT # 7
TREATMENT OF RESULTS
TRIAL 1 Charl
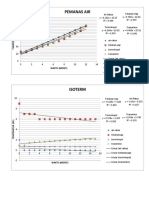
Temperature (deg C) Volume (mL) 20
Ice-Brine 4 11 18 f(x) = 0.0937375283x +
Ice-water 5 13 16 R² = 0.8971111152
14
Tap water 27 15.25
Volume (mL)
12
Heated Water 40 16
10
70 18 8
6
Trial 2 4
Temperature ( deg C) Volume (mL) 2
Ice-Brine 4 11 0
0 10 20
Ice-Water 5 12.75
Tap Water 27 15.25
Heated Water 40 16
76 18.3
Trial 1 Char
Equation of the Line : y= 0.0937x +11.913 20
At abs zero temp: V = 0: 0= 0.0937x +11.913 18 f(x) = 0.0913366617x +
16 R² = 0.9066368144
x= -127.14
14
R^2 = 0.8971 Volume (mL)
12
Trial 2
10
Equation of the line : y= 0.0913x + 11.883 8
0=0.0913x + 11.883 6
x= -130.15 4
R^2 =0.9066 2
0
0 10 20
Relationship of V and T : Direct relationship
As Volume Increases , the temperature increases
SOURCE OF ERROR: the Syringe used
1 half of the marks are not visible (faded) which resulted to an inexact reading
2 it was just clogged by something that may cause the plunger to be stuck which resulted to inconsistent results
*The syringe the we sealed with an epoxy did not dry
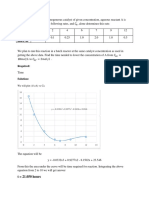
Charles' Law V vs T graph
20
18 f(x) = 0.0937375283x + 11.9128641745
16 R² = 0.8971111152
14
Volume (mL)
12
10
8
6
4
2
0
0 10 20 30 40 50 60 70 80
Temperature(deg C)
Charles' Law V vs T graph
20
18 f(x) = 0.0913366617x + 11.8833654828
16 R² = 0.9066368144
14
Volume (mL)
12
10
8
6
4
2
0
0 10 20 30 40 50 60 70 80
Temperature (deg C)
esulted to inconsistent results
Vous aimerez peut-être aussi
- Ion Beams for Materials AnalysisD'EverandIon Beams for Materials AnalysisR. Curtis BirdPas encore d'évaluation
- Doc2 IPDocument1 pageDoc2 IP.Pas encore d'évaluation
- Pengadukan (RPS) Vs Waktu PencampuranDocument2 pagesPengadukan (RPS) Vs Waktu Pencampuranrujikulo hunterPas encore d'évaluation
- Leeds Mass BalanceDocument32 pagesLeeds Mass BalanceAmrikPas encore d'évaluation
- Tekanan UapDocument2 pagesTekanan UapmridhotriadiPas encore d'évaluation
- Data Dan Perhitungan P11Document5 pagesData Dan Perhitungan P11Shinta SetyowatiPas encore d'évaluation
- Wa0002Document2 pagesWa0002aliyahnahdaPas encore d'évaluation
- Wa0002Document2 pagesWa0002aliyahnahdaPas encore d'évaluation
- ID 17,25 Inch Number and Length 95, 18'0'' Baffle Space 3 Phases 2 Phases 1 OD, BWG, Pitch 1inch., 18BWG., 1,25inch. SquareDocument3 pagesID 17,25 Inch Number and Length 95, 18'0'' Baffle Space 3 Phases 2 Phases 1 OD, BWG, Pitch 1inch., 18BWG., 1,25inch. SquareErwin FirmansyahPas encore d'évaluation
- Ch12 AddendumDocument14 pagesCh12 AddendumReza SadeghiPas encore d'évaluation
- Result and CalculationDocument4 pagesResult and CalculationjohncenaPas encore d'évaluation
- Given: DV/DT 1000 V/ S and I: Chapter 9 - ThyristorsDocument8 pagesGiven: DV/DT 1000 V/ S and I: Chapter 9 - ThyristorsAbd Alkader AlwerPas encore d'évaluation
- ChE Day 2Document6 pagesChE Day 2JHuvieCLairePas encore d'évaluation
- Top of LEAD Tidak Diketahui, Jadi Asumsikan Kolom LEAD Adalah Dari Top of TAIL S/D SURFACEDocument7 pagesTop of LEAD Tidak Diketahui, Jadi Asumsikan Kolom LEAD Adalah Dari Top of TAIL S/D SURFACEBimantara PrayogoPas encore d'évaluation
- 1 B Cambiar ColoressssDocument2 pages1 B Cambiar ColoressssSolda GamasPas encore d'évaluation
- TPP Mini ProjectDocument2 pagesTPP Mini Projectraden adibPas encore d'évaluation
- Reactor Type: Reactor With Specified Constant Temperature of 150 CDocument8 pagesReactor Type: Reactor With Specified Constant Temperature of 150 CJay MaradiyaPas encore d'évaluation
- Grafik Hubungan Antara PH Terhadap Volume (Sampel 2)Document2 pagesGrafik Hubungan Antara PH Terhadap Volume (Sampel 2)Laely PermanasariPas encore d'évaluation
- Group 2 CRE Lab (Autosaved)Document54 pagesGroup 2 CRE Lab (Autosaved)ahmad RaoPas encore d'évaluation
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagPas encore d'évaluation
- Lab Exe GarlandDocument5 pagesLab Exe Garlandichiwaaa sanPas encore d'évaluation
- Ideal Regenerative Cycle - Sample ProblemDocument8 pagesIdeal Regenerative Cycle - Sample ProblemJohn David DivinagraciaPas encore d'évaluation
- University of Sabratha Faculty of Engineering Department of Chemical EngineeringDocument8 pagesUniversity of Sabratha Faculty of Engineering Department of Chemical EngineeringMarPas encore d'évaluation
- Taprop CobaDocument146 pagesTaprop CobafaridPas encore d'évaluation
- Lab Report #4Document2 pagesLab Report #4Mad BasblaPas encore d'évaluation
- Solution To ChECal MRII BookDocument15 pagesSolution To ChECal MRII BookKristinePas encore d'évaluation
- Problem Set #3 - "Due" September 21st, 2015Document10 pagesProblem Set #3 - "Due" September 21st, 2015DechenPemaPas encore d'évaluation
- Distillation Column: Bubble Point ComputationDocument11 pagesDistillation Column: Bubble Point ComputationChua RhickPas encore d'évaluation
- PTD Assignment 2 - Andreas K 217069363Document9 pagesPTD Assignment 2 - Andreas K 217069363Andreas KanimePas encore d'évaluation
- Semibatch KRD LabDocument7 pagesSemibatch KRD LabPritiPas encore d'évaluation
- Determination of Relative PermeabilityDocument8 pagesDetermination of Relative PermeabilityAamir AwaanPas encore d'évaluation
- Crank Nichlson: Condiciones LimiteDocument9 pagesCrank Nichlson: Condiciones LimiteMayra RiosPas encore d'évaluation
- 06 Summative-Gases KeyDocument2 pages06 Summative-Gases KeyENSANO, RHYNS G.Pas encore d'évaluation
- Romulo, Aleck Gio N. HW3 Odd 6.1Document15 pagesRomulo, Aleck Gio N. HW3 Odd 6.1Kenneth SablayPas encore d'évaluation
- Sockets:: BasementDocument10 pagesSockets:: BasementSamuel AdamuPas encore d'évaluation
- Section N 5-9 How Canwec Check ?Document3 pagesSection N 5-9 How Canwec Check ?ericleePas encore d'évaluation
- Yarmouk University Civil Engineering Department Fluid Mechanics and Hydraulics Laboratory CE 354Document9 pagesYarmouk University Civil Engineering Department Fluid Mechanics and Hydraulics Laboratory CE 354Mohammed MigdadyPas encore d'évaluation
- Grafik Perbandingan PH Antar Titik Sungai: Sungai Cipamokolan 6 Sungai Cipamokolan 7 Sungai Cipamokolan 8Document5 pagesGrafik Perbandingan PH Antar Titik Sungai: Sungai Cipamokolan 6 Sungai Cipamokolan 7 Sungai Cipamokolan 8zeihan niswanurrahimPas encore d'évaluation
- Objective:: Feed Tanks Batch ReactorDocument5 pagesObjective:: Feed Tanks Batch Reactorfareeha saeedPas encore d'évaluation
- Uitm Lab ReportDocument4 pagesUitm Lab ReportShark MJPas encore d'évaluation
- Grafik Kadar Air Vs Laju PengeringanDocument2 pagesGrafik Kadar Air Vs Laju PengeringanSindi SagitaPas encore d'évaluation
- CRE Exp10Document3 pagesCRE Exp10kabali007123Pas encore d'évaluation
- Hydrodynamic Calculation Butterfly Valve Lenticular Disc $S - D 0.26$Document30 pagesHydrodynamic Calculation Butterfly Valve Lenticular Disc $S - D 0.26$Eng-CalculationsPas encore d'évaluation
- Problem Solution ch15Document7 pagesProblem Solution ch15윤희상Pas encore d'évaluation
- TPP Excel BaruDocument7 pagesTPP Excel BaruAtika HapsatiPas encore d'évaluation
- 2 Condenser 3: CompressorDocument16 pages2 Condenser 3: CompressorLeslie CatindigPas encore d'évaluation
- School of Geology, Petroleum and Mining Engineering Petroleum Engineering DepartmentDocument9 pagesSchool of Geology, Petroleum and Mining Engineering Petroleum Engineering DepartmentУлдана ЖанабековаPas encore d'évaluation
- Terrasse 7 - 5: P1 Section 15x30: A-A B-BDocument5 pagesTerrasse 7 - 5: P1 Section 15x30: A-A B-BDrancyPas encore d'évaluation
- Gambar SkemaDocument2 pagesGambar SkemaCrown HuntPas encore d'évaluation
- Experiment 3 Conductometry HCLDocument5 pagesExperiment 3 Conductometry HCLMayank BajajPas encore d'évaluation
- Perhitungan Biokim Asam Amino ProteinDocument6 pagesPerhitungan Biokim Asam Amino ProteinSiti SainidahPas encore d'évaluation
- APPENDIX C ComputationsDocument9 pagesAPPENDIX C ComputationsNicole Anne BorromeoPas encore d'évaluation
- Allen: Target: Pre-Medical 2024Document18 pagesAllen: Target: Pre-Medical 2024Rishu kumarPas encore d'évaluation
- Cálculos Químicos: Cálculos Del Experimento 1: "Volumen Molar de Un Gas"Document4 pagesCálculos Químicos: Cálculos Del Experimento 1: "Volumen Molar de Un Gas"Carl AcuñaPas encore d'évaluation
- Qave Π D Time Q1+Q 2+Q 3 Ρxdxvave Μ: Inside Diameter For All Pipes Is 25 Mm = 2.5 CmDocument4 pagesQave Π D Time Q1+Q 2+Q 3 Ρxdxvave Μ: Inside Diameter For All Pipes Is 25 Mm = 2.5 CmJohn Pierre JerusalemPas encore d'évaluation
- Lampiran A Perhitungan: Vc2H5Ohxpc2H5Oh BM C 2 H 5 Oh GR ML GR MolDocument4 pagesLampiran A Perhitungan: Vc2H5Ohxpc2H5Oh BM C 2 H 5 Oh GR ML GR MolNfwciwaPas encore d'évaluation
- Banco de Tubos 3 BolilloDocument2 pagesBanco de Tubos 3 BolilloThomasPas encore d'évaluation
- Binary LiquidsDocument8 pagesBinary LiquidsSuzanne Clariz M. BaltazarPas encore d'évaluation
- Lesson 2 Exercises Problem 2Document3 pagesLesson 2 Exercises Problem 2esclitoarhonPas encore d'évaluation
- Procon Project EDITED 1Document4 pagesProcon Project EDITED 1Erwin CabangalPas encore d'évaluation
- CSTR 1Document2 pagesCSTR 1Erwin CabangalPas encore d'évaluation
- Quiz 8 - Engg Mechanics and Strength of MaterialsDocument11 pagesQuiz 8 - Engg Mechanics and Strength of MaterialsErwin CabangalPas encore d'évaluation
- Experiment A3: Series/Parallel Centrifugal Pumps: Department of Chemical EngineeringDocument1 pageExperiment A3: Series/Parallel Centrifugal Pumps: Department of Chemical EngineeringErwin CabangalPas encore d'évaluation
- Ust Che Batch: Chemical Engineering DepartmentDocument1 pageUst Che Batch: Chemical Engineering DepartmentErwin CabangalPas encore d'évaluation
- Letter For Plant VisitDocument1 pageLetter For Plant VisitErwin CabangalPas encore d'évaluation
- Batch Shirt Order FormDocument1 pageBatch Shirt Order FormErwin CabangalPas encore d'évaluation
- Analysis Exp 1 Version 2Document3 pagesAnalysis Exp 1 Version 2Erwin CabangalPas encore d'évaluation
- Parts of An IntroductionDocument3 pagesParts of An IntroductionErwin Cabangal100% (2)
- Experiment C1: Permeability: Department of Chemical EngineeringDocument1 pageExperiment C1: Permeability: Department of Chemical EngineeringErwin CabangalPas encore d'évaluation
- Experiment 6 Lab ReportDocument3 pagesExperiment 6 Lab ReportErwin CabangalPas encore d'évaluation
- 4Che-B Class Directory: Name Contact NumberDocument2 pages4Che-B Class Directory: Name Contact NumberErwin CabangalPas encore d'évaluation
- TMP - 28983-Physics 202L - Schedule - 1st Term 2016-205974324Document1 pageTMP - 28983-Physics 202L - Schedule - 1st Term 2016-205974324Erwin CabangalPas encore d'évaluation
- Problems in Getting F12Document1 pageProblems in Getting F12Erwin CabangalPas encore d'évaluation
- Fundamental Electrical Measurements: Experiment # 1Document8 pagesFundamental Electrical Measurements: Experiment # 1Erwin CabangalPas encore d'évaluation
- Transport ProblemsDocument4 pagesTransport ProblemsErwin CabangalPas encore d'évaluation
- Evaluation of View FactorDocument1 pageEvaluation of View FactorErwin CabangalPas encore d'évaluation
- TMP - 28983-PHYSICS 202LAB - Handouts Aug 2016198621344Document36 pagesTMP - 28983-PHYSICS 202LAB - Handouts Aug 2016198621344Erwin CabangalPas encore d'évaluation
- Psychology - Scientific Study of Behavior and MentalDocument2 pagesPsychology - Scientific Study of Behavior and MentalErwin CabangalPas encore d'évaluation
- 1st Sem AY 2015-2016: University of Santo TomasDocument2 pages1st Sem AY 2015-2016: University of Santo TomasErwin CabangalPas encore d'évaluation
- Centripetal Force Is Related Directly To Velocity Squared and Mass, and Is Related Inversely To RadiusDocument3 pagesCentripetal Force Is Related Directly To Velocity Squared and Mass, and Is Related Inversely To RadiusErwin CabangalPas encore d'évaluation
- Schottky DiodeDocument23 pagesSchottky DiodeRavi KiranPas encore d'évaluation
- ThAnalysis Catalogue enDocument44 pagesThAnalysis Catalogue enHanLe DuyPas encore d'évaluation
- ViscoelasticityDocument11 pagesViscoelasticitymchilwesaPas encore d'évaluation
- Effects of PH On Shear Thickening Behaviour On Fumed Silica SuspensionDocument7 pagesEffects of PH On Shear Thickening Behaviour On Fumed Silica SuspensionEHTESHAMUL ISLAMPas encore d'évaluation
- Gujarat Technological UniversityDocument2 pagesGujarat Technological UniversityShivam PanchalPas encore d'évaluation
- Hudson, J. A. and Harrison, J. R. - Rock Mechanics (Part1) - 99Document1 pageHudson, J. A. and Harrison, J. R. - Rock Mechanics (Part1) - 99Khatri NiwasPas encore d'évaluation
- Shear Strength of Soil-1Document21 pagesShear Strength of Soil-1Kavita NaikPas encore d'évaluation
- Chemical Engineering Thermodynamics Solution Manual PDFDocument725 pagesChemical Engineering Thermodynamics Solution Manual PDFNaveen100% (1)
- Department of Mining, Metallurgical and Materials EngineeringDocument24 pagesDepartment of Mining, Metallurgical and Materials EngineeringDrakePas encore d'évaluation
- NBTS-08Document23 pagesNBTS-08ShrutiPas encore d'évaluation
- Hot Working Cold WorkingDocument2 pagesHot Working Cold WorkingBivas Panigrahi100% (1)
- Handbook of Polymer Reaction EngineeringDocument1 131 pagesHandbook of Polymer Reaction Engineeringtflmckenna100% (10)
- Fluoroelastomer DAIEL Selection Guide: Peroxide Cure SystemDocument1 pageFluoroelastomer DAIEL Selection Guide: Peroxide Cure Systemkyeong cheol leePas encore d'évaluation
- STK400Document2 pagesSTK400irne83Pas encore d'évaluation
- Bands MoS2Document5 pagesBands MoS2Theodore Berlin100% (1)
- Cavity Writeup ExpDocument7 pagesCavity Writeup ExpKr PrajapatPas encore d'évaluation
- Reseach Project On Zircnonia Doped AluminaDocument2 pagesReseach Project On Zircnonia Doped Aluminatushargoelrkl1131Pas encore d'évaluation
- Stainless Steels 430F: MartensiticDocument2 pagesStainless Steels 430F: MartensiticRavindra ErabattiPas encore d'évaluation
- InTech Nano Imprint LithographyDocument38 pagesInTech Nano Imprint LithographyBhavik PatelPas encore d'évaluation
- Title: Latent Heat of FusionDocument4 pagesTitle: Latent Heat of FusionJanine Anne De VeraPas encore d'évaluation
- Data Sheet API 2W Grade 50Document3 pagesData Sheet API 2W Grade 50sagitrosePas encore d'évaluation
- Chapter ThreeDocument38 pagesChapter ThreeAbi DemPas encore d'évaluation
- Investigation of Transistor Characteristics of N-P-N and P-N-PDocument9 pagesInvestigation of Transistor Characteristics of N-P-N and P-N-PMOKAYAPas encore d'évaluation
- 06 Well Testing 201102Document27 pages06 Well Testing 201102Hosni Ben Mansour100% (2)
- Composite Leaf SpringDocument19 pagesComposite Leaf SpringsreedhilmsPas encore d'évaluation
- Electronics Device PDFDocument38 pagesElectronics Device PDFFariz Azhar AbdillahPas encore d'évaluation
- Design of FRP-Profiles and All-FRP-StructuresDocument67 pagesDesign of FRP-Profiles and All-FRP-StructuresjavierPas encore d'évaluation
- Physical Chemistry: José Mauricio Rodas RodríguezDocument10 pagesPhysical Chemistry: José Mauricio Rodas RodríguezJuan Camilo MejíaPas encore d'évaluation
- SPE 29543 Drilling Mud Rheology and The API Recommended MeasurementsDocument9 pagesSPE 29543 Drilling Mud Rheology and The API Recommended Measurementskmskskq0% (1)
- E205Document3 pagesE205Levi PogiPas encore d'évaluation