Académique Documents
Professionnel Documents
Culture Documents
CH11SB061
Transféré par
Rez Caitlina0 évaluation0% ont trouvé ce document utile (0 vote)
7 vues1 pageThis chapter summary covers key concepts about the periodic table including:
1. It describes periodic trends such as atomic radius, ionization energy, and electronegativity and how they are explained by electron arrangements and forces within atoms.
2. It defines terms such as atomic number, mass number, atomic mass, isotope, and radioisotope and their relationships.
3. It relates the reactivity of elements to their position in the periodic table and analyzes periodic property data to recognize trends.
Description originale:
Chemistry 11
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentThis chapter summary covers key concepts about the periodic table including:
1. It describes periodic trends such as atomic radius, ionization energy, and electronegativity and how they are explained by electron arrangements and forces within atoms.
2. It defines terms such as atomic number, mass number, atomic mass, isotope, and radioisotope and their relationships.
3. It relates the reactivity of elements to their position in the periodic table and analyzes periodic property data to recognize trends.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
7 vues1 pageCH11SB061
Transféré par
Rez CaitlinaThis chapter summary covers key concepts about the periodic table including:
1. It describes periodic trends such as atomic radius, ionization energy, and electronegativity and how they are explained by electron arrangements and forces within atoms.
2. It defines terms such as atomic number, mass number, atomic mass, isotope, and radioisotope and their relationships.
3. It relates the reactivity of elements to their position in the periodic table and analyzes periodic property data to recognize trends.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 1
Chapter 1 Summary
Key Expectations Key Terms
Throughout this chapter you have had the actinide law of constant composition
opportunity to do the following: alchemy line spectrum
• Demonstrate an understanding of the periodic law, and alkali metal mass number (A)
describe how electron arrangements and forces within alkaline earth metal mass spectrometer
atoms can explain periodic trends such as atomic analogy metal
radius, ionic radius, ionization energy, electron affinity, atom metalloid
and electronegativity. (1.1, 1.2, 1.4, 1.5) atomic mass model
• Identify chemical substances and reactions in everyday atomic number (Z) neutron
use or of environmental significance. (1.1, 1.3) atomic radius neutron number (N)
• Define and describe the relationship among atomic carbon-14 dating noble gas
number, mass number, and atomic mass, isotope, and compound nonmetal
radioisotope. (1.3) continuous spectrum nucleus
• Identify and describe careers associated with matter. (1.3) diagnostic test orbit
• Relate the reactivity of a series of elements to their posi- electron period
tion in the periodic table. (1.5) electron affinity periodic law
electron cloud periodic trend
• Analyze data involving periodic properties such as ion-
electronegativity principal quantum number
ization energy and atomic radius in order to recognize
element proton
general trends in the periodic table. (1.5)
empirical definition quantitative
empirical knowledge quantum mechanics
energy level radioactive
Make a excited radioisotope
Summary fission representative element
flame test SATP
Summarize the concepts that you have learned ground state STP
in this chapter by creating a concept map. Begin group theoretical knowledge
with the word “element” and use as many words
half-life theory
as possible from the list of key words.
halogen transition
ion transition metal
ionization energy transuranic element
Reflectyour
on
Learning
isotope
IUPAC
trend
triad
Revisit your answers to the Reflect on Your Learning ques- lanthanide unified atomic mass (u)
tions at the beginning of this chapter. law of conservation of valence electrons
mass
• How has your thinking changed?
• What new questions do you have?
The Nature of Matter 61
Vous aimerez peut-être aussi
- A Book of SecretsDocument311 pagesA Book of Secretsfrankie5791% (11)
- Longevity Forever BookDocument32 pagesLongevity Forever Bookavatar1959100% (1)
- Physics NotesDocument131 pagesPhysics NotesshridharPas encore d'évaluation
- How To Write ClearlyRules and Exercises On English Composition by Abbott, Edwin A.Document77 pagesHow To Write ClearlyRules and Exercises On English Composition by Abbott, Edwin A.Gutenberg.org100% (1)
- 5 Studyguide KeyDocument3 pages5 Studyguide KeyAnonymous PersonPas encore d'évaluation
- Sokolov - Quantum MehcanicsDocument566 pagesSokolov - Quantum MehcanicsGianniNicheli100% (1)
- Aqa A Level Chemistry Cheatsheet 3Document24 pagesAqa A Level Chemistry Cheatsheet 3David AdigboPas encore d'évaluation
- Organic Spectroscopic AnalysisDocument186 pagesOrganic Spectroscopic AnalysisNguyễn Ngọc Phước VươngPas encore d'évaluation
- Servant Leadership QuestionnaireDocument3 pagesServant Leadership QuestionnairedeepikaPas encore d'évaluation
- Fundamental Principles of Modern Theoretical Physics: International Series of Monographs in Natural PhilosophyD'EverandFundamental Principles of Modern Theoretical Physics: International Series of Monographs in Natural PhilosophyPas encore d'évaluation
- Shinmai Maou No Keiyakusha - Volume 11Document435 pagesShinmai Maou No Keiyakusha - Volume 11Bablu Dexter100% (1)
- 2009 Book ModelsOfComputationDocument188 pages2009 Book ModelsOfComputationDeepika100% (2)
- BONEFELD, Werner (Org.) Open MarxismDocument191 pagesBONEFELD, Werner (Org.) Open MarxismDiogo CarvalhoPas encore d'évaluation
- Guozhen Wu - Tsinghua University Press - Vibrational Spectroscopy-De Gruyter (2019)Document206 pagesGuozhen Wu - Tsinghua University Press - Vibrational Spectroscopy-De Gruyter (2019)tlhnabb2016Pas encore d'évaluation
- Year 9 End of Year Assessment 2023 PLCDocument1 pageYear 9 End of Year Assessment 2023 PLCVaidile JonikasPas encore d'évaluation
- B.Sc. III - Atomic and Nuclear PhysicsDocument3 pagesB.Sc. III - Atomic and Nuclear PhysicsDr. Satish MahadikPas encore d'évaluation
- Syllabus PH 5118 & 5218Document5 pagesSyllabus PH 5118 & 5218Findx proPas encore d'évaluation
- SyllabusDocument11 pagesSyllabusSavitri BhandariPas encore d'évaluation
- Phymerge PDFDocument181 pagesPhymerge PDFvenkateshPas encore d'évaluation
- Course: Department Specific Elective 2-: HPHDS5021T Nuclear and Particle PhysicsDocument3 pagesCourse: Department Specific Elective 2-: HPHDS5021T Nuclear and Particle PhysicsGrim Reaper Kuro OnihimePas encore d'évaluation
- Bscpe Sci1 SLM 6Document36 pagesBscpe Sci1 SLM 6April Joy GonzalesPas encore d'évaluation
- AL Chemistry SyllabusDocument33 pagesAL Chemistry Syllabusapi-3734333Pas encore d'évaluation
- Electronic Structure of Atoms: Topic 1Document6 pagesElectronic Structure of Atoms: Topic 1Brandon OoiPas encore d'évaluation
- Faraday Research Article Reactive Scattering Atoms and RadicalsDocument22 pagesFaraday Research Article Reactive Scattering Atoms and RadicalsgiampieroPas encore d'évaluation
- H Chem Electron STructure Test Prep WKSHTDocument4 pagesH Chem Electron STructure Test Prep WKSHTErin RiegoPas encore d'évaluation
- Chapter 1 NMR TheoryDocument63 pagesChapter 1 NMR TheoryabhiPas encore d'évaluation
- Decay of Radioactivity: Robert Miyaoka, PHDDocument9 pagesDecay of Radioactivity: Robert Miyaoka, PHDTieku NoblePas encore d'évaluation
- Scheme DSCE 1st Yr UG Scheme 2016 17Document119 pagesScheme DSCE 1st Yr UG Scheme 2016 17Nandan MaheshPas encore d'évaluation
- Scheme DSCE 1st Yr UG Scheme 2016Document61 pagesScheme DSCE 1st Yr UG Scheme 2016Saathvik Bhat100% (1)
- Chemistry Study Guide MYP 4Document1 pageChemistry Study Guide MYP 4JamesMartinPas encore d'évaluation
- Topic 3 - Periodicity SLDocument20 pagesTopic 3 - Periodicity SLnikes 1Pas encore d'évaluation
- Silabus Material Teknik ElektroDocument4 pagesSilabus Material Teknik ElektroNajmi KertasafariPas encore d'évaluation
- Periodic Trends Think Ib SlidfesDocument10 pagesPeriodic Trends Think Ib Slidfesamaya chopraPas encore d'évaluation
- 07AMSCH23 - Physical Chemistry - IIDocument260 pages07AMSCH23 - Physical Chemistry - IIALPHY100% (1)
- Chemistry - Atomic Structure - Gaseous State - Complete ModuleDocument122 pagesChemistry - Atomic Structure - Gaseous State - Complete Moduleruchir angraPas encore d'évaluation
- Unit 2+12 TARGET SHEET HL Atomic StructureDocument2 pagesUnit 2+12 TARGET SHEET HL Atomic Structurediya joshiPas encore d'évaluation
- CH 7 Atomic TheoryDocument73 pagesCH 7 Atomic TheoryMurk FatimaPas encore d'évaluation
- Seperation TechniquesDocument17 pagesSeperation TechniquesJagadeesh YPas encore d'évaluation
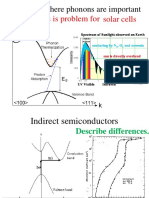
- Solar Cells Energy Loss Is Problem For: Examples Where Phonons Are ImportantDocument51 pagesSolar Cells Energy Loss Is Problem For: Examples Where Phonons Are Importantjose mirandaPas encore d'évaluation
- 1.1 Revision Guide Atomic Structure AqaDocument10 pages1.1 Revision Guide Atomic Structure AqadodoPas encore d'évaluation
- Paper V Unit-I: Classical Mechanics Ii (25 Marks) LECTURES 25 + 5 TutorialDocument5 pagesPaper V Unit-I: Classical Mechanics Ii (25 Marks) LECTURES 25 + 5 Tutorialzoo zooPas encore d'évaluation
- Tenorio 2005Document10 pagesTenorio 2005LuisOctavioJojoLosadaPas encore d'évaluation
- Lesson 20-10-2020-Nuclear ChemistryDocument10 pagesLesson 20-10-2020-Nuclear ChemistryAlemkeng BrendaPas encore d'évaluation
- Intro To Phonons-CastepDocument57 pagesIntro To Phonons-CastepgadasPas encore d'évaluation
- Chem 113E Module 5 Nuclear ChemistryDocument20 pagesChem 113E Module 5 Nuclear ChemistryKenneth John FerrarizPas encore d'évaluation
- A Level Chemistry Two Year Linear Course Scheme of WorkDocument64 pagesA Level Chemistry Two Year Linear Course Scheme of WorkRehan RafiqPas encore d'évaluation
- Topic 3 Periodicity SLDocument21 pagesTopic 3 Periodicity SLLyv SantosaPas encore d'évaluation
- Particle Interaction Matter UploadDocument79 pagesParticle Interaction Matter UploadvictorPas encore d'évaluation
- m1 Properties Structure of Matter ChemistryDocument8 pagesm1 Properties Structure of Matter ChemistrysarahsyedazakiPas encore d'évaluation
- CHEM 1 - MODULE 4 (Periodic Table, Valence, LEDS)Document5 pagesCHEM 1 - MODULE 4 (Periodic Table, Valence, LEDS)Joseph ZafraPas encore d'évaluation
- Materials 10 01148Document20 pagesMaterials 10 01148Lin TianPas encore d'évaluation
- Syll Btech. 1st Year New Syllabus FINAL 2008 BPUTDocument22 pagesSyll Btech. 1st Year New Syllabus FINAL 2008 BPUTkamalkantmbbsPas encore d'évaluation
- Btech. 1st Year New Syllabus FINAL 2008 BPUTDocument22 pagesBtech. 1st Year New Syllabus FINAL 2008 BPUTRajesh KumarPas encore d'évaluation
- Time-Dependent Density-Functional Theory: Carsten A. Ullrich Neepa T. MaitraDocument98 pagesTime-Dependent Density-Functional Theory: Carsten A. Ullrich Neepa T. MaitraFahd ElmourabitPas encore d'évaluation
- Fundamentals: The Quantum-Mechanical Many-Electron Problem and The Density Functional Theory ApproachDocument56 pagesFundamentals: The Quantum-Mechanical Many-Electron Problem and The Density Functional Theory ApproachAaron ArvindPas encore d'évaluation
- PhysicsDocument126 pagesPhysicsKishanJhaPas encore d'évaluation
- Atomic Structure and The Periodic TableDocument95 pagesAtomic Structure and The Periodic TableSarah 丹鳳Pas encore d'évaluation
- Periodic Table Lab: Part 1: Exploring Periodic Trends (Data Collection)Document4 pagesPeriodic Table Lab: Part 1: Exploring Periodic Trends (Data Collection)Sasha Lucero QuintanaPas encore d'évaluation
- Periodic Table (Chemistry)Document25 pagesPeriodic Table (Chemistry)bm OUREMPas encore d'évaluation
- Physics: Year: I Semester: IIDocument3 pagesPhysics: Year: I Semester: IIHercules ShahPas encore d'évaluation
- Lectures 10-11: 9.0 OverviewDocument18 pagesLectures 10-11: 9.0 OverviewHusna TajPas encore d'évaluation
- Phy1001 Engineering-Physics LTP 1.0 1 Phy1001-Engineering PhysicsDocument3 pagesPhy1001 Engineering-Physics LTP 1.0 1 Phy1001-Engineering PhysicsDevarsh ShahPas encore d'évaluation
- Soft X-Ray Photoionization of Atoms and Molecules Svensson2005Document19 pagesSoft X-Ray Photoionization of Atoms and Molecules Svensson2005jbmacielPas encore d'évaluation
- Che101 Chap 8Document68 pagesChe101 Chap 8Ruhi AfsaraPas encore d'évaluation
- Toward A Modern Atomic Theory: Sections 1.2-1.3 QuestionsDocument12 pagesToward A Modern Atomic Theory: Sections 1.2-1.3 QuestionsRez CaitlinaPas encore d'évaluation
- Trends in The Periodic Table: Section 1.4 QuestionsDocument13 pagesTrends in The Periodic Table: Section 1.4 QuestionsRez CaitlinaPas encore d'évaluation
- Covalent Bonding: Formation of Covalent BondsDocument8 pagesCovalent Bonding: Formation of Covalent BondsRez CaitlinaPas encore d'évaluation
- Ionic Bonding: AnalysisDocument7 pagesIonic Bonding: AnalysisRez CaitlinaPas encore d'évaluation
- Classifying Compounds: Investigation 2.1.1Document3 pagesClassifying Compounds: Investigation 2.1.1Rez CaitlinaPas encore d'évaluation
- CH11SB062Document2 pagesCH11SB062Rez CaitlinaPas encore d'évaluation
- CH11SB064Document2 pagesCH11SB064Rez CaitlinaPas encore d'évaluation
- 1984 SummaryDocument3 pages1984 Summaryflorin94Pas encore d'évaluation
- Eisner 2003 The Arts and The Creation of MindDocument6 pagesEisner 2003 The Arts and The Creation of MindAli KucukcinarPas encore d'évaluation
- DLL New GRADE 1 To 12 With SampleDocument25 pagesDLL New GRADE 1 To 12 With SampleencarPas encore d'évaluation
- Projek RC 1Document5 pagesProjek RC 1Hani BarjokPas encore d'évaluation
- 1Document26 pages1chaitanya4friendsPas encore d'évaluation
- Math Grade 1 TG Q1 and Q2Document169 pagesMath Grade 1 TG Q1 and Q2Sheryl Balualua Mape-SalvatierraPas encore d'évaluation
- Terapia Sistemica de Familia.Document15 pagesTerapia Sistemica de Familia.Oscar GonzalezPas encore d'évaluation
- (Walter Benjamin, Howard Eiland (Transl.) ) Berlin (B-Ok - CC) PDFDocument208 pages(Walter Benjamin, Howard Eiland (Transl.) ) Berlin (B-Ok - CC) PDFOvidio RíosPas encore d'évaluation
- Usulan Judul Skripsi 2 (Acc)Document2 pagesUsulan Judul Skripsi 2 (Acc)Rido WahyudiPas encore d'évaluation
- Five Codes of Barthes in Shahrazs StoryDocument14 pagesFive Codes of Barthes in Shahrazs Storymuhammad faheemPas encore d'évaluation
- PG II CC-7 The Theory of Rasa by DR Ravi K SinhaDocument3 pagesPG II CC-7 The Theory of Rasa by DR Ravi K SinhaShobhit MishraPas encore d'évaluation
- MPN 5 Tube MethodsDocument4 pagesMPN 5 Tube Methodsnewbonchu9291Pas encore d'évaluation
- Coek - Info The Fabric of RealityDocument5 pagesCoek - Info The Fabric of RealityFredioGemparAnarqiPas encore d'évaluation
- Ecclesiastes 12 9-14Document4 pagesEcclesiastes 12 9-14Samuel XiaoPas encore d'évaluation
- Methodology SrinivasDocument3 pagesMethodology SrinivasBidisha DasPas encore d'évaluation
- TrigonometryDocument16 pagesTrigonometryMark Lyndon M OrogoPas encore d'évaluation
- HCIDocument7 pagesHCIChethan.M100% (3)
- Adlerian TheraphyDocument2 pagesAdlerian TheraphySuparman SuaRez IIPas encore d'évaluation
- Shatakaalu Vemana Telugubhakthi ComDocument30 pagesShatakaalu Vemana Telugubhakthi ComkomireddyPas encore d'évaluation
- 현대 국어 감정동사의 범위와 의미 특성에 대한 연구Document26 pages현대 국어 감정동사의 범위와 의미 특성에 대한 연구Jennie ImPas encore d'évaluation
- A1 ArtsDocument4 pagesA1 ArtsJennifer AdvientoPas encore d'évaluation
- Foundations of Group BehaviourDocument36 pagesFoundations of Group BehaviourniginkabrahamPas encore d'évaluation