Académique Documents
Professionnel Documents
Culture Documents
Units Translation of Nanotechnology
Transféré par
afaqunaDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Units Translation of Nanotechnology
Transféré par
afaqunaDroits d'auteur :
Formats disponibles
Table of Units and Unit Conversions
The fundamental unit system is MKS, although cm are often used in this course. The
fundamental MKS units are:
• m = meters
• s = seconds
• kg = kilograms
• C = Coulombs
• K = degrees Kelvin (Temperature)
• Amount of “stuff” = mole = NAV of stuff (unitless)
Although not officially an MKS unit, Volts (V) are often used. Volts are defined in terms
of the potential to move charge. One volt appears across a resistance of one ohm
when a current of one ampere flows through that resistance. Hence volts are defined in
terms of current and resistance. Potential (measured in volts) is how much energy is
required to move charge from one point in space to another.
The column listing MKS units will also use other units at times such as Joules or
Newtons when they are common and help to clarify meaning.
Bold values represent vectors or tensors
Some Useful Rules for Working with Units
1) The units on both sides of “=” must be the same.
2) In any expression ef ( x ) , f(x) must be unitless.
3) Any two expressions that are added (+) or subtracted (-) must have the same
units.
∂A
4) Taking the differential does not change the units. For example has the same
∂t
A
units as .
t
desired
5) To convert from one set of units to another multiply by = 1 . For example
current
⎛ [1m ] ⎞
2
2 2
to convert from cm to m multiply by ⎜⎜ ⎟⎟ since 1 m = 100 cm.
⎝ [100cm ] ⎠
Units used in Spectroscopy
Symbol Name Units/Value MKS units
amu Atomic mass unit 1.66054×10-27 kg
c Velocity of light 2.9973×108 ms-1
D Debye [unit of dipole 3.336×10-30 Cm
moment]
e Electron charge 1.6022×10-19 C
eV Electron volt [unit of energy] 1 eV = 1.602×10-19 J
1 eV = 8062.4 cm-1
E, U Energy J = Nm kgm2s-2, CV
E Electric field Vm-1
h Planck’s constant 6.626×10-34 Js
I Intensity Wm-2 Js-1m-2
J Joule [unit of energy] Nm = kgm2s-2 = CV
k Wavevector k = 2πλ−1 = ωc-1 m-1
kB Boltzmann’s Const. kB = 1.3807×10-23 JK-1
me Electron mass me = 9.1095×10-31 kg
mp Proton mass mp = 1.6726×10-27 kg
NAV Avogadro’s Number NAV = 3.022×1023 Unitless
µ Dipole moment Cm
λ Wavelength m-1
ν, f Frequency s-1 = Hz
Spectroscopic Unit Conversions
Different disciplines of science and engineering often use different units to represent
photon energy or frequency. Chemists use cm-1, engineers like Hz (s-1), and physicists
use nm (10-9 m)or Ǻ (10-10 m) for wavelength and eV for energy. Since nanotechnology
is an interdisciplinary science, you will often be called on to convert from one set of units
to another.
Three constants need to be memorized or written down in order to be able to do these
conversions:
c = 3×108 ms-1, the speed of light
h = 6.62×10-34 Js, Planck’s constant
e = 1.6×10-19 C, the charge on an electron
The equations below show the relationship between frequency, f or ν; wavelength, λ;
and photon energy, E:
c = fλ
E = hf
Two more facts will be helpful:
To convert between an energy in Joules (J) and electron volts (eV) the value in electron
volts is multiplied by the electron charge, e: X(J) = e × X(eV)
The unit of cm-1 commonly used by chemists is the inverse of the wavelength, λ,
expressed in cm- λ-1
Combining these simple rules allows you to convert between any commonly used units.
Some examples are shown below:
Example 1: Convert from 500 nm to Hz
o Step 1, unit conversion from nm to m: 500 nm × 1 m / 109 nm = 500×10-9 m
o Step 2: convert from wavelength to frequency: f = c/λ: 3×108 ms-1 / 500×10-9 m = 6×1014 Hz
Example 2: Convert from 0.45 eV to cm-1
o Step 1, energy unit conversion: 0.45 eV × e = 7.2×10-20 J
o Step 2, convert from energy to frequency: E/h = f → 7.2×10-20 J / 6.62×10-34 Js = 1.09×1014 Hz
o Step 3, convert from frequency to wavelength: λ = c/f → 3×108 ms-1 / 1.09×1014 Hz = 2.76×10-6 m
o Step 4, unit conversion from m to cm: 2.76×10-6 m × 100 cm/1 m = 2.67×10-4 cm
o Step 5, take inverse of wavelength to get cm-1: (2.67×10-4 cm)-1 = 3625 cm-1
As you become more proficient at converting between units you can eliminate many of
the intermediate steps shown in these examples.
Vous aimerez peut-être aussi
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsD'EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsPas encore d'évaluation
- EEE2203 PHYSICAL ELECTRONICS II Lesson1Document11 pagesEEE2203 PHYSICAL ELECTRONICS II Lesson1FrancisPas encore d'évaluation
- Quantum Quest KeyDocument4 pagesQuantum Quest KeyAnonymous 7CxwuBUJz3Pas encore d'évaluation
- Strength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresD'EverandStrength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresÉvaluation : 4 sur 5 étoiles4/5 (1)
- AHL Calculating 1st IonizationDocument2 pagesAHL Calculating 1st IonizationMarilee HuntPas encore d'évaluation
- PART 1 SHORT LECTURE K30 21dec 2020Document27 pagesPART 1 SHORT LECTURE K30 21dec 2020Hgwergweg GwqegwPas encore d'évaluation
- Problems in Quantum Mechanics: Third EditionD'EverandProblems in Quantum Mechanics: Third EditionÉvaluation : 3 sur 5 étoiles3/5 (2)
- Re Materi Fisika Listrik 1bDocument46 pagesRe Materi Fisika Listrik 1bHeny AnggorowatiPas encore d'évaluation
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenD'EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillPas encore d'évaluation
- Lecture 12Document14 pagesLecture 12JayeshAtreyaPas encore d'évaluation
- Mathematical Solution Unifying the Four Fundamental Forces in NatureD'EverandMathematical Solution Unifying the Four Fundamental Forces in NaturePas encore d'évaluation
- Physical Quantities, Units and DimensionsDocument12 pagesPhysical Quantities, Units and Dimensionsdavid ama100% (1)
- Advanced Beam Dynamics & Hamiltonian Formalism: Rick Baartman, TRIUMF January 29, 2015Document23 pagesAdvanced Beam Dynamics & Hamiltonian Formalism: Rick Baartman, TRIUMF January 29, 2015s gPas encore d'évaluation
- Physics HandbookDocument10 pagesPhysics HandbookNational Physical Laboratory100% (1)
- Chapter 1: Basic Concepts: Today's ObjectivesDocument16 pagesChapter 1: Basic Concepts: Today's ObjectivesWan RodiePas encore d'évaluation
- Lecture21 Electromagnetic WavesDocument39 pagesLecture21 Electromagnetic WavesTaqi ShahPas encore d'évaluation
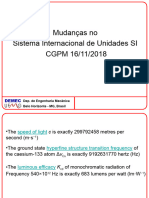
- Aula 2 Sistema SI NovoDocument17 pagesAula 2 Sistema SI NovoIuri ChavesPas encore d'évaluation
- 2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave EquationsDocument29 pages2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave Equationsjoao pedro GomesPas encore d'évaluation
- Chapter 10 Systems of Particles: Center of Mass (CM)Document15 pagesChapter 10 Systems of Particles: Center of Mass (CM)Nitin DasPas encore d'évaluation
- Physical Quantities and UnitsDocument5 pagesPhysical Quantities and UnitsLaud FumhandaPas encore d'évaluation
- Chapter 1 Fund E Eng DR MoenesDocument54 pagesChapter 1 Fund E Eng DR Moenesmahmoudaliabdelaziz11Pas encore d'évaluation
- Lecture 21Document39 pagesLecture 21ekta39Pas encore d'évaluation
- Relativistic UnitsDocument3 pagesRelativistic UnitsMarc Edzel Gercin DanaoPas encore d'évaluation
- Basic Concepts in ChemistryDocument124 pagesBasic Concepts in Chemistryara_anjoPas encore d'évaluation
- Tutorial Set 1Document2 pagesTutorial Set 1elisee tsokezoPas encore d'évaluation
- Physics For EngineersDocument14 pagesPhysics For EngineersEmilPas encore d'évaluation
- P N NucleusDocument14 pagesP N NucleusSerene AryalPas encore d'évaluation
- Lecture-3: Instruments & MeasurementsDocument46 pagesLecture-3: Instruments & MeasurementsWalid IqbalPas encore d'évaluation
- 13 Oscillatoryor Periodic Motion Rev 2Document12 pages13 Oscillatoryor Periodic Motion Rev 2Eslam HashimPas encore d'évaluation
- MIT2 57S12 Lec Notes 2004 PDFDocument177 pagesMIT2 57S12 Lec Notes 2004 PDFGerman ToledoPas encore d'évaluation
- PHY 102 - Wk2SlidesDocument11 pagesPHY 102 - Wk2Slidesrajiaishat69Pas encore d'évaluation
- V 4 Chap 1Document16 pagesV 4 Chap 1gurveer sainiPas encore d'évaluation
- Electrical BasicSDocument77 pagesElectrical BasicSsenthilanviewPas encore d'évaluation
- Tarea 1Document12 pagesTarea 1Fred RVPas encore d'évaluation
- Quantum AnsDocument12 pagesQuantum AnslemathaPas encore d'évaluation
- CPC - 1 Chp. Lec.Document17 pagesCPC - 1 Chp. Lec.Latu BharaliPas encore d'évaluation
- DR TMMP (Quantum)Document50 pagesDR TMMP (Quantum)Tmmp SmilePas encore d'évaluation
- TD1-EX Part 1 Corrections-V2Document9 pagesTD1-EX Part 1 Corrections-V2Эмиль БабашовPas encore d'évaluation
- II. Coulomb's Law - Worked ExamplesDocument26 pagesII. Coulomb's Law - Worked ExamplesToluPas encore d'évaluation
- 2015 JC2 MYA H2 SectionBC SolutionsDocument14 pages2015 JC2 MYA H2 SectionBC SolutionszhangPas encore d'évaluation
- Chapter 1 - 240Document13 pagesChapter 1 - 240Nurul AinPas encore d'évaluation
- Chapter 2 Waves and Oscillation (Compatibility Mode) PDFDocument77 pagesChapter 2 Waves and Oscillation (Compatibility Mode) PDFVaibhavPas encore d'évaluation
- Physics Equations and Definitions SheetDocument6 pagesPhysics Equations and Definitions SheetOkkar AungPas encore d'évaluation
- Studymate Solutions To CBSE Board Examination 2015-2016: Series: ONS/1Document19 pagesStudymate Solutions To CBSE Board Examination 2015-2016: Series: ONS/1ujjwalgoelPas encore d'évaluation
- Measurement - 2019 - Lecture - Slides (For Students - Shared Folder)Document117 pagesMeasurement - 2019 - Lecture - Slides (For Students - Shared Folder)Wee Chee LimPas encore d'évaluation
- Chapter One: Basic Principles of PhysicsDocument18 pagesChapter One: Basic Principles of Physicsm46960580Pas encore d'évaluation
- Physics Notes Class 11 CHAPTER 2 UNITS AND MEASUREMENTS PDFDocument7 pagesPhysics Notes Class 11 CHAPTER 2 UNITS AND MEASUREMENTS PDFLYF WITH AK KUMAR67% (3)
- محاضره ثانيهDocument14 pagesمحاضره ثانيهsajadjudo871Pas encore d'évaluation
- Electrical CircuitsDocument56 pagesElectrical CircuitsfangyuanreverendinsanityPas encore d'évaluation
- P102 Assignment2 2111158Document8 pagesP102 Assignment2 2111158Hrishikesh MalladiPas encore d'évaluation
- Spectroscopy - Rotational Spectroscopy - Wikiversity PDFDocument36 pagesSpectroscopy - Rotational Spectroscopy - Wikiversity PDFKishore KishorePas encore d'évaluation
- Spectroscopy - Rotational Spectroscopy - WikiversityDocument36 pagesSpectroscopy - Rotational Spectroscopy - WikiversityKishore KishorePas encore d'évaluation
- 1 2-ProblemSetSolutionsDocument4 pages1 2-ProblemSetSolutionsbobnh100% (3)
- Arthur Bieser Solution Chap 2Document21 pagesArthur Bieser Solution Chap 2Sma Shamsi47% (19)
- Bansal Classes Physics Study MaterialDocument21 pagesBansal Classes Physics Study Materialjaykay33Pas encore d'évaluation
- PHY401 - Nuclear and Particle PhysicsDocument10 pagesPHY401 - Nuclear and Particle PhysicsMohamed ElsayedPas encore d'évaluation
- Easurements: Course: Diploma Subject: Applied Science Physics Unit: IDocument28 pagesEasurements: Course: Diploma Subject: Applied Science Physics Unit: Ipankaj baviskarPas encore d'évaluation
- Lecture 3 Electrical InstrumentsDocument38 pagesLecture 3 Electrical Instrumentsf2020019015Pas encore d'évaluation
- JD 624h Loader Tc62h Operator Sec WatDocument20 pagesJD 624h Loader Tc62h Operator Sec Watalbert100% (51)
- Anp 1106 Midterm 1 NotesDocument10 pagesAnp 1106 Midterm 1 NotesKristyPas encore d'évaluation
- EN 16407 HOIS PresentationDocument26 pagesEN 16407 HOIS PresentationEsin DenizPas encore d'évaluation
- Motor Cd20Document152 pagesMotor Cd20Maciej Sobczyński100% (1)
- Lab ManualDocument96 pagesLab ManualASWANI RAJAN50% (2)
- OTS60SX2 UG en-medidorRigidezDielectrica PDFDocument14 pagesOTS60SX2 UG en-medidorRigidezDielectrica PDFblancaPas encore d'évaluation
- Tech Spec For Plate Heat ExchangersDocument37 pagesTech Spec For Plate Heat ExchangersSHIVAJI CHOUDHURY50% (2)
- Compression Springs: Dimensions According To DIN 2098Document9 pagesCompression Springs: Dimensions According To DIN 2098Андрей МеренковPas encore d'évaluation
- Engineering Guide Custom Battery PacksDocument16 pagesEngineering Guide Custom Battery PacksAndrew PPas encore d'évaluation
- Victron Pylontech Up2500 Us2000 Us3000 Us2000c Us3000c Us5000 Us5000b Us5000c Pelio-L Up5000 Phantom-S Force-L1 l2Document15 pagesVictron Pylontech Up2500 Us2000 Us3000 Us2000c Us3000c Us5000 Us5000b Us5000c Pelio-L Up5000 Phantom-S Force-L1 l2Warren MorsePas encore d'évaluation
- Riello Burner Handbook PDFDocument169 pagesRiello Burner Handbook PDFfsijest50% (2)
- Dynamics of Fixed Marine Structures N. D. P. Barltrop A. J. AdamsDocument3 pagesDynamics of Fixed Marine Structures N. D. P. Barltrop A. J. AdamsEri KaPas encore d'évaluation
- Diamer Basha DamDocument6 pagesDiamer Basha DamKashif Ali Hasnain0% (1)
- Type SSM Medium Voltage 2300 - 13,800V: SoftstartersDocument5 pagesType SSM Medium Voltage 2300 - 13,800V: SoftstartersJavier AffifPas encore d'évaluation
- Shanghai Greenport MasterplanDocument146 pagesShanghai Greenport MasterplanApriadi Budi RaharjaPas encore d'évaluation
- Autopage C3-RS665 PDFDocument34 pagesAutopage C3-RS665 PDFRafael Lizano RodriguezPas encore d'évaluation
- Maintenance and Safety of Stationary Lead Acid BatteriesDocument20 pagesMaintenance and Safety of Stationary Lead Acid BatteriessaikatkhanPas encore d'évaluation
- MSZ-FH09-15NA Operation JG79A806H02 03-14Document44 pagesMSZ-FH09-15NA Operation JG79A806H02 03-14JordanPas encore d'évaluation
- Ford Egr ValvesDocument4 pagesFord Egr ValvesTravis SincombPas encore d'évaluation
- Asme Boiler & Pressure Section ViiiDocument22 pagesAsme Boiler & Pressure Section ViiiHesham ismail SeddikPas encore d'évaluation
- CV6312 S1 OverviewDocument6 pagesCV6312 S1 Overviewlim kang haiPas encore d'évaluation
- Fab 03 Non - Destructive TestingDocument25 pagesFab 03 Non - Destructive TestingRaghu vamshiPas encore d'évaluation
- Abb LV139Document20 pagesAbb LV139Ahmad Shaikh NooruddinPas encore d'évaluation
- Percent Yield Definition and ExampleDocument3 pagesPercent Yield Definition and Exampleqwertydude123Pas encore d'évaluation
- Bucyrus HD495Document8 pagesBucyrus HD495Ivan100% (1)
- Exploring Science Edition © Pearson Education Limited 2008Document2 pagesExploring Science Edition © Pearson Education Limited 2008Victor Barber SanchisPas encore d'évaluation
- C848 88 (2016)Document7 pagesC848 88 (2016)werrtePas encore d'évaluation
- Design of High Heat Release Slinger Combustor With Rapid Acceleration RequirementDocument18 pagesDesign of High Heat Release Slinger Combustor With Rapid Acceleration RequirementFernando TaleroPas encore d'évaluation
- Propane Workshop PPTDocument71 pagesPropane Workshop PPTch.kashifpervez100% (1)
- Accenture 5G Municipalities Become Smart CitiesDocument20 pagesAccenture 5G Municipalities Become Smart CitiesEkastrielPas encore d'évaluation
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyD'EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyPas encore d'évaluation
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceD'EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceÉvaluation : 4 sur 5 étoiles4/5 (51)
- A Brief History of Time: From the Big Bang to Black HolesD'EverandA Brief History of Time: From the Big Bang to Black HolesÉvaluation : 4 sur 5 étoiles4/5 (2193)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldD'EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldÉvaluation : 3.5 sur 5 étoiles3.5/5 (64)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseD'EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseÉvaluation : 3.5 sur 5 étoiles3.5/5 (69)
- Bedeviled: A Shadow History of Demons in ScienceD'EverandBedeviled: A Shadow History of Demons in ScienceÉvaluation : 5 sur 5 étoiles5/5 (5)
- Quantum Physics: What Everyone Needs to KnowD'EverandQuantum Physics: What Everyone Needs to KnowÉvaluation : 4.5 sur 5 étoiles4.5/5 (49)
- AP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticeD'EverandAP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticePas encore d'évaluation
- Summary and Interpretation of Reality TransurfingD'EverandSummary and Interpretation of Reality TransurfingÉvaluation : 5 sur 5 étoiles5/5 (5)
- Lost in Math: How Beauty Leads Physics AstrayD'EverandLost in Math: How Beauty Leads Physics AstrayÉvaluation : 4.5 sur 5 étoiles4.5/5 (125)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterD'EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterÉvaluation : 4.5 sur 5 étoiles4.5/5 (410)
- Packing for Mars: The Curious Science of Life in the VoidD'EverandPacking for Mars: The Curious Science of Life in the VoidÉvaluation : 4 sur 5 étoiles4/5 (1396)
- The End of Everything: (Astrophysically Speaking)D'EverandThe End of Everything: (Astrophysically Speaking)Évaluation : 4.5 sur 5 étoiles4.5/5 (157)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeD'EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifePas encore d'évaluation
- The Beginning of Infinity: Explanations That Transform the WorldD'EverandThe Beginning of Infinity: Explanations That Transform the WorldÉvaluation : 5 sur 5 étoiles5/5 (60)
- Vibration and Frequency: How to Get What You Want in LifeD'EverandVibration and Frequency: How to Get What You Want in LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (13)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessD'EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessÉvaluation : 4 sur 5 étoiles4/5 (6)
- What If?: Serious Scientific Answers to Absurd Hypothetical QuestionsD'EverandWhat If?: Serious Scientific Answers to Absurd Hypothetical QuestionsÉvaluation : 5 sur 5 étoiles5/5 (5)
- A Natural History of Color: The Science Behind What We See and How We See itD'EverandA Natural History of Color: The Science Behind What We See and How We See itÉvaluation : 4 sur 5 étoiles4/5 (13)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldD'EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldÉvaluation : 4.5 sur 5 étoiles4.5/5 (54)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessD'EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessÉvaluation : 4.5 sur 5 étoiles4.5/5 (57)