Académique Documents
Professionnel Documents
Culture Documents
(Exxon) - Exxon PDC Vol I
Transféré par
ChakerZagrouba100%(2)100% ont trouvé ce document utile (2 votes)
302 vues281 pagesExxon
Titre original
[Exxon] - Exxon Pdc Vol i
Copyright
© © All Rights Reserved
Formats disponibles
PDF ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentExxon
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF ou lisez en ligne sur Scribd
100%(2)100% ont trouvé ce document utile (2 votes)
302 vues281 pages(Exxon) - Exxon PDC Vol I
Transféré par
ChakerZagroubaExxon
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 281
PROCESS DESIGN
COURSE
VOLUME I
PRESENTATION
ZS
EXXON ENGINEERING
PROCESS DESIGN
COURSE
VOLUMEI
PRESENTATION
EXXON ENGINEERING
is
1.
2.
3.
DATA St
Given:
Given:
Given:
05309101. Pom
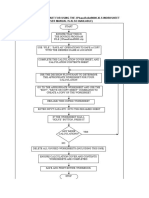
TRAINING PERFORMANCE OBJECTIVES
EXXON ENGINEERING PROCESS DESIGN COURSE
SOURCES
+ A.gas stream of three defined components
+ The mol. fraction of each component
+ Temperature and pressure of gas stream
Determine the following properties of the stream:
Molecular weight
Weight and mola] average boiling points
Pseudocritical temperature
Pseudocritical pressure
Absolute viscosity
Compressibility factor
Density in pounds/ft3 at conditions.
eteaoce
+ A liquid stream of three defined components
* Volume fraction of each component
+ Temperature and pressure of liquid stream
Determine the following properties of the stream:
Molecular weight
Mean, molal, volume, and weight average boiling points
Characterization factor
True critical temperature
True critical pressure
Density in pounds/ft3 at 60°F
Density in *API at 60°F
eroance
* A liquid stream of three defined components
* The weight fraction of each component.
* Viscosity of each component at 100°F and 210°F
Determine:
a - The viscosity of the mixture at 100°F and 210°F via the Blending
Index procedure.
b - The viscosity of the mixture at 150°F using
viscosity-temperature charts.
c- The difference in enthalpy between the stream as a liquid at
100°F and 30 atmospheres and the stream as a vapor at 600°F and
1 atmosphere.
d- The latent heat of vaporization at 600°F.
4.
5. Given
6. Given:
. Given:
05309101.POM
+ A gas stream of three defined components
+ The mol fraction of each component
+ Temperature and pressure of the gas stream
Flow rate of stream in MegaSCF/SD
Determine:
a
b
- The brake horsepower required to compress the stream
adiabatically to twice the given pressure
- Temperature of the compressed stream
+A stream of three defined components
+ The mol fraction of each component
+ Pressure of the stream
Estimate the following properties of the stream using Vapor
Pressures:
a
b
* A stream of three defined components
- Bubble point temperature
- Dew point temperature
The mol fraction of each component
The equilibrium instants for each component
+ Temperature and pressure of the stream
Determine the following properties of the stream:
a
b
- Mole percent liquid at given conditions
= Liquid and vapor phase compositions
ASTM Distillation of pipestil] gas oil cut
API gravity
Determine:
a
- Specific heat of stream at 200°F, 300°F, and 400°F
(correct for Ky)
- Latent heat of vaporization of stream at 200°F, 300°F, and 400°F
- 15/5 Distillation Curve
- The "names" of an arbitrary number of pseudocomponents that will
define the stream
Cx
DISTILLAT! (OLOGY AND TOWER DESIGN
1. Given:
+ Temperature, pressure, and rate of C3-430°F refinery stream
+ Composition of the stream through C5 material
+ Product specifications for Cq in the bottoms and Cs in the
overhead
* ASTM distillation of the C5+ material
Conditions of utilities that are available
Be able to
- Determine a quickie preliminary design using Fenske-Gilliland
procedure
2. Be able to define the following tray design terms:
a - Blowing g - Single Pass Tray m - Downcomer Clearance
b - Dumping h - Bubble Area n - Two Pass Tray
¢ - Jet Flooding i - Hole Area o - Antijump Baffle
d - Liquid Gradient dj - Perforated Area P - Downcomer Seal
e - Weeping k - Waste Area @ - Downcomer Filling
f - Ultimate Capacity 1
Free Area
3. Be able to classify the following types of trays:
- Bubble Cap - det
~ Sieve - Valve
In order of increasing:
a - Capacity c - Fractionation Efficiency
b - Cost per Unit Area d - Flexibility
4. Given:
~ A liquid stream’ s--
+ Flow rate (pounds/hour) and composition
+ Surface tension
* Viscosity
+ Temperature
- A vapor stream’s -~
* Flow rate (pounds/hour) and composition
+ Viscosity
+ Temperature
+ Pressure
- Required flexibility of the service
Be able to design a sieve tray by hand for this service
IIL. HEAT TRANSFER FUNDAMENTALS
1. Given:
+ Shell and tube exchangers for concurrent, countercurrent, and
mixed flow
Be able to:
a - Identify each
b - Explain the differences among them
2. Given:
+ Temperature in and out for both sides of S & T exchanger
+ Number of tube and shell side passes
«No change in phase for either fluids
Be able to:
a - Calculate the ATLM
b - Calculate Fn
¢ - State the temperature approach
d - State the temperature cross
e - Explain why one should not design an exchanger that results in
outlet temperatures giving Fy <0.8
3. Given:
* Temperatures in and out for both sides of a water condenser
* 1/Q curve for the HC being condensed
Be able to:
- Calculate the effective temperature driving force (Ate)
4. Given:
« Temperatures in and out for both sides of S & T water cooler
* Rate of HC on the shell side
+ API gravity and MABP of the HC
+ Exchanger geometry (tube sizes, area, etc.)
Be able to:
Calculate the individual film coefficients and the overall
coefficient
- Calculate the total resistance due to fouling
- Calculate tube wall temperature on the water side
Specify which, if either, film coefficient is controlling
- Estimate the effect on the overall coefficient if the rate of HC
is doubled
eane
05309101.POM
5.
\T_EXCHANGER DESIGN CONSIDERATIONS
Given:
Be ab]
Be abl
and wa‘
Given:
Given:
(05309101. POM
* An exchanger design with less than the maximum allowable AP on
both sides
Be able to:
a - Explain why low pressure drop is undesirable
b - List steps that could be taken to increase AP
fe to list two advantages for double-pipe exchangers.
e to list the factors that enter into the choice between air-cooled
ter-cooled exchangers.
+ The total duty of a $ & T exchanger (Btu/h)
+ The characteristics at the two fluids in the exchanger
« The temperatures and pressures of both fluids
+ Type of service
* Tube side and shel] side fouling factors
Be able to:
a - Determine the minimum number of shells and explain reason for
this minimum
b - Determine which fluid should be on the tube side
c - Specify whether a fixed tube sheet, U-tube, or pull-through
floating head design should be used
d - Specify the tube layout
- Specify tube type (bare or low fin)
© The task of designing aS & T exchanger with specified shell side
pressure drop
Be able to:
Specify minimum and maximun baffle spacing
Explain reason for maximum limitation
¢ - Specify the most desirable baffle orientation if the exchanger
has condensing vapor on shell side
List advantages and disadvantages for high pressure drop through
the shell side
a
6. Given:
Shell side hydrocarbon rate (no change of phase)
Shel] side fluid properties
Temperatures and pressures
Allowable pressure drop
Water side inlet temperature
Water side allowable AP
Be able to:
- Estimate the area of the exchanger
5309101 .POM
e Vv. FURNACES
1. Given
2. Given
+ The design of a furnace from a furnace supplier
+ The process information and requirements for the furnace
+ Type of fuel and percent excess air
Be able to:
a - Determine radiant heat flux
b - Calculate heat transferred in the radiant section
¢ - Calculate bridgewall temperature
d - Calculate heat transferred in the convection section
e - Calculate the stack temperature
f - Calculate maximum tube metal temperature in radiant section
g - Calculate fin-tip temperature in the convection section
h - Calculate film temperatures
‘* Fuel gas type and rate to a furnace
« Stack temperature
+ Flue gas analysis
+ Anbient air temperature
Be able to:
a - Calculate the percent excess air
b - Calculate the furnace efficiency
3. The following information is given for a 100 M Btu/hour (heat to oi1)
furnace:
05309101. POM
* 75% of heat load is obtained with 15° API fuel oi]
+ 25% of heat load is obtained with 1000 Btu/scf fuel gas
O11 burners operate at 25% excess air
* Gas burners operate at 15% excess air
* 0.6 pounds of atomizing stean (150 psig saturated) is required per
pound of fuel oi] fired
* Stack temperature is 60°F
Determine:
a - Furnace efficiency
b - Pounds per hour of flue gas
VL. _FLUID FLOW, PUMPS, COMPRESSORS
1. Given
+ A piping system handling a liquid hydrocarbon stream
+ Stream flowrate and properties
+ Length and size of pipe
«+ Number and type of fittings
Be able to:
a - Estimate equivalent length of pipe
b - Estimate pressure drop
2. Be able to define the term "cavitation"
3. Given the following data for each of four pumping services:
Rate
Specific gravity and viscosity
NPSH available to the pumps
Solids content
Differential head required
Viscosity
Be able to select from the following list the type of pump to be
used in each service
a - Centrifugal
b - Rotary screw
¢ - Reciprocating
4. Given the following data for a pumping service:
Horizontal centrifugal pump with motor driver
Suction drum is an overhead distillate drum
Elevation of drum and pump centerline
Size, length, and number of fittings in the suction line
Rate, specific gravity and viscosity of the liquid being pumped
Be able to determine the NPSH available to the pump
05309101 .POM
@ 5. Given the
same data as TPO Number 4 plus
* Pump characteristic, efficiency, and NPSH curves
+ Pump impeller diameter
«© Flow control valve pressure drop
« Piping system downstream of pump
« Downstream controlled pressure
Be able to determine
fo
b-
c-
Brake horsepower required by pump
System resistance curve
Diameter to which impeller should be trimmed to allow for a
control valve pressure drop equal to 20% of the line friction
pressure drop
Be able to list process information required to specify pump and
spare
6. Given, for a centrifugal compressor:
« Composition and rate of gas stream feeding the compressor
+ Discharge pressure
» Suction pressure and temperature
Be able to estimate:
ance
05309101.PON
Temperature rise exponent
Discharge temperature
Polytropic compression exponent
Brake horsepower
-10-
WIL. PROCESS CONTROL
1. For Item under Fluid Flow Section, be able to determine the control valve
size.
2. Given
3. Given
4. Given
5. Be abl
05309101. Pom
* A list of services such as feed from tankage to tower,
fractionator reflux, etc.
Be able to:
a - List the four fundamental types of control
b - Select the type or types of control which would be most
frequently used for each service
¢ - Select the control valve action on instrument air failure for
each contro] valve
‘An incomplete process design flowplow for a multipass process
furnace
Properties and operating conditions of process stream
Fuel gas pressure
Pilot gas source is natural gas (pipeline)
Coil outlet temperature control is to be used
Furnace pressure drop
Be able to prepare sketch showing typical instrumentation for
controlling furnace operation
* Compressor suction knockout drum with automatic liquid drawoff to
blowdown drum
Be able to:
~ Sketch drum and compressor showing all instrumentation needed for
the drum, including alarms and compressor shutdown
je to:
a - Define the term rangeability
b - State the maximum rangeability for a control valve (double
seated, globe) and for an orifice meter.
© - Specify when a rotameter, turbine meter and venturi are used
’
e
-u-
DRUM.
Given:
05309101.POM
* Existing P&ID for a tower that is to be expanded
* Detailed Heat and Material Balance for the new conditions
Be able to:
a - Calculate the maximum velocity permitted in the vapor-liquid
distillate separator drum
- Determine the volume required in the drum for the liquid
- Determine the length and diameter of the drum
- Prepare a detailed process design drawing of the drum suitable
for submission to a vendor
aoc
-12-
IX. GENERAL PROCESS DESIGN CONSIDERATIONS
1. Be able to define the following terms:
a - Operating temperature
b - Maximum fluid temperature
c - Design temperature
d - Operating pressure
e - Design pressure
f - Short time design basis
2. Be able to explain why it is very important to minimize the difference
betwee
operat:
3. Given
4. Given
Given
05309102. Pom
n operating temperature and design temperature for carbon steel
‘ing above 650°F
* The task of designing a depropanizer, a butane splitter and a
gasoline rerun tower
Be able to
a - List the factors that should be considered in specifying the
design pressures and the minimum design temperatures for the
vessels.
b - Explain how the vendor will know what internal
pressure the bottom of the towers will have to withstand
+ Temperatures and pressures in and out of two exchangers in series
in discharge from pump
+ Pump curves
+ Normal and maximum operating conditions upstream of the pump
* Exchangers have bypasses
Be able to
a - Specify the design temperatures for each exchanger
b - Specify the design pressure for each exchanger
+ A carbon steel piping system with uninsulated flanges
* Design temperature
+ Design pressure
Be able to
a - Determine the flange rating for the pipe
b - Calculate the maximum design pressure for the system using the
selected flanges
c - Calculate the maximum pressure to which the system may be
subjected at the given temperature
-B-
e 6. In addition to the onsite process design considerations, be able to list
at least three other systems that need to be considered in the complete
design of a project.
In the preparation of a plot plan layout, be able to specify the desirable
location for:
a - Furnaces with respect to other equipment
b - Cooling towers with respect to the unit
c - Air fin exchangers with respect to fractionating towers and
furnaces
d - Pumps with respect to fractionating towers and furnaces
05309101 POM
-u-
X._ SAFETY. CONSIDERATIONS
1, Be able to list the references used in the design of emergency release
systems.
2.
Be able to
Sorusse-heance
3. Given
© The process flow sheet for a unit
Explain the meaning of the following
Autoignition Temperature
Contingency
Emergency
Explosive Limits
Flash Point
- High Flash Stocks
- Light Ends
Low Flash Stocks
Pyrophoric Material
Single Risk
Toxic Material
Fire Zone
Pressure Relief Valve
Spacing standards
Be able to
N&xE
MeABP, °F (of Fraction) ——»
CRITICAL PROPERTIES
+ Our Primary Use
— Compressibility of Gases
— Correlations for Equilibrium Constants
+ True-Critical - for Pure Compounds.
+ Pseudo-Critical - for Mixtures
PSEUDO-CRITICAL TEMPERATURE (T,,)
Light Hydrocarbons Only
400
¢ 350
Ls Mixtures
3 Base Curve -
= Mol ABP Vs Tpc
& 300 Gr. Curve -
WABP Vs Tyo
Maxwell p. 70
Blue Book p. 6.16
0 25 50 75 100
Average Bolling Point °F ——»
PSEUDO-CRITICAL TEMPERATURE (Tec)
Petroleum Fractions
APL 10 ar
Tec V8 Mol. ABP
Maxwoll p. 72
Blue Book p. 6.30
Critical
Temp.
Average Bolling Point
For Narrow Bolling Fret. Vol. ABP = Mol. ABP
PSEUDO-CRITICAL PRESSURE (Ppc)
Light Hydrocarbons - Normal Paraffin Mixtures
Poo Maxwoll p. 71
Blue Book p. 6.19
Molecular Weight
PSEUDO-CRITICAL PRESSURE (Ppc)
Petroleum Fractions
‘API
Pre SK fe
ese na
MABP, °F
GAS DENSITY
PV =npRT = uRT for 1 Mol
Volume of 1 Mol = HRT
‘Weight of 1 Mol = M
=the M
Pe ie aRT P=PSIA
P T=R
p=
PM = Ibs
HRT” te?
112
DENSITY OF LIQUIDS
+ See Section 8 of Maxwell
+ Density Calculations
Do Not Blend API.
Blend Specific Gravity, or Ibs/t?
THERMAL EXPANSION OF LIQUIDS
1000
Mol ABP
Vol @T
Vol. @ 60°
‘Maxwell Section 8
Blue Book Section 8
1.0,
°F
THERMAL PROPERTIES
‘Section 7 of Maxwell and Blue Book
+ Specific Heat
+ Latent Heat
+ Enthalpy
145
VISCOSITY
‘Section 9 of Maxwell and Blue Book
+ Pure Compounds
+ Pet. Fractions
+ Visc. - Temp. Charts
+ Vise. Conversions
+ Vise. Blending
+ VBI
VISCOSITY OF LIQUID MIXTURES
Page 173 Maxwell
Page 9.61 Blue Book
Vise. @
Comp, Vol. Fr, 50°F. CS vel
A 0s 15.0 36.4
B 03 31.5 31.8
c 22 131.0 247
1.0 IVF x VBI = 32.68
Vol. Average
Viscosity,CS 26.7
447
VISCOSITY OF GAS MIXTURES
+ Viscosity Charts - Section 9 of Maxwell & Blue Book
+ Calculations:
Za NeZs¥Mi + NaZ2Vil +.
NVM + NoMa +...
Z = Visc. of Mix. at Atmos. Press.
Ny, No = Mol. Fr. of indiv. Components
21s Zz = Vise. of Indiv. Components
M,, Mz = Mol. Wt of Components
* Correct for Pressure (Maxwell Page 177, Blue Book 9.87)
118
VAPOR-LIQUID EQUILIBRIUM
+ Whenever a Liquid and Vapor Exist in Equilibrium, It Is.
Possible to Determine the Composition of Each of the
Phases. V-L-E Relationships Are the Basis for Bubble
Point, Dew Point and Flash Calculations.
+ For Any Specific Component at V-L Equilibrium, its
Concentration in the Vapor Phase Divided by its
Concentration in the Liquid Phase Is Known as its
Equilibrium Constant, or K.
IDEAL SYSTEM — EQUILIBRIUM CONSTANTS
Raoult's Law: p, = P,X,
Dalton's Law: 9, =nY,
PX, =2Y,
‘NeoPM eK,
xX
K, = 4(T,P)
= Partial Pressure of i in Vapor
P, = Vapor Pressure of i @ Temp.
‘otal System Pressure
Mol. Fret. of i in Liquid
Y, = Mol. Fret. of iin Vapor
K = Equilibrium Constant
Good up to About 4 Atmospheres
for Systems Comprised of Similar
Compounds
NON-IDEAL SYSTEM
+ Activity Coetticlent Corrects for Non-Ideal Liquid Solutions
— Neccessary In Calculations involving Polar Compounds;
@.g. Sour Water Strippers
+ Equilibrium Constants for Non-Ideal Vapor Phase Are Obtained
from a Varlety of Computerized Correlations, e.g.
— Benedict Webb Rubin
— Chao Seader
— Redlich Kwong
FUGACITY FUNCTIONS
+ Fugacity Function May be Considered a Corrected Vapor Pressure.
+ Honee K, = (Fd . Fugacity Function
Total Pressure
+ When K, is Determined by a Rigorous Methods such as Chao -
‘Seader, then the product of K, and x may be Called a “Fugacity
Function".
+ Maxwell Chapter 5 Gives Graphs for Pure Component Fugacity
Functions. These Are Only Approximate Values (Non Composition-
Dependent). They Cannot be Used for Definitive Designs.
BUBBLE POINT CALCULATION USING GAS LAWS
‘System Pressure = 120 PSIA = 8.17 ATM =x
(See Maxwell Chapter 4)
Vapor Pressure = P, K= Pin
10E 18 14 BOE PE ur
Cs 18.30 19.50 20.70 2.240 2300 «(2530
ey 7.46 8.00 851 0.913 0.979 1.040
ny 5.46 5.90 6.36 0.668 0.722 0.779
Y= Kix
S_ 1e er
Cs 0.0133 0.0298 0.0318 0.0336
Wy 0.9516 0.8688 0.9316 0.9897
nC, 9.0351 9.0234 9.0283 9.0273
1.0000, 0.9220 0.9887, 1.0506
Linear interpolation Gives Bubble Point = 126°F
FUGB = 135.0°F
CHAO = 137.5°F
RKAZ = 135.8°F
} maorou aod
DEW POINT CALCULATION USING IDEAL GAS LAWS
‘System Pressure = 132 PSIA = 8.982 ATM = x
Vapor Pressure = P,
(SPE SSF 160
Cy 9.71 10.40 11.00
nCe 7.270 «(7.75 8.30
ICs 310 3.34 3.60
a
IC, 0.4590
nC, 0.8393,
ICs 9.0017
1.0000
K=Pi/n
ASYE 185°F
1.061 1.160
0.809 «(0.863
0.345 = 0.372
X= WK;
IPE 18 EOF
0.425 0.396 0.376
0.667 0.625 0.584
9.005 9.005 9.004
1.007 1.028 0.984
Linear interpolation Gives Bubble Point = 157°F
FUGB = 157.5°F
CHAOs 159.0°F } Rigorous Methods
KAZ = 157.2°F
16°F
1.220
0.924
EQUATIONS FOR FLASH VAPORIZATION
F = Mols of Liquid Feed
Vs Mols of Vapor Product
L = Mols of Liquid Product
2, = Mol Fraction of i in Feed
y= Mol Fraction Vapor
X= Mol Fraction of i in Liquid
+ Material Balance: Fz, = Vy, + La %;
+For 1 Mol of Feed: z, = Vy, + Lx,
+ By Equilibrium: =x = h
7
EQUATIONS FOR FLASH VAPORIZATION
(Continued)
2, = Vy. + Ly (Previous Silde)
Divide by vy,:
Zt ate ety Le
wi wy Ke
ue wi(t+ ae)
Vie 2
te
VK
FLASH VAPORIZATION CALCULATION
tan lang
ve > vie —_a_
a1 it+t
VK
STEP 1. Assume a Value of V.
Then b= 1-V, and = 1:¥.
STEP 2. Calculate Vy, for Each Component
STEP 3, Sum to get V. iterate until Vessumed = Vestcutated
Convergence Can be Difficult. Agreement of Vessumed With Vesicutated
Should be within 0.1% to Assure a True Solution.
FLASH CALCULATION EXAMPLE
P=200PSIA T= 180°F
a
compe kn) 1+LvkK i+LWK
c2 0.08 4.1710 1.3311 0.0601
c3 0.22 1.8650 1.7405 0.1264
NC4 0.53 0.8233 2.6774 0.1980
NCS 017 0.3760 4.6729 9.0364
1.00 0.4209
0.0964
0.4289 OKI
(1) K Values Are From Rigorous Caloulation, Method RKJ2.
1.28
DISTILLATION CONVERSIONS
Definitions
$155 — 15 Trays/5:1 Reflux
— Approx. of Actual Bolling Point of Components.
+ TBP — Ambiguous Connotation; Preferable Term Is 15/5
+ Stem Correction © — When Thermometers Are Used All the Stem Is
Not Immersed. Need to Correct for This.
+ ASTM Distillations — Handout 1.01
+ GC Distillation — Gas Chromatography ("GC's") has Largely Replaced
the 15/5. For GC's, the IBP Is Taken as 0.5 Vol.%
Point; the FBP Is Defined as the 99.5 Vol.% Point.
1.29
CONVERSION OF ASTM DISTILLATION
TO A 15/5 DISTILLATION
+ Establish Type of Data Reported
— Stem Corrected?
— Procedure Name?
* Plot Data to Arrive at Smooth Curve and Reject Sports
* Stom Correct the Smooth Curve, if Not Done by Laboratory
+ Use Charts in Handout 1.01, Pages 2-3, to Convert This Stem
Corrected Curve to 15/5
DISTILLATION CONVERSION EXAMPLE
‘Stem Corrected ASTM D-86 (Naphtha)
ASTM
TEMP
Lv% °F
5 347
10 356
50 374
90 401
95 410
FBP 425
50% - 10% = 374 - 356 = 18°F
90% - 50% = 401 - 374 = 27°F
50%-10% 90%-50%
increment Increment
FO ro
-33
20
ant g
16
7
@ Addendum 1.01, Page 4
@ Addendum 1.01, Page 5
PSEUDO - COMPONENTS
: Ita Component Analysis Is not Available, a Composition In Terms
of "Pseudo" Components Can be Obtained from the Distillation
Curve and Gravity.
+ How Do We Represent the Components?
350 A 45.0
350K 11.6
350 8 0.80
+ How Do We Get Pseudo-Components?
te
15/5
314
326
374
47
PSEUDO-COMPONENT BOILING POINT
+H Only an ASTM Dist. Curve is Available, Convert it to a 15/5 Distillation.
+ Divide the Curve Into Rectangles, Each Rectangle Representing
Pesude Component.
+ Tope of the Rectangle are the Vol. Avg. BP's: 50% of each “Component”
Bolla Lower than the Top at Rectangle, 80% Bolle Higher.
010 20-30 40 50 60 70 80 9% 100
Vol. % D +L
12
PSEUDO COMPONENT GRAVITY
+Have Tyagp and Vol% of Each Fraction
+Have Gravity of Total
+Calc. Char. Factor, K, of Total Feed
k =_/MeABP, “°R
2 ‘SP. GR.
{for Narrow Bolling Fractions, Can Assume Tyapp = Tweasp)
+ Use Same Equation, Solved for S.G., to Calculate S.G. of
Each Pseudocomponent.
Page 1
‘Addendum 1.01
amsseid Bunerade wy 5
‘1gerj21un fyje10488 eed “10/8u3 wnnven wu OT parie> K\uoWNOD “powIaU UNnDEA DazIpIePUELS A00d “parenbNUY
paiayset wnnaen iayjo ay) “sorsagse yuim pajeinsur avo “speay 10den
wo10}n1p om, 40 ouo uItM pouiquios (wulgz "4990 Tous 10 “wu OOT "¥290
aden ou1 “pow
2M v) panturad su
uoiyy "wowainseau
24, “paUTIsap Aj100¢ pue pat
1uarenindo anrs v0
osn sajdnosowiayy “Seimeroduiey oni jou oie s8uipess s2uay ‘e2eds 10den ut
so
WASY 1819 0} Ao Kidde y>tus “SIeNBVeW ALII0G-9p1m Pu LnIPOW 14 “W'L°S"y oun Aa pelonb sonten Jo ue! 2)
—— —
“2a r
vonvoyrsads on] x2 | auon | 0st |yatty | 1029) “oy | 090-06 | enpsoy pue| 24
— — — ~—}— | sayeiinsia
5H “Dad soot
ost ~ Ot w= =| auoN wor | 2029] “Oy> 0g yori eORLTO
: i ~ | T ot )ee-aebuew |
sags gas) sods on ---| 2 | auon} ose | vuny | 2845 1°45 | goy-o¢ bux esn)| 851-0
on oN on | on avgjosqg| NISY
ean ae a a clarz<| ooee |. ie] 2980
ors 89 ora] ee cz v2] 2 | avon] set | any | zev<]ziz<| o92-0€ |acoeunsia nin] anon
z zav<|ziz<| 080 seu2s0r]
ors 89 or} 2 Ee wet—— awn] car | ony |= =}————] ‘eden “hny] 2
st zav>|2t2> | 085-0 |sarennsic wor] SY]
eereanesliemicries 5 5 “3ad5 | paca, ) AABOH 20 abueyl 298-4
ors 09 os] oz |x v2] st | aon] ser | un | zer>] 25] ogs-oe | “NoHo ciel) | 298-0
HO dad dal] ain
do ad siog por
eAitjiqovoedoy “1989 4 Pesn
sige gerd “PS
SOOHLAM NOLLVTTLSIO WASY LN3YUND JO SAUNLVI4 TWdlONINd
STEM-CORRECTED THERMOMETER READINGS FOR ASTM D-86 NAPHTHA DISTILLATION
TREADING CORRECTED FOR THERMOMETRIC ERROR;
| eer PE SEH
Lae Se ae a
tae ie at a
7 le ie oe ee ec l oe oo)
aie |e gp # #lel|e-e se
ee) es 8 b BR |e oR
|e ile)e 2 oe gleleg g BR
(om fae | Be Be) | se eb
|
| mw | | mm «© mlm | m om mw om
| 2 |e |B # 8 #1 |B R BB
me | | i m is @ |i | im bm BR
Ble iB BR BB S|a'S BR B
2 Ble 8 8 12/8 8 8 B
=» lel me os mw mime | mom
a l|g|# 8 @ BIR |B # B B
BUS 8 & R BR BS # & B
[ela ) eg @ gS Be i
Ss |e|e = 2 BIS 2 @ B
ee eee ee
Ze es ee Ble eg Be B
gooey eR FS Blk eg oR eR
eee se ee ae eee
Be |e B # BRIS # OB
vw fel wow woe lw mw
Oise 8 of Blaise & oe
ee) ge pg Big gs # 8 &
Biggie = B Bie |e B BF
e|k(S 2 B BI|B|2 & 2 8
40, boa | az a3 a 415 ae ar ae ay 40
sia; 8 # giglg 2 g @
| 2 & |e & & B&B | a mw
ieee ce ee se Sean Boe oy fo
|S '8)8 8 # S'S) Ss 2 8 8
|
450 | be 465 466 4 as | ae | a0 42 a3 am
op te ae a) te eo) ol ce |
|# |@/@ £€ & 2 e#\|se #8 8 &
1 ago | a6 | a7 8 500, so | 502 | 503. soa 505, 506
je ksh) Rehr
mee ee eee eee
ep eee a ie enol ie aoe Se
woe |e 2 # Ble 8 # BB
Bp) B Bo ele] gos Bt
BPE 8 2 #/8)/8 # R R
wo [am | me gs oe ais wom
|e fijaile 2 # aig og 2 8
elf] 8 8 8 Bie Ao
2/8 & & & e|f 8 @ @
Bg /fi 8 8 8 2/3 82 ¢ 8
wo oa foe taste eet ie alee ase esse ar
Page 2
‘Addendum 1.01
Page 3
Addendum 1.01
STEM-CORRECTED THERMOMETER READINGS FOR ASTM GAS OIL DISTILLATIONS, D-86 AND 0-158
saad READING CORRECTED FOR THERMOMETRIC RIOR *F
eae (Ghclunte 19 ee OF seed
a a ee a a
‘ay ae | ae
BR |e es BR BR |e | eo om Re
mf | Me | | mM Be
ms (om mmm | we
2) S BoM Bo ne
mo oom | mo moms m | in _ mm
mom | BB mm | mt | BOR
| Bo jm lm ROR | m a
oo | om | ome aes | a | oma
Mf | | bh Oe
|B ie Bo me me | mw |e RR
| BB Re BR OS | we |e eM Ge
ee ee ee
wo | am | eae awe awe | ww
ee a”
eo | BG Be eat
ee ee a ee
Sf ees | mw | me
| mo | we me wm om mw | momma
eS | 2G @ & S12 |e 8 2
eee eR Be |e
Be Sm | me | em ee
op |e me BB | |
\
2 le e w mw eo mw wow
& i$ 8 ££ 8 8 oe Bo RM
= = © @ © | me me we
e 2 2 @€ S & & & so
so ee
Fw | oe fe ge gts) ge gee
Ble) h 2 B RR fF 2 es
Bo] | BOBS | Se eh eo
w]e | | Se eee
Ee
i |
ee ey eh ee pe ceateectels
er (8 @ 2 8
oe | oe | Ss ke Se
| Ss | se te et
| | ee mt os
wm | | a oe a we ww
| & | we | te ge Se oe
& | & | ee a te et
ee a
| me | at | te po re)
wo lim l im mos am wm wm
|) Be ee & 8 &
| i | fe ee re ee
| | ome mo om hs
| % | oR | mom te moO BO
me) om lm om om om | m | me momo
| BB MM) me | es
m | ie | emote os | me | oe Mt
m |e | om Sm | me | me mom me
BB] RR Room | om | mm om
1 Ling mm oe | m | mm mis
oF To Be Added To Stem-Comrected ASTM Tenoerature, °F
Page 4
Addendum 1.01
DISTILLATION CONVERSION,
CORRECTED ASTM
086 OR D158 70 15/5
-20
-10
50 00 SO te 200 eee 250 zea 900) 350 400
‘Ster-Corrected ASTM 50% Minus 10% Tenveratures, °F
20 20 30 20 50 60 70
Stem-Corrected ASTM 50% Minus 107. Tenperatures, *F
°F To Be Added To Sten-Corrected ASTM Temperature, °F
EES
TEE
CORRECTED ASTM
086 OR 0158 TO 15/5
DISTILLATION CONVERSION,
4.00
150 200
Stem-Corrected ASTM
250 300 350
100
Stem-Correctes ASTM 90% Minus 507s Temperatures, °F
Page 5
‘Addendum 1.01
Page 6
‘Addendum 1.01
4+ "404 Sri os o9tt0 MASH
ooe___ ow ove ome _—oze_—ooz__—owt’_——gat bt ot oot =o)
sommandens O9UTO WAS 8
CL Le ee ee, aC ope rere c2)
44 "Hos Sn Hoe o9TTO MASY
S/s¢.01 0919 wisy ayvanvis.
or SWSANBOW2ANT NOLL HLSI0 WAROVA
ow
02+
ocr
ove
ost
ov
ou
o9tta WIS¥ 4 300 39 01
Page 7
‘Addendum 1.01
“F To Be Added To 15/5 Vacer Tenoeraure
Page 8
Addendum 1.01
DISTILLATION CONVERSION,
15/5 To STem-consected [FH
[ASTM 086 OR D158
60
50 Ee
ao
30
20
ao fy
50 Too 150 200° 250~«*SDSCSC« SO
15/5 50% MINUS 10% Vapor Temperatures, °F
15/5 50% Minus 10% Vasor Terwertures, °F
ALIAVYDS Idv
Ss °
°Q
+
30
20
10
40 60 60 70 80 90 100
10 20 30 4
°
Jd - 3univeadWaL
ALIAVYD Id¥
Ss ° °
oO R o o
°
+
20
10
°
o
60 60 70 80 90 100
10 20 30 40
°
ad - 3uNivwadWaL
1000
800
700
600
500
8
Temperature - °F
_
8
50
20 30 40 50 60 70 8 9 95
Per Cent Over
IGeeem 2aoe4St810) + z,
e 1000
700
600
250
Temperature - °F
200
150
T
Tt {
50 {
20 30 40 5060 70 80 90
Per Cent Over
we 2345 10 987 =P
SECTION 2
DISTILLATION
GENERAL PROBLEMS
(Ref. EEDP—Section III)
Maxwell-Chapter 14
1. Design of Fractionation Column to Make Desired
Separation of Feed
A. Screening
B. Definitive Design
y
- Checking a Given Column for Desired Separation
of Feed
A. For New Service
8. For Existing Service
it We Can Handle the 1st Class, We Will Not Have
Trouble With the Second Type. 2ot
FRACTIONATION COLUMN
(Mole Flow Rates)
Condenser Ge
Map. Dist.
ve
Accumulator
OHD. Lig. Product
°
Xo
Enrichi hp
Section
.
f,—feed_|
be
Low Boling Comp.
Sipping Heme, ame.
DEFINITIONS
1. Key Components
— Major Feed Components Between Which Split
Is to be Made.
2, Minimum Reflux
— Reflux Required for Desired Separation With
an Infinite Number of Plates.
3. Total Reflux
— Reflux at Infinite Reflux Ratio. Reflux Required
for Separation With Minimum Number of Trays.
4, Minmum Cost Requirement
— Cost Optimization for Best Return on Investment.
PLATES vs. REFLUX
‘Thore is an economic balance between plates and reflux.
Tot. Cost
Oper.
Cost
10 15
0/D1(01 0) min.
Operating Reflux Ratio/Minimum Reflux Ratio |
PLATES VS, REFLUX (Continued)
* In standard Tower Systems, Economics Not
Too Sensitive. Sensitive for Complex System.
Can Use Shortcut Procedures
Can Use Simplified Plate-to-Plate Calc,
* With Utility Cost Going Up, the Optimum.
Favors More Trays and Lower Reflux.
205
BASIC STEPS IN COLUMN DESIGN
Know—
* Feed Rate & Composition
* Desired Separation—Recovery & Purity
* Battery Limit Conditions on Feed,
Products, and Utilities
206
STEPS IN COLUMN DESIGN (Continued)
1. Determine Key Components Which Represent
the Desired Separation
2. Determine Operating Conditions
3° Use Short Cut Distillaton Techniques to Define
the Extremes—Min. Stages & Min. Reflux
4° Select an Operating Reflux Ratio
5." Use Gilliland or Similar Correlation to Determine
No. of Theoretical Stages
6. Pertorm Plate-To-Plate Calc. on Computer to
Define the Tower
7. Optimize Tower Design
—Reflux
—Number of Stages
—Level of Feed Preheat
—Feed Tray Location 207
INITIALIZING COMPUTER PLATE-TO-PLATE
CALCULATIONS TO MODEL THE TOWER
* Steps 3 to 5 May Be Eliminated by Use of Information From a
Similar Operations
PROCESS—COLUMN Has a Short Cut Distillation Procedure to
Help Initialize a More Detailed Plate-To-Plate Tower Simulation,
(Uses a Generalized Fenske Method)
* Addendum 2.2 Provides More Details on Short Cut Distillation
Techniques
208
OPERATING PRESSURE
FACTORS TO CONSIDER
1, Disposition of the Overhead Product
2, Is the Overhead to be Totally Condensed?
3. Temperature of Available Cooling Medium
to Overhead Condensers.
It Cooling Water Available at 95°F is Used,
the Min. Pressure for BP Distillate Would
be That Corresponding to the Pressure at
a BP Temperature of About 110°F
(15° Approach).
209
OPERATING PRESSURE (Continued)
4. The Max. Pressure Might be Limited by
A. Tower Bottoms Temperature. A Very High
Pressure Could Result In Excessive
Bottoms Temp. (Cracking, Color)
B. Temperature of Heating Medium for Reboller
C. A Bottoms Temperature too Close to the
Critical Temperature Results in Poor
Separation—Bottoms Temperature Should
be at Least 50°F Below T,
210
OPERATING TEMPERATURE.
1. Fixed Once the Separation and Pressure are Specified.
2. Temperature Estimates.
A. BP Calculation on OHD at Accumulator Pressure Gives
Reflux Temperature for Total Condenser. Flash Calculation
lf Not a Total Condenser,
8B. OP Calculation on Tower Overhead Will Give Tower
Overhead Temperature.
C. BP Calculation on Bottoms Will Give Bottom Temperature.
0. Flash Calculation on Feed to Get Temperature & Fraction
Vaporized.
2m
FEED CONDITION
Factors to Consider—
1. Balancing of Tower Loadings to Take Advantage
of Avaliable Column Diameter Above and Below
the Feed
2. Cost of Column Reflux (Water or Refrigeration)
Cost of Column Rebolling
4. Heat Available From Exchange With Products or
Overhead Vapor
5. Not Unreasonable to Start With BP Liquid
2
222
RELATIVE VOLATILITY
(USED TO MODEL SEPARATION)
Comp. Ky a= KyKe
A Ky KaiKe
8 Ka KelKe
Heavy Key —> c Ke 10
Component
D Ko KpKe
Ky. Pale Vapor Pressure Component A
= Ka 2 Palt . py jp, — Vapor Prossure Component
“Ac = Ko = Bein * °&!Pe Vapor Pressure Component C
MINIMUM REFLUX (UNDERWOOD’S EQN)
=N xix,
O A,
(Baum be x in6 = ( “oan oat
= Min.
Reflux
Ratio
Where: Xi < @ < Ox
f=N iz,
«
it
i Moles Reflux
(O/D) MIN = Minimum Reflux = jouer
Mole Fraction Liquid in Feed
Moles Fraction I in Distillate.
Mole Fraction | in Feed
Relative Volatility of i
(ip Xp Xp)" + Geometric Mean Average 214
MINIMUM STAGES (FENSKE EQN)
( (% Uses:
onl G2) Ge2)] Estimate Minimum No. of
MN Trays Required
—Estimate Feed Tray Location
Sy = Minimum Number of Stages (At Total Reflux)
Xixo = Mole Fraction Light Key in Distillate
Xixe = Mole Fraction Light Key in Bottoms
Xo = Mole Fraction Heavy Key In Distillate
Xyxe= Mole Fraction Heavy Key in Bottoms
Relative Volatility of Light Key
(Averaged Over the Entire Tower)
= (Xing X Cine x Xixy)"> = Geometric Mean Average
t t t
Tower Tower Tower
Bottoms Feed —_—Distita
215
ESTIMATING ACTUAL REFLUX RATE
AND ACTUAL NUMBER OF STAGES
General “Rule of Thumb"—
(3) cua = (1.1810 128) x (Baan
Use Gilliland Correlation to Calculate Actual
Theoretical Stages for Selected O/D (P, 244
Maxwell). Use Safety Factor of 10% or 3
Trays, Whichever is Larger.
216
FEED TRAY LOCATION
1, Locete so That Ratio of Key Components in Feed Liquid
is About Same as Liquid on Feed Tray. (Rule of Thumb)
2, Compare With Plate-To-Plate Printouts of Similar System
3. Use of Fenske EQN. at Total Reflux
4, Parametric Study on Plate-To-Plate Calculation (e.q.
PROCESS-COLUMN) or Parametric Study Using a “Short
Cut” Distillation Tower Model (e.g. PROCESS-SHORTCUT)
2a
FEED TRAY LOCATION (MODIFIED FENSKE EQN.)
‘Approximate Feed Tray
Location Set by the Ratlo—
Sap
Su
Number of Stages Above Feed (At Total Reflux)
Mole Fraction Light Key In Distillate
Mole Fraction Light Key In Feed
Xtxp = Mole Fraction Heavy Key In Distillate
Xyxr = Mole Fraction Heavy Key in Feed
o'x = Relative Volatility of Light Key
(Averaged Over the Enriching Section of the Tower)
= (euxeX ouxo)2
4
Tower Tower
Feed Distillate ate
FEED TRAY LOCATION (KIRKBRIDE METHOD)
This Is the Correlation Used by the
Shortcut Distillation Technique in PROCESS/PRO II
Sar [ Kane) (Xuxe ’]
og =F = 0.206 log | Bip (uxe|
109 Spp = 0208 log [Pi ie) Xan)
Sarigg, = (Ky)?20 where K, = [Bip (ZHt*) Xua)']
Xue’ \Xako
COMPARISON OF KIRKBRIDE METHOD
WITH FENSKE METHOD:
(MOLE FLOW RATES AND COMPOSITIONS)
XicrH0.25 Oy,
Kore 7025 Sic =148 (Entire Tower) Xixo 20.60 Xixg=0.03
xp =OL LK
(Enriching Section) Xj =0.03 Xjxa=0.10
Bip=153
FENSKE METHOD KIRKBRIDE METHOD
S=12.22 Sap=3.42 Sar o
Ser=Sy-S,e=8.80 logg;, 70.206 logl(.63\(0.20y1.0)"]
Sarj = 0-78
Spr
220
TRAY CHARACTERISTICS
(REF. EEDP—SECTION III-A, TABLE 1)
Type Capacity Efficiency __Cost Flex.
Sieve Med.to High Lowest Med, to Good
High ee ON eee
BC. Low to Med. to High Med. to Good
Med. High 200% Sieve 3/1
Valve Goodas Good as Med. 10- High Up
Sleve. Sieve 20% >Sieve to 5/t
Jet Highest-HP Lowto 5%>Sieve Low. 1.5/1 or
Med-LP = Med. 2n
221
SINGLE PASS
Dc.
Clearance
Liquid
Sieve Tray
(Shut Down)
FR
TWO-PASS TRAY
Weir Height
“Tot pod “T “oat
TRAY NOMENCLATURE — SINGLE PASS TRAYS
sLOPED DOWNCOMERS SHOWN)
fae NOMENCLATURE.
Moa Mat he ma, A, = Free (vet Aron = Beaten to det
lood Point
= Bubble Area
= Tower Cross Sectional Area
rrp
‘Ag = Down Gomer Inlet Area
‘Ay = Down Comer Outlet Area
AL = Waste Area
TRAY NOMENCLATURE — TWO PASS TRAYS
(CHORDAL DOWNCOMERS SHOWN)
“ oe
il ya rhode
a Lie
Aa
:
ore
fersuneore en
Cc a w | For Design Caics
\ Use the Smaiier
eee aa, | ofthe hwo"
+ = inboard ey
PRESSURE BALANCE FOR A TWO PASS SIEVE
Pressure Balance for Inboard Downcomer Filling:
Lg = Pe + PAH Lhe
Pressure Balance for Outboard Downcomer Filling:
Lg = Pag + Py tb be
KS
Total Tray
Pressure Drop
TYPICAL TRAY PERFORMANCE DIAGRAM
Poor Ett.
Jet Flooding
Below Design
ticleney
Increasing Vap. Rate—=
Dumping
Increasing Liq. Rate—=
TOWER SIZING
For Sieve Trays
A. Calc. a Trial Tower Diameter With
Assumed Tray Spacing
. Check Liquid Carrying Capcity
Repeat (A) if Necessary
Size D.C. & Set Other Tray Details
. Calculate Tray Hydraulics
Calculate Perforated Area
. Calculate Flexibility
|. Calculate Efficiency
ZO™moo®
228
COMPUTER PROGRAM SELECTION
FOR PLATE-TO-PLATE CALCULATIONS
* PROCESS-COLUMN or COPE-TOWER
— Good for Simple and Complex Systems
— Direct Access to EDL
— Can Set Product Specs
— Can Run With Minimum Data
— Maybe Difficult to Converge With Complex Systems
Depending on Nature of the Complex System, Either
TOWER or COLUMN May be Preferred
ASPECT Il
—Fast
— Can Use Steam Stripping
— Wider Range of Temperature and Pressure
— Good for Heavy Hydrocarbon Systems
(Vacuum Pipe Stills)
* CHEMDIST
— For Severely Non-ideal Systems
— Limited in Number of Components
USE OF PACKINGS
Vacuum Systems Where Low AP is Critical
Debottlenecking Existing Trayed Towers
Light Ends Towers at High Liquid Loadings
Absorption and Stripping Services (High Liquid Loadings)
Small Diameter Towers (Tower Diameters Under About
Three Feet)
Corrosive Services Where Ceramic Materials or Plastic
Materials are Attractive. Corrosive Services Where
Packing Cost is Less Than Alloy Tray Cost
220
229
PACKING CHARACTERISTICS AND TYPES
(REF. EE. DP-SECTION III-A, TABLE 2)
Type Capacity _Etticlency Cost Flex.
indom Dumped
Packing (eg. Pall
Rings, Metal Intallox,
Nutter Rings, ete.)
Structured Packing Med, to. Med. to High (at Med. to Good
(eg. Flexipac, Montz, Very High Very High Least 2x > 3
Gempak, etc.) ROP Cost)
Grids (eg. Giltsch Very High Poor (Good = Med. to. = Low < 24,
Grid, Flexigrid, For Entrain- High
‘Snapgrid) ment Removal
and Heat
Transfer)
Based on Efficiency, Capacity, Tumdown and Cost, the Two Inch Metal Pall Ring
Is an Economic Packing for Most General Petroleum Distillation Services.
231
ADDENDUM 2.1
Section 2
DISTILLATION
Nomenclature
= Area, ft?
= Bubble area, ft?
= Downcomer clearance area, in?
Downcomer inlet area, ft?
= Downcomer outlet area, ft?
= Average tower free area, ft” (superficial area minus arithmetic aver-
age of inlet and outlet areas of downcomer (s) above the tray minus
the waste area); for multipass trays, use smallest value of Ay.
= Hole area, ft
= Superficial (total) tower area, ft?
Waste area, ft? (normally zero for sieve trays)
= Clearance between tray and downcomer at tray inlet, inches
Diameter, ft
Trial diameter, ft
Overall efficiency, %
Peeper
"
rMg0°PP>P
"
Tray spacing, ft
he = Clear liquid height on tray, inches of hot liquid
= Downcomer filling, inches of hot liquid
heq = Effective dry tray pressure drop, inches of hot liquid
hy = Try inlet head, inches of hot liquid
hy = Total tray pressure drop, inches of hot liquid
tug = Head loss under downcomer, inches of hot liquid
hy = Inlet weir height, inches
two = Outlet weir height, inches
Ky. = Tray spacing—liquid rate capacity factor, dimensionless
Kyp = Liquid height—-pressure drop factor, dimensionless
Koz = Surface tension—viscosity capacity factor, dimensionless
Kjp = Hole diameter—pressure drop factor, dimensionless
Ky = Clear liquid height—density difference weeping factor,
dimensionless
Ke = Tray spacing—percent hole area weeping factor, dimensionless
Kg = Surface tension—hole diameter weeping factor, dimensionless
L = Liquid rate, gphitt of weir per pass
u = Liquid rate, gphitt of diameter per pass
& = Liquid load, ft°/s at conditions
Lug) = Minimum liquid load, t%s at conditions
mmosaon0 Page 1
Nomenclature (Continued)
f
4
6
fa
M
M,
Np
Q
Vai
vr
Me
Vesti
Vo
Voguin)
Vocweep)
A
AL
Pv
Oo
Isto
jossoazon
Flow path length (distance between inlet and outlet downcomers),
feet
Inlet weir length, inches
Outlet weir length, inches
= Length of bottom edge of downcomer, inches
= Liquid loading, Ib/s
Vapor loading, Ib/s
= Number of liquid passes
Liquid rate, gpm at conditions
Allowable velocity of clear liquid in downcomer inlet, ft
Vapor velocity based on average tower free area, ft/s
8
Design vapor load = ® Ve at conditions
Ultimate vapor load dependent on system properties, f°/s at
conditions
Vapor velocity through holes, ftis
Vapor velocity through holes at minimum vapor rate, fts
Calculated vapor velocity through holes at which tray will start to
weep, fts
Capacity factor, TN a
Liquid viscosity at conditions, cP
Liquid density at conditions, Ib/tt?
Vapor density at conditions, Ib/ft?
Liquid surface tension at conditions, mN/m
Standard liquid surface tension, mN/m
oe)
10 ww
Hole diameter, inches
(160 -
Page 2
ADDENDUM 2.2
Section 2
DISTILLATION
In order to simplify the work involved in making stepwise calculations for the rectification of bi-
nary and multicomponent systems, Gilliland’ has presented an empirical correlation between
theoretical steps and reflux ratio. To use the Gilliland correlation to predict the number of theo-
retical plates for a given reflux ratio, the minimum number of steps at total reflux and the mini-
mum reflux ratio are required.
Minimum Number of Theoretical Steps
When a separation is specified with respect to only two components of a multi-component mix-
ture, the lower boiling of these two components is designated the light key component and the
higher boiling the heavy key component, and the minimum number of steps can be calculated
by the well-known Fenske equation? as follows:?
lolita) Gt)
Su = Tog ak )
or
x,
lau = (te) a (ra)
After equation (1) is solved for Sy, the latter may be substituted in this equation along with the
distribution of either key component to predict* the distribution of the other components, or
tog (42) tt) = Sy tog @
Likewise,
tog ($E24-)(RU) = sy tog (4) ®
In any of the above equations, moles per 100 moles of feed may be replaced by total moles,
or volume or weight units since in any of these conversions the multiplying factors cancel out.
When the degree of separation is specified for more than two components, equation (1) must
be applied to all critical combinations of these components and the maximum Sy determined
for the most difficult case. If the separation is specified with respect to the total quantity of two
‘of more components, as in the case of Example 1, trial and error is required for the solution
of Su.
It should be pointed out that the concentrations calculated by equations (2) and (3) actually ap-
ply only to the separation at total reflux and, with the exception of the two key components, there
will be some variation of the degree of separation with the reflux ratio, As the reflux ratio decreases,
there is some improvement in separation between light and heavy components boiling outside
the range of the key components and some deterioration in the separation of components boil-
ing intermediate between the key components. However, in so far as the present procedure is
concerned, the distillate and bottoms compositions for other reflux ratios are assumed to be
the same as those calculated for total reflux.
‘Gilliland, ind. Eng. Chem. 32, 1220 (1940). 2A table of nomenclature is given on page 10.
*Fenske, Ind. Eng. Chem. 24, 482 (1932). “This equation may be used for any pair of components.
cecoezere Page 1
Minimum Reflux Ratios
Gilliland® has proposed several different formulas for predicting minimum reflux ratio and all
have the disadvantage of being composed of a number of complex terms in addition to requir-
e ing trial and error for solution. Although all these equations appear to give satisfactory results,
‘the terms are so complex that it is difficult to be certain that there are no numerical errors in
their application.
In order to apply the Gilliland method with greater facility, the following equation was developed
for predicting the minimum reflux ratio of a multicomponent system:
(Oy + + = (ae ~Xino)
- Pac ani Qin ~ WXxxo) + Faxon meee - 20) @
(O/D)y can be calculated for two arbitrary states of feed vaporization:
1. “‘Liquid” feed, corresponding to vaporization of the feed equivalent to the fraction of the feed
lighter than the light key component. For the components lighter than the light key, I. = Zi/ay,
and for the light key and heavier components, lx = Zix, and Iy = Zy.°
2. “Vapor” feed, corresponding to vaporization of the feed equivalent to the fraction of the feed
Consisting of the heavy key component and lighter. For the components lighter than the heavy
key, IL = Zila and Ix = Z,x/c%,« and for the components heavier than the heavy key, ly = Zy
After the minimum reflux ratios have been calculated for “liquid” and “vapor feeds, the mini-
mum refiux ratio for the actual vaporization of the feed can be calculated by direct interpolation
e or extrapolation, However, extrapolation beyond 50% of the difference between “liquid” and
“vapor” feed may lead to serious deviations.
The first term of the right-hand side of equation (4) is the same as for binary mixtures, and the
equation reduces to the equivalent of a binary mixture when alll light components other than
the light key have infinite volatility and all heavy components other than the heavy key have
zero volatility. Under these circumstances the equation is exact when I, is taken as the ratio
of the two components in the liquid phase of the feed. That is, if the feed is introduced as a
liquid at its bubble point, Ix = Ziq, which is the ratio of the two components in the feed, if
the feed is introduced as a vapor at its dewpoint, I, = Z,x/o1,x, which is the ratio of two com-
ponents in the equilibrium liquid, For intermediate stages of vaporization I, can be calculated
from the flash vaporization formula, although direct interpolation of the minimum reflux ratio
of the basis of percentage vaporization between the saturated liquid and saturated vapor feeds
gives values only slightly in error on the conservative side.
In the case of multicomponent mixtures, equation (4) is semi-empirical since it was necessary
to make simplifing approximations in its derivation. Furthermore, the exact values of the various
I's cannot be calculated directly from the composition and state of vaporization of the feed, since
the liquid on the feed piate is not identical to the liquid phase of the feed as in the case of a
binary mixture. As a result, it was necessary to define the I's empirically for two states of feed
vaporization, arbitrarily chosen to simulate a binary mixture of the two key components, and
then interpolate or extrapolate to the minimum reflux ratio corresponding to the actual vaporiza-
tion of the feed.
e SGilliland, Ind. Eng. Chem. 32, 1001 (1940).
Sif components, intermediate between the two key components, are present, they are considered either
light or heavy components depending upon which key their volatility more nearly approaches. In the
case of “liquid” feed, I, = Z, and ly = Zy for these intermediate components; in the case of “vapor”
feed, IL = Z,/ay and hy = Zyoin
soso Page 2
Equation (4) has been checked for a number of multicomponent systems on which the mini-
mum reflux ratio was determined by stepwise trial and error calculations. Generally, unusual
systems were chosen with respect to composition and relative volatility in order to reveal the
maximum deviations ever likely to be encountered in practice. The agreement was quite satis-
factory as the average deviation was less than +5% and the maximum about 10%. The latter
occurred at the limit of extrapolation relative to the arbitrary feed states.
Also, the minimum reflux ratio was calculated for these same systems by the Colburn method”
with about the same degree of accuracy. It should be pointed out that the latter gave better
results than equation (4} when the relative volatilities and compositions were not so abnormal
as the system selected. However, under these circumstances both methods were quite accurate
as the deviations seldom exceeded a few percent, and the present equation has a distinct ad-
vantage in that it is explicit and does not require trial and error.
Both methods are quite sensitive to the selection of key components, and the selection of the
wrong key components can lead to a much greater error than is inherent in either method. If
the desired separation is between adjacent components, there is usually no doubt about select-
ing these as the key components. However, if there are additional specifications relative to other
components, it may be necessary to try two or more combinations of key components to make
sure that the minimum reflux ratio is sufficient to fulfull all specified conditions.
Correlation of Theoretical Steps With Reflux Ratio
As mentioned at the beginning of this section, Gilliland correlated the results of a large number
of stepwise calculations on various binary and multicomponent mixtures by plotting
[IS - Sy] /[S + 1] = $(S) against [((O/D) - (O/D)y] / [O/D + 1] = F(O/D)
and found that all points could be represented by a single curve irrespective of the type or degree
of separation. These points, along with about half again as many additional points, were replot-
ted, and the best curve through them was essentially the same as Gilliland’s original correlation.
In arriving at the coordinates for the additional points the minimum reflux ratio was calculated
by equation (4); therefore these points are a criterion of the present method as well as the curve
itself. In no case did the deviations exceed either 3 theoretical steps or 15%, and the average
deviation was less than 1 theoretical step and also less that +5%. To take care of the maximum
deviation it is recommended that in any design the number of theoretical steps predicted from
the correlation on page 13 be increased by either 3 theoretical steps or 10%, whichever is greater.
Plate Efficiency
Because of the large number of factors which undoubtedly influence the plate efficiency of a
fractionating tower, any fundamental formula accounting for even the most important variables
must necessarily be quite involved. For this reason, a simple empirical correlation of the limited
data on hydrocarbon mixtures seemed to be the most promising method of predicting plate
efficiency.
Gunness® correlated the results of several tests on petroleum mixtures on the basis of vapor
pressure of the liquid. As he points out, this is a method of indirectly correlating plate efficiency
with liquid viscosity since viscosity of pure hydrocarbons and narrow boiling fractions is an ap-
proximate function of vapor pressure over a fairly wide range of vapor pressures.
7Colbum, Trans. Am. Inst. Chem. Engrs. 37, 805 (1941).
®Gunness, Sc.D. Thesis, Mass, Inst. Tech. (1936).
oasoazone Page 3
In view of the consistent results obtained by Gunness, plate efficiency was plotted directly against
fluidity (reciprocal viscosity) for a number of tests on commercial towers including those upon
which Gunness based his curve. The curve on page 12 represents this correlation. While the
over-all plate efficiency exceeds 100% at fluidities greater than 9 cm™", this is not inconsistent
as the flow of the liquid across the plates results in concentration gradients which may achieve
a greater degree of fractionation than predicted by stepwise calculations in which the liquid is
assumed to leave the plate in equilibrium with the composite vapor. Lewis® has shown theoret-
ically that different combinations of liquid and vapor concentration gradients across the plate
may give over-all plate efficiencies as high as 200-300% when based on stepwise calculations.
There is no reason to believe that this correlation applies to mixtures other than hydrocarbons,
and with the exception of alcohol-water mixtures there are too little data available to afford a
comparison. Although there is considerable variation in the alcohol-water data, there is some
indication that plate efficiencies are somewhat greater than for hydrocarbons of the same viscosity.
Location of the Feed Plate
As a simple approximation for locating the feed plate, it may be assumed that the proportion
of actual plates above the feed will be the same as that required to effect the same separation
between the key components at total reflux. That is, the number of theoretical steps at total reflux
is calculated for the concentration then assumed that the ratio of this to the total number of
theoretical steps at an infinite reflux ratio is the same as the ratio of actual plates above the
feed is to the total number of plates. Application of this method is illustrated by Example 1.
In some cases where there are critical components other than the two key components, it may
be necessary to check the total reflux steps above and below the feed on the basis of these
components, since the optimum location of the feed plate will be different with each pair of com-
ponents. Usually the separation of components other than the key components is so complete
that only the latter need be considered.
Example
At an operating pressure of 100 psig determine the number of plates and reflux ratio required
to separate the mixture given below so that the bottoms contain at least 90% of the butenes-2
present in the feed and at the same time have an isobutene content not greater than 5%:
Feed
Component (Mole %)
i-CgHi0 40.0
iC,He 200
CyHert 15.0
CaH10 50
t-CyHyr2 100
o-CyHy2 10.0
100.0
Lewis, Ind. Eng. Chem. 28, 399 (1936).
ooooszo Page 4
(1) Dewpoint of Distillate and Bubble Point of Bottoms
In order to calculate the average volatilities, the dewpoint of the distillate and bubble point
of the bottoms must be found by trial and error using assumed compositions. These are tabu-
lated below.
co Moles Per 100 Moles of Feed Mole Fraction
an Feed Distillate [ Bottoms | Distillate | Bottoms
+-G4Hio 400 293. =| OF (0530 0027
iCjHg 200 187 13 253 050
CaHet 150 130 20 176 or7
CoH 50 10 40 014 184
tO,Hr2 100 15 a5 020 327
o-CyHg-2 100 05 95 007 365
7000 74.0 260 7.000 7.000
As a first trial, assume the dewpoint of the distillate is 140°F at 78 atm (114.7 psia).
First Tal
‘Component Yo a - i
140°F 140°F Tye
-CuHio 0530 1.29 a4 0.493
FC yHy 253 1.155 75 263
Cae 176 1.13 735 187
CaHio 014 1.00 65 017
tOyHe2 020 os7 63 025
oC He2 007 ost 59 009
7.000 0994
Relative volatilities to C4H yo or (c's aro used as a matter of convenience; then, the (a’'g,)'s are
converted to (cqy)'S, the relative volatilities to t-C,Hg-2, which will be selected as the heavy key
‘component.
**Computed from the fugacity function of butane multiplied by the relative volatilies.
Since the sum of the x's is 0.994 instead of 1.000, the assumed temperature should be lower
slightly, but the difference would be so small (less than 1°F) that the change in the (x‘p)'s
would be imperceptible. Consequently, 140°F will be used as the dewpoint of the distillate.
p
Page 5
The bubble point of the bottoms is assumed to be 165°F at &0 atm" for the first trial.
First Trial Second Trail
Component] Xy awe FY y awe Pe
tesr | 165°F | Fem | 160°F | 160° y
iCHi | 0.027 126 | 107 | 0036 1.265 | 10.25 | 0035
i-C4Hy 050 144 97 061 1.145 93 058
CyHet 077 1115 95 091 112 94 088
Cio | 154 1.00 a5 164 1.00 at 156
tCHye2 | 327 og7 325 | 337 097 785 | 321
CC He2 | _365 oss | 78 356 oss | 74 338
1.000 1.045 0.996
"Relative volatilities to CgHo or (cr)'s are used as a matter of convenience; then, the (%'gy)'s are
converted to (%ay)'s, the relative volatilities to t-C,Hg-2, which will be selected as the heavy key
component.
**Computed from the fugacity function of butane multiplied by the relative volatilies.
The bubble point of the bottoms will be taken as 160°F. The relative volatilities are averaged
and converted to t-C,Hg-2 as the heavy key in the following table:
c 1 | 1408 ‘eo 1500 Oy
-omponent °F PF pone ee a,
78 atm 80 atm 79 atm _|(2'oa!wa'a) 7
-CyHig 1.29 1.265 1.275 1.275 1.315
i-C,Hg 1.155 1.145 1.15 1.15 1.185,
CyHp-1 113 1.12 1.125 1.125 1.16
Caio 1.00 1.00 1.00 1.00 103
Cater? og7 os7 os7 os7 100
c-C,He-2 0.91 0915 091 0.91 0.94
(2) Minimum Theoretical Steps (Total Reflux)
The minimum number of theoretical steps by which the desired separation can be accom-
plished is calculated as follows:
Let t = moles of t-C,H,-2 in the distillate per 100 moles of feed
10 — t = moles of t-C,Hg:2 in the bottoms per 100 moles of feed
Since 90% of the butenes-2 must be retained in the bottoms, the cis-butene-2 content of
the distillate and bottoms will be:
and (2 — t) moles in the distillate per 100 moles of feed
(@ + 1) moles in the bottoms per 100 moles of feed
"After allowing 3 Ib/sq in. as the approximate pressure drop through the tower.
Page 6
Using the previously assumed values of 18.7 moles of isobutene in the distillate and 1.3
moles in the bottoms, the following equations must be satisfied:
(8) (a) «ses
,
(8) (ge) = (ay
A trial and error solution of these equations shows that they are satisfied by Sy = 255
andt = 1.62.
The distribution of the other components can be calculated from Sy and the distribution
of tC,Hy-2.
t-C,Hio: Let u = moles of t-C4Hjo in bottoms
(4)
) (238) = (raters = 1075
= 0.19 moles of t-C,Hio in the bottoms
CyHe-t: Let v = moles of C,Hg-1 in the bottoms
v) (8.38 255
C3) (FR) = ase = 44
V = 158 moles of C,Hg-2 in the bottoms
CaHio: Let w = moles of CyHyo in the bottoms
(S—w\ (8.38) _ 255 _
( ) (838) = (1.03) = 2.12
W = 355 moles of C,Hio in the bottoms
The percentage of -C,Hg in the bottoms will be:
13
(onaneeisree) = 100 = 53%
In order to meet a maximum of 5.0% t-C,Hg specified for the bottoms, it is necessary to
reduce the 1.3 moles to 1.22 moles in the bottoms. This would require an increase in Sy
to 25.8 which would modify the distribution of the other components. However, the latter
change is so slight that it can be neglected. The composition of the overhead and bottoms
will then be:
mt Moles Per 100 Moles of Feed Mole Fraction
Se Feed Distillate | Bottoms | Distillate | Bottoms
iC aHio 400 3981 0.19 0528 0.008
i-CyHy 200 1878 1.22 229 050
CyHe-t 150 1342 158 178 064
Caio 50 1.45 355 019 145
tCHy2 100 162 838 021 342
c-CyHs-2 100 038 62 005 391
75.46 2454 4.000 4.000
(3) Minimum Reflux Ratio
‘Since the critical separation is between isobutene and the butenes-2, the former is naturally
selected as the light key component and trans-butene-2, since it is more volatile than the
cis-butene-2, as the heavy key component. Butene-1 is considered a light intermediate com-
ponent because of the proximity of its relative volatility to that of isobutene; normal butane
is considered a heavy intermediate component since its relative volatility is nearer to the
heavy key than the light key. The following tabulation gives the necessary information for
calculating the minimum reflux ratios for the two arbitrary states of feed vaporization:
Mole Fraction 1
Component] Type = ay | “Liquid” | “Vapor”
Feed | Distillate | Bottoms eat | feed
FOHio L a400 | 0528 | 0008 1315 | 304 304
1-C,He uk .200 249 050 1185 | 2.00 169
CHet | ob 150 178 064 1.16 150 1.29
Cato H 050 019 145 1.03 400 348
tC,Hy2 | HK 100 021 342 1.00 = =
eCyHy2 | H 100 005 391 094 2.00 2.00
1.000 | 1000 | 1.000
“Liquid” feed—40% vaporized
(1D + 1 = (13955200510)
+ (438) (0528 - 304 x 0021) + 448 (0.178 - 150 x 0021)
+ sabe (BP - oor) + reBBbur (BBP - 008)
(OlD)y = 188 + 194 + 107 + 0.29 + 046 - 1 = 464
“Vapor” feed—90% vaporized
(O1Dyy + 1 = MBSR LO (0289 _ ooo1)
+ 194 + $48 (0.178 - 1.29 x 0021)
+ tres CRB - o019) + 046
(Oly = 206 + 194 + 1.40 + 035 + 046 - 1 = 491
‘Assume that the feed is sufficiently preheated to vaporize a percentage equivalent to the
distillate or 75.46%. By interpolation, the minimum reflux ratio corresponding to this feed
vaporization is:
(O1D)y = 464 + (7848549) (401 — 464) = 483
Slama Page 8
(4) Theoretical Steps vs Reflux Ratio
Using the values determined in preceding sections for minimum theoretical steps, 258, and
for minimum reflux ratio, 4.83, the number of theoretical steps for various reflux ratios can
@ be predicted trom the correlation on page 13.
F(O/D) (8) s [Theoretical Plates"
= = o 5
0.067 0570 613 603
136 502 52.7 51.7
223 430 460 45.0
314 366 413 403
- _ 258 248
*The reboiler is considered the equivalent of one theoretical step. With a partial instead of a total
condenser, a second theoretical step also could have been deducted.
(6) Number of Actual Fractionating Plates
To predict the number of actual plates it is necessary to determine the average viscosity of
the liquid on the plates. Since the temperature difference between the top and bottom of
the tower is so small, the average viscosity may be taken as the viscosity at the average
temperature. For this purpose the viscosity of butane at 150°F will be used.
Viscosity of CyHyo @ 150°F = 0.216 cs ~ 0.216 x 0523 = 0.113 cp
Fluidity = 10.113 = 89 cp~'; Plate efficiency = 99%
e Using a plate efficiency of 99% the number of actual plates is computed for various reflux
ratios:
) s Theoretical Steps | Actual Plates
483 oo oo oo
5.25 613 603 609
575 527 517 52.2
650 460 450 455
750 413 403 40.7
oo 258 248 25.0
The number of actual plates is plotted against reflux ratio in Figure 1.
Areflux ratio of 6.50 to 1, or 1.35 times the minimum is selected. The number of actual plates
corresponding to this reflux ratio is 45.5 so that a 50-plate tower would be required.
(©) Location of the Feed Plate
‘The number of plates above the feed is based on the proportion of theoretical steps at
total reflux which would be required to effect the change in concentration of the key com-
ponents between the feed and distillate. This proportion is applied to the actual number
of plates (including the reboiler) to determine the number above the feed plate.
In order to take into account any appreciable difference in relative volatility above and
e below the feed, the relative volatility used for calculating the steps at total reflux between
feed and distillate is the geometric mean of aip and cg OF,
on = (HEP x 889)" 110
osooszaa0 Page 9
‘The number of total reflux steps which would be required between the feed and distillate
is calculated by the following equation:
(3) () = 1.19" = 5.78;n = 10.1
Number of actual plates above the feed would then be:
BA 0 + 1) = 20
The vaporization of the feed can be taken into account by adding the fraction vaporized
to n since 100% vaporization would be equivalent to a theoretical ‘step at total reflux.
This would change the proportion of plates above the feed as follows:
(144975) (5041) = 21.4 plates above the feed
Feed lines would probably be installed above the 24th, the 28th and 32nd plates from
the bottom of the tower.
Nomenclature
X moles of any component in distillate or bottoms per 100 moles of feed
x mole fraction of any component in liquid
y mole fraction of any component in vapor
D _ moles of distillate per 100 moles of feed
O moles of reflux per 100 moles of fee
o/D retlux ratio
(O/D)y, minimum reflux ratio corresponding to S = oo
S "number of steps from still to distillate
Sq minimum number of steps corresponding to O/D = oo
P number of theoretical plates; with a partial reboiler and partial condenser, P = S
~ 2, and with a partial reboiler and total condenser, P = S — 1
Zz atio of mole fraction of any light component to heavy key component in the feed
Z4 —_Tatio of mole fraction of light key component to any heavy component in feed
2 relative volatility of any component to heavy key at the dew point of the distillate
yw relative volatility of any component at the bubble point of the bottoms
%, _relative volatility of any component at the arithmetic average temperature of the dew
Point of the distillate and the bubble point of the bottoms
gy mean relative volatility of any component, (ap-aw - a4)“
LK used as a subscript to refer to the light key component
HK used as a subscript to refer to the heavy key component
L used as a subscript to refer to any light component
H used as a subscript to refer to any heavy component
D used as a subscript to refer to the distillate
W = _used as a subscript to refer to the bottoms
n used as a subscript to refer to the Plates above the feed
m —_used as a subscript to refer to the plates below the feed
a Pana 10
FIGURE 1
= 25
Actual Plates
3
5
3
E
3
z
5
£
=
=
e8'y = ney xnjey WNWTUIW
‘Sa1e]1q feNoy JO JequiNN
Reflux Ratio
60;
3 $ +
ry + °
Page 11
Overall Plate Efficiency — %
120,
0
FIGURE 2
OVERALL PLATE EFFICIENCY VS
FLUIDITY OF LIQUID ON PLATES
Only Data on Hydrocarbon Mixtures
Were Used in this Correlation, and
There Were Insufficient Data on Other
‘Types to Justify a More General Use.
However, There was Some Evidence
that the Curve is a Little Conservative
for Alcohol-Water Mixtures
1
23°45 67 8 9 10 11 12 13 14
Average Fluidity of Liquid on Plates — (Centipoise)-'
Page 12
FIGURE 3
GILLILAND CORRELATION
CORRELATION OF THEORETICAL STEPS WITH REFLUX RATIO
MULTICOMPONENT AND BINARY MIXTURES
ub & Roo NN ® bo
Fe
>
Reference: Gilliland, Ind. Eng. Chem. 32, 1220 (1940)
R-Rm, R F(R)+Rm
FOID) = FR) = "Ray Am * Rmft-FR)]
s-sm, S (S)+Sm
OS) = ~SEr Sm = Smlt- GO
@ &(S)
ub oa ON & ©
12345678 910
F(R) or F(O/D)
Page 13
accrion(ai FRACTIONATING TOWERS
DISTILLATION DEVICE SELECTION AND BASIC CONCEPTS
e TABLE 1
TRAYS - A SUMMARY OF CHARACTERISTICS
[rare a
= mane | ees | mae
Bee. ee Weim | ponte
™ data avedadie.
wa a
aaa | coe Seen [aT Soom
so Saget
me
arias — Taare ae | ee, Same
fees [Sars [sor [Se [Sat
ae Sure, | Sen
See ace
= ae em ae Semon
=a tee |e mca
ie ead See
- ae meer
PCR
tao Ge irene a een ona eee
=F a ce et
=7s. Tore |S Peer
ia STs
a eR Rg ee
o Eanom aon
sme ae
an
Sor,
=
soa
LL
@ TABLE 2
COUNTER-CURRENT DEVICES "A SUMMARY OF CHARACTERISTICS
Seer — ase 1 eisoey tat rant res Tes? Te
= ro a wpe
ao Ss ae
an. =. SS
ts = reas
= rr
a a a sooo
Merce” [Saacst [Mnasst | ron‘ =e
ee. [oo [so er
=
Soe ae ES oro ee ae
= poses Ea Seams
= re Pte)
roa i=
x GoTo ae
Sees
| oe
ae ae = am aon eae
= was wiares
one Sacto «
Pins
on ln cane
See [Rae Sa" |sre Sane m
a ax =e, pS
sa =
a
TA eR SRT Ee SRST HT Raa ae Ta be
|
|
SODIAIOS Joysuely Jeay ‘Burjog ‘Hbuljno4 -
Aouaioyjy Mo ‘Ayoedes ybipy, +
puBdeus seynN
DESIGN PRACTICES EXON
‘Section Pope FRACTIONATING TOWERS
me 40 of 62 PACKING AND GRID
PROPRIETARY INFORMATION — For Authorized Company Use Only
EXXON
ENGINEERING
Figure 4
ANCILLIARY TOWER INTERNALS.
NEEDED IN PACKING INSTALLATIONS
‘Vapor Out
VL
wus"
(Only If Deentrainment
Required)
Remove Existing ~~)
Tray Supports and
(To Within 4" to 4”
e [6-13 mm] of Wall)
Gas Injection
‘Support Plate
Liquid Product
onmney oY or Drawott
(For Sidestreams,
Pumparounds, etc.)
Liquid Distributor
(For All-Liquid Feed)
Non-interfering
Bed Limiter
Gas Injection
Support Plate
‘Vapor Distributor” Vapor In
e Notes:
For Height Dimensions Associated
With Each Device, See Table 5
aa/NdD “oVeH PINbIT,
oe oz oo °
—_—ToooOTO oo TOF
Xxnyou [e001
VISd 00€ ’oU/*9!
s6uly ited .Z
oz
ma NN, 3
soInqUIsiG Ued dug MON $
ax
2m
aa
ex
a3
aS
ss
2
L
AONAIOISSa NO LOWd ~”?
; 7g NVOMINGIS. 3AVH Nvosoinarisid
Figure 2
TYPES OF TRAYS
(SCHEMATIC)
Sieve
a) Fr
vatve 7 ——
(ee) ho}
ae Fully
= eo
(1-0)
Co) Standard Vacuum
ap cap
EXXON RESEARCH AND ENGINEERING COMPANY — FLORHAM PARK, Nu.
EXON
XX
@reaineernc
DESIGN PRACTICES
FRACTIONATING TOWERS Tecton
EXXON PACKING AND GRID ms mace
PROPRIETARY INFORMATION — For Authorized Company Use Onty Pate Nov, 1989
Figure §
TYPICAL LIQUID DISTRIBUTORS
b-v pajeeg-eiqnog yosyi5 ev YOSHI, Ledhy yooy,
Hae JOUNN A USSHID v edk| yooy
Sap
shell aajeA
e e e
ADDENDUM 2.4
SECTION 2
DISTILLATION
SELECTION AND DESIGN OF
COMMERCIAL FRACTIONATION EQUIPMENT
BY P. W. BECKER AND R. K. NEELD
EXXON RESEARCH AND ENGINEERING COMPANY
Although Chemical Engineering technology has grown tremendously in
the past ten years, unit operations stil] remain at the heart of the chemical
and petroleum industries. And, within the unit operations area, distillation
remains the chief means of the various physical separation techniques.
Distillation as an art is old; but distillation as a science is much newer,
and a proportionate share of the R&D effort involved in developing new
technology has been and will continue to be expended in this area.
The purpose of this handout is to discuss what some of this
technology is, especially from the standpoint of the design engineer. First,
why is it important. Second, what changes have taken place in recent years.
Third, what tools do the designers have to work with and how do they go about
selecting and designing conmercial distillation equipment.
Importance of Distillation
The large annual investment in distillation equipment is a major
reason why work in the fractionation area is important to us, and why we
undertake research and development work; spending both time and money trying
to perfect our technology in this area. For example, during the past several
years, Exxon Corporation alone has spent over ten million dollars annually for
towers and trays. Obviously, then, the possibility of even small improvements
in the selection, design, and performance of our distillation units can be
readily justified.
A second reason for a continuing effort in this area is that
distillation is usually the final processing step that determines whether
on-spec products are being produced. And, thirdly, the purity specifications
for both new and existing products are constantly being raised, thereby
requiring more accurate tower design techniques. Furthermore, we could cite a
variety of system physical properties, yield-purity relationships, liquid and
vapor rates, temperature sensitive materials, and fouling requirements that
create special problems affecting hardware design.
Since the oi1 embargo of 1974, and the subsequent quadrupling of oi]
prices, another important consideration has arisen--that of conserving energy.
Distillation is a large energy consumer in refineries and chemical plants.
Heat must be put in at a high temperature level at the bottom, and removed at
a lower level at the top. This introduces thermal inefficiency, and
necessitates the use of lower reflux and lower pressure drop internals.
08159001.c¥S
DESIGN PRACTICES
EXON | FRACTIONATING TOWERS Section Page
EXXON DEVICE SELECTION AND BASIC CONCEPTS WA 29 0147
Date
ENGINEERING PROPRIETARY INFORMATION — For Authorized Company Use Only Dec. 1968
e aes
‘TYPES AVAILABLE (PACKING, GRIDS, BAFFLE SECTIONS, DUALFLOW TRAYS) (Cont)
Figure 20
PHOTOS OF DUMPED PACKINGS
PALL RING NORTON METAL
INTALOX (MTP)
a
NUTTER RING
‘There are a number of other packing types available but they are not widely used in Exxon towers and therefore
‘not been included here. 4)
b) Structured packings (also called ordered packings). These devices are fabricated in bundles from crimped sheet
‘metal and installed in the tower in layers having a fixed orientation. Since they provide more surface area per unit
e volume they are more efficient than random packings. However, they cSBTZ-4 Trn@s as much, Since the crimp height,
can be changed, the capacity, efficiency, pressure drop and cosl car also be varied Thus, the optimum choice must
be determi ‘economies
of
the contacting doves ayalabe, ney provide the lowes pressure cro per theoretical stage of contacting as |
well as the best capacity/etticiency combination. This feature makes them especialy attractive in vacuum towers
‘There are several suppliers including: Flexipac by Koch Engineering, Gempak by Gitsch, Intalox Structured by
Norton, Montz by Nutter Engineering, and Meliapak by Sulzer. One of these devices, by Koch Engineering, is shown
in Figure 21.
Figure 21
PHOTOS OF STRUCTURED PACKING
{BY KOCH ENGINEERING)
ISOMETRIC VIEW
EXXON RESEARCH AND ENGINEERING COMPANY — FLORMAM PARK, NJ.
Why New Devices Outperform
‘The Bubble Cap Tray
We must also remember that the variety of equipment (or hardware)
available to handle these process-oriented problems is also constantly growing
and being steadily improved. For instance, in the early 50’s and for many
years before, the bubble cap tray was the workhorse of the distillation field.
This is no longer true, and we now have:
* devices with greater capacity and lower pressure drop than the bubble
cap,
* devices with efficiencies that are equal to or better than the bubble
cap,
* devices that are lower cost,
* devices that possess almost equal flexibility to handle varying liquid
and vapor loads without loss in effectiveness, and finally,
© devices that are easier to maintain.
Tray Performance Diagram
And Design Limitations
A number of physical boundary conditions limit our final tray
design. The final tray design should eliminate or, at the very least,
minimize the impact of each of these limitations. The correlations available
for calculation a "performance diagram" for each contacting device vary from
company to company. Contractors have correlations obtained from various
sources, tray vendors generally have developed their own, as some companies
have also, while other companies may use Fractionation Research Inc. or the
literature sources. Regardless of the source, these design limitations are
calculable, and the various correlations themselves will not be discussed.
The physical boundary conditions include:
Weeping
The weep point is that vapor rate at which liquid starts to leak
through the tray openings. It is not necessarily the lower operation limit
for good tray efficiency. For systems with high liquid-to-vapor ratios, a
smal] amount of liquid bypassing will not seriously reduce tray efficiency,
providing it is only a small fraction of the total liquid on the tray.
Dump ing
Dumping is excessive leakage of liquid through the tray openings.
It is characterized by a sudden and significant drop in tray efficiency. The
minimum vapor rate for acceptable tray performance is equal to or greater than
that at which dumping occurs.
08159001.c¥5
Blowing
Blowing is a fine dispersion of fog of tray liquid which becomes
entrained to the tray above. It is caused by excessive vapor velocity through
the tray openings at relatively low liquid loadings. The high hole velocity
is caused by a high vapor loading or low percent hole area, or both. Blowing
results in poor contacting since it lifts the liquid or froth phase off the
tray and the vapor phase thus becomes the continuous phase. This limitation
does not necessarily result in flooding.
Flooding is an unstable condition in which the liquid height on the
tray and in the downcomer builds up until the tower is essentially full of
dense foam. The two principle causes of flooding are, first, excessive
entrainment and, second, excessive downcomer filling.
Entrainment Flooding or Jet Flooding
Entrainment or "jet" flood is a condition where a vapor handling
limitation is caused by the carryover or "jetting" of liquid droplets from one
tray to the tray above because of excessive vapor velocity through the tower
free area. This represents the maximum capacity of the tray at a given tray
spacing.
Excessive Downcomer Filling
High tray pressure drop or insufficient disengaging of vapor in the
downcomer results in a buildup of froth in the downcomer and eventual tray
flooding. It can occur at any liquid at any liquid rate if inadequate
domcougr clearance, inadequate downcomer area, or inadequate tray spacing is
provided.
iguid Gradient
Liquid gradient may also cause problems. The change is depth of
liquid on a tray, from the inlet toward the outlet, is the liquid gradient.
Depending on the type of tray (that is, the resistance to flow) ad the motion
of the vapor, the static head represented by the liquid gradient may furnish
anywhere from a negligible to a major part of the driving force that moves the
liquid across the tray. Gradient problems are worst on bubble cap trays and
least on jet trays.
loci sen
The liquid velocity into the downcomer must be low enough to allow
vapor to disengage and travel up and out of the downcomer against the flow of
incoming froth. If this velocity is exceeded, the increased downcomer level
08159001..crs
due to excessive aeration may cause flooding of the tray. In addition, the
vapor in the froth mixture may be of such magnitude that when it disengages on
the tray and result in premature jet flooding.
timate Capacity
Ultimate capacity is another limitation that is occasionally
encountered. The ultimate capacity is the upper limit of vapor loading which
the tower can handle. It depends mainly on the physical properties of the
system. This vapor load cannot be increased by changing the tray design or
increasing the tray flooding occurs first; but, in certain cases, an ultimate
capacity limitation may be reached before jet flooding. Thus, each tray
design should always be checked for ultimate capacity.
Flexib) yr Turndown Ratio
Flexibility is the ratio of maximum to minimum vapor loads which
bound the range of operation conditions over which the tray will perform
satisfactorily; i.e., tray efficiency remains above, roughly, 90 percent of
its maximum value.
The flexibility of a typical tray is depicted in Figure 1. We see
that tray efficiency remains relatively constant in the range of loadings from
40 to 90 percent of the flood point. Below 40 percent of flood, efficiency
decreases due to weeping, dumping, and poor contacting due to low interfacial
area. Above 90 percent of flood, efficiency rapidly. drops off due to high
entrainment. If better turndown that this is required, a special device might
be required. Therefore, it is important that you, as a designer, carefully
determine the minimum loading conditions that the tower will actually see.
Quite often, arbitrary or unrealistic turndowns are specified, and the cost of
the tower increases appreciably; for example, shifting to a costlier device.
Now that we briefly reviewed the various tray limitations, let’s
look at the various types of trays available to us. The contacting devices
can be broken into three broad categories. These include: downcomer Type
Trays, Downcomerless Type Trays, and Packing. Category 1 - The Downcomer Type
Trays which include bubble cap, sieve, and valve trays. Some of the major
characteristics of each device include:
tegory ncomer_Type Trays
+ Bubble caps - There are many, many types of bubble cap tray designs
available to the industry. And, surprisingly enough, there is probably
very little difference in the performance of those designs that might be
called the best. The better designs are characterized by being
relatively high in efficiency, in capacity, and in flexibility. In
general, however, they do possess a higher pressure drop than the other
devices that we'll talk about. But, their biggest deficiency is that of
cost. Other types of trays are as much as 50 percent cheaper than bubble
cap trays; and this is, perhaps, the main reason why bubble caps have
lost their popularity.
08159001..c¥5
Sieve trays - We find that they are currently the most widely-used of
all the trays available. A typical, modern sieve tray is shown on the
next Slide 6. This tray exhibits good capacity, excellent efficiency,
and good flexibility--that is, it will operate quite efficiently at
loadings which are one-half to one-third of design values. And, perhaps
most importantly, it is the cheapest device currently available. One
reason for the increasing popularity of sieve trays can be traced to the
work of an organization called Fractionation Research Inc. (FRI). This
organization has been in operation for over 20 years as a non-profit,
industry-sponsored research cooperative. It has published performance
data on almost all of the important contacting devices currently
available. These data have allow not only pretty good comparative
evaluations to be made, but have also allowed design procedures to be
established.
Valve trays - These trays contain proprietary devices manufactured by
three concerns: Koch Engineering, F. W. Glitsch & Sons, and Nutter
Engineering. These trays consist of a number of discs (or valves)
suspended above the holes of a perforated tray at a height which depends
upon the vapor flow rate to the hole. The trays themselves are
characterized by a high turndown ratio. Capacity and efficiency are both
high, and are similar to sieve trays, while their cost averages about ten
percent more than sieve trays. They are generally used where wider
flexibility than that obtained from sieve trays is required. By the way,
this is a "two-pass" valve tray. That is, the liquid flows from left to
center, or from right to center. On the trays directly above or below
this one, liquid flows from the center to either side.
Jet trays are similar in construction to a standard sieve tray except the
punched holes are replaced by inclined tabs. The tabs are punched from
the tray deck and inclined to the plate at a typical angle of 20° from
the horizontal. This tray is characterized by having a high liquid and
vapor handling capacity. Liquid handling capacity is high because the
vapor assists or “pumps” the liquid across the tray. The tray is used in
heavily-liquid loaded services where multi-pass convential trays are
normally required. For most distillation towers (where liquid rate is
normally low), the tray is not generally used since its capacity
advantage becomes marginal, and its efficiency is less than a sieve or
valve tray.
V-Grid tray - A typical V-Grid tray is a convential crossflow tray.
Distributed throughout the tray deck are V-Grid units, which can best be
described as rectangular valves which are always in the fully-open
position. The main difference between the operation of the V-Grid tray
and a typical sieve tray is in its vapor flow patterns. While vapor
always flows in a vertical direction through the sieve tray, it must flow
horizontally through the V-Grid units. Because V-Grid units are laid out
on a rectangular (rather than triangular) pitch, vapor jets leaving
adjacent units oppose each other, thereby promoting increased turbulence
and mass transfer.
8189001 .crs
e The operation of the V-Grid was compared to that of a sieve tray during a
laboratory test, program conducted by Nutter at their facilities in Tulsa,
Oklahoma(1). These data indicated that this increased action on the
V-Grid tray resulted in its having somewhat higher fractionation
efficiency than a sieve tray. Also, liquid did not weep as readily
through the V-Grid tray, which indicated that V-Grid has a better
turndown ratio than the sieve tray although certainly not as good as a
capacity advantage over a sieve tray under most conditions, it did
exhibit higher entrainment than the sieve tray at very low liquid rates.
Thus, at moderate and high liquid rates, the V-Grid tray’s performance is
at least equivalent to, and possibly somewhat better than, that of a
sieve tray. At low liquid rates, however, V-Grid trays should be used
cautiously since they may entrain excessively.
The V-Grid tray should also be particularly attractive in fouling
services. The violent tray action (caused by the opposing vapor jets)
should make the V-Grid tray a self-cleaning device. Nutter claims that
their trays have been used successfully in services which were previously
plagued with tray fouling problems.
ategory ‘The Downcomerless Trays
© Dual flow is a generic term and describes, basically, a downcomerless
sieve tray in which both liquid and vapor flow takes place through the
same holes. This could well be the sieve tray you just looked at with
e the downcomers completely blocked off and the area Filled with more of
the perforated sheet that comprises the active area of the sieve tray.
© Ripple tray - This is a proprietary device that’s been patented by the
Stone and Webster Engineering Company. They fabricated this device by
crimping a standard flat dualflow tray into sine waves of a desired
frequency and amplitude.
We have some data on the performance of both the dualflow and ripple tray
and have found they possess very high capacity and moderate efficiency.
However, the flexibility or turndown ratio over which good efficiency can
be obtained is generally poor. As a matter of fact, unless the tray is
properly designed for the load at which it is to operate, it won’t do the
job. It is normally considered only when higher capacity is desired and
Some sacrifice in efficiency and turndown ratio can be tolerated. This
‘is the case in some debottlenecking studies.
(1) The results of this program were discussed in detail in A.I.Ch.E.
Preprint 49c (Ammonia Stripping Efficiency Studies), Sixty-Eighth
National Meeting, March-April, 1971 by D. E. Nutter.
8159001. cys
Categor: in
Four of the more familiar packings that are widely used today are
the Intalox Saddle, the Pall ring, the Raschig Ring, and the Berl Saddle.
Both the Raschig Ring and the Beri Saddle, while still used in some limited
services, have been largely replaced by the Pall ring and the Intalox Saddle.
This is due to the higher capacity and efficiency of these newer packings.
The Raschig Ring is nothing more than a short cylinder. When made from
ceramic material, its walls are usually quite thick. The Pall ring, on the
other hand, has thin, slotted sides with fingers that protrude inward. This
permits much more of the ring’s surface to be used in the mass transfer
process by producing more turbulent contacting and reducing stagnant zones.
Likewise, the new Intalox Saddle is more efficient than the older Berl Saddle
it has largely replaced. On an overall basis, i.e., efficiency, capacity,
turndown, and cost, we have found the two-inch metal Pall ring to be the most
economic’packing to use in most of our distillation services.
Packed towers are primarily used in the following applications:
vacuum systems where low pressure drop is critical,
+ debottlenecking existing trayed towers,
* light ends towers at high liquid loadings,
* absorption and stripping services (high liquid loadings).
In addition, packing is also used frequently in small diameter towers (under ~
three feet) and in corrosive services where a ceramic material of construction
is required.
Are These All of the Contacting
Devices Available
The trays and packings discussed previously will usually fill more
than 95 percent of the designer’s needs. However, there are certain
applications where some of the more non-conventional or high cost devices are
justified. Although there are many, only a few more commonly-used ones are
described here. These are:
+ The MD Tray - This tray is a perforated fractionating device which
features a multiplicity of narrow downconers distributed over the entire
tray surface. Hence, the name "multiple downcomer". The Linde(2) tray
is a high capacity device which makes it especially attractive for
debottlenecking a tower. While its efficiency is somewhat lower than a
(2) Detnicki, W.V., and Wagner, J. L., “Performance Characteristics of Linde
Multiple Downcomer Distillation Trays", Preprint 24c A.I.Ch.E.
Sixty-Second Annual Meeting, November 16-20, 1969.
osisso01 cys
standard cross-flow sieve tray, its high capacity may mean that extra
trays can be added to a given tower or the reflux rate increased somewhat
to offset any fractionation debit. For a specific case, you should
contact Linde to determine whether the MD tray is attractive for your
application.
Sulzer Packing - This packing is manufactured by Koch Industries, Inc.
The packing is a woven wire fabric material which is completely
self-wetting. The packing is a high efficiency device (i.e., low HETP)
with a very low pressure drop. It has special application in distill ing
heat-sensitive materials under vacuum conditions. Its main disadvantage
for more gengral use is its cost, being about $450/ft3 (as compared to
about $20/ft3 for stainless steel two-inch pall rings).
Pe Kontakt Tray - This device is made from pieces of expanded
metal and contains a number of intermediate weirs. In addition, the
metal is expanded in such a manner that the vapor helps "push" the liquid
across the tray. Because of this feature, the tray is claimed to have a
high capacity and is, therefore, recommended for debottlenecking as well
as new designs. Unfortunately, due to tis newness in the U.S.,
relatively little data is publically available for comparison purposes.
The vendor should, however, be contacted for more specific details and
recommendations on the application of this tray(3).
What is Involved in Tower Design?
Having discussed some of the characteristics of the equipment that
designers, might use, let’s briefly go through the steps involved in designing
a distillation tower. With the advent of computers, this has becone more
rigorous in recent years.
The steps involved are summarized as follows:
1, Define the key separations - Product specifications or degree of
separation can be given to us in a number of ways; perhaps as a flash
point or an amount of material boiling below or above a certain
temperature. On simpler towers, where individual components can be
identified, the amount of purity is given, We must, by some means, tie
this into a yield and quality description. Although we don’t need, at
the start, to have a complete definition of the products (that is, we
don’t need a complete material balance on all components), we do need to
know the key requirements of the separation, that is, the yield and the
purity level of our products.
For further information, contact Ferrostaal Overseas Corporation, 17
(3)
Battery Place, New York, New York. (212) 422-6535, Attention Mr. Alfred
B. Walter, Manager.
08159001..c¥s,
2. jate vapor-liqui ilibri enthalpy data - A
number of basic data correlations are available to use. At last count,
Exxon had over a dozen different correlations or techniques in use for
different types of problems. One of our big jobs as designers is to
define the limits and proper application of each of these correlations.
3. Calculate heoretical plates requi! iff f1 ios -
The calcuTation techniques for determining the right combination of
theoretical trays and reflux ratio has improved tremendously in recent
years, and these techniques are limited now only by the accuracy of the
basic data. Today, we have plate-to-plate programs that can handle up to
150 components, five sidestreams, and five heat removal sections or
pumparounds. A complete printout gives us tray and produce vapor and
liquid rates, temperatures, compositions, and certain physical property
information.” If we run several cases with different numbers of trays and
different reflux ratios, we can then define the shape of the theoretical
trays vs. reflux ratio curve.
Before we can select the optimum combination, we need to:
4. Estimate the tray efficiency - Lacking specific data on a similar
system, there are a number of correlations available to us to do this,
‘the most fundamental being the A.I.Ch.E. method for bubble cap trays.
Since this correlation was developed from bubble cap data on binary
systems, most of them aqueous solutions, there is some question as to its
applicability to the multicomponent hydrocarbon systems frequently
encountered in the petroleum industry. Exxon has, therefore, derived our
own correlations for predicting sieve tray efficiency.
With our estimated plate efficiency, we can now go back and calculate the
actual number of trays that corresponds to each reflux ratio. We now
have the basis for an economic balance to determine which is the proper
combination to use. In general, we have found that reflux ratios in the
range of 1.2 to 1.5 times the minimum are usually optimum.
5. Define the minimum and maximum feed rates that our tower must handle -
Tt is important that the designer be given the range of loadings over
which his tower must operate, and this is a number that is frequently not
easily obtained. In general, an assumption of a2 to 1 rangeability fits
most normal operations. When greater flexibility than this is required,
the degree of flexibility becomes one of the key items in determining
both the type of tray to use and how it should be designed.
The next step in designing towers should be:
6. Selecting the best tray for the given set of process conditions.
Choosing the best tray type goes back to our earlier discussion on the
merits of the various trays. For about 90 percent of new designs, sieve
trays have proved adequate to cover both the range of loadings and the
various services required. Of course, such items as coking tendency,
allowable pressure drop, flexibility requirements, and, in the case of
bottleneck removal, required capacity and/or efficiency will have sone
bearing on tray selection. By and large, however, sieve trays have
og1sg001.cys
-10-
become the design engineer’s first choice among the available contacting
devices. If packing is used, it is hard to beat the two-inch Pall ring.
7. Tower sizing and tray hydraulic calculations complete our tower design.
Most companies have their own design methods for this phase of tower
design; and, in almost all cases, towers are designed to flood by
entrainment; that is, liquid is carried from one tray to the tray above
by the ascending vapor. But, it is also essential to check the overall
tray pressure drop, downcomer liquid velocity, and downcomer Filling to
assure that the trays will be hydraulically stable. While the hydraulic
calculations are pretty well standardized, tray capacity correlations
are, by and large, of the generalized empirical variety. Principal
variables governing the allowable vapor velocity are: liquid and vapor
density, liquid surface tension, viscosity and rate, and certain tray
hardware factors; namely, tray spacing and percent hole area.
8. Process = In general, tower control schemes were pretty well
standardized until a few years’ ago when so-called super fractionators,
and by this we mean towers with large nunbers of trays and high reflux
ratios, appeared on the scene. This has given rise to a number of
studies of control schemes involving lag time estimates,
temperature-composition sensitivity, (which, incidentally, is usually
poor), and chromatographic analyzer applications. In addition, small
analog computers seem to offer promise in this area to optimize utilities
and to standardize product output of these towers since large lag times
make manual control difficult. Tower control issues may thus influence
the final tower design.
08159001.cys
SECTIONS 3 & 4
HEAT TRANSFER AND HEAT EXCHANGERS
What We'll Cover:
SECTION 3
© Heat Transfer Theory-Review
* Relation of Heat Transfer Theory to Shell and
Tube Heat Exchangers
* Design of a S&T Exchanger—Procedure Outline
SECTION 4
* Design Features and Parameters of Shell and
Tube Exchangers
BASIC HEAT TRANSFER CONCEPTS
* Flow of Heat Behaves Like Flow of Fluids
and Flow of Electrons
|, Driving Force
Rate a K « SAVING FORE (General)
. Pressure Drop
Resistance
10+ penta (Electricity)
Qak (Fluids)
__ Temperature Difference
oo Resistance Ae)
COMPARISON WITH FLUIDS
2
Fluids: (2)" = «« Pe-Pr (Section 6 wit!
A ( & 7 Treat Fluid Flow)
D
Dat. Toth
Heat: 2 = 1-6 TE
FiDs HEAT
@ = Volume/Second Q = Btu/Hour
P,, Py = Higher, Lower Ta, Ty = Higher, Lower
Pressures Temperatures
A = Area Available for Flow Ry = Total Specific Resistance
fe A = Area Available for Flow
(4 )= Number of Fluid of Heat
Flow Resistance
Units
BASIC HEAT TRANSFER EQUATION
Qay-.y.ar
A Ry Ry
Ry = Total Resistance, HeFT?-°F/Btu
ie = Total Conductivity = U, Btu/Hr-Ft?-°F
Det-uar
Q = U,+ A> AT Btu/Hr
U, Is Referred to as the “Overall Heat Transfer Coefficient”
TOTAL RESISTANCE TO HEAT
FLOW—HEAT EXCHANGERS
* There Are Two Areas Through Which Heat Must Flow:
the Inside Tube Area and the Outside Tube Area.
Resistance Occurs at Both Areas.
* The Industry Standard Reference Area Is the Outside
Tube Area,
INDIVIDUAL COMPONENTS OF THE TOTAL RESISTANCE
Inside Film Resistance = Ri. = Fi( %)
Inside Fouling Resistance = 14, = 5, ( *)
Tube Wall Resistance = 1, = é
Outside Fouling Resistance = r,
Outside Film Resistance = R,
Ro + fo + fw + fo + Ry = Rr = 7
&, = Wall Thickness, Feet
K, = Thermal Conductivity, Btu/Hr-Ft?- zt
f= Resistances, Hr-Ft?-°F/Btu
TYPICAL RESISTANCE VALUES
Very Low _Typical_ Very High
Film Resistances (Each) 0.00050 0.004 0.04
(inverse = h) (2000) (250) (25)
Fouling Resistance (Each) 0.001 0.002 0.01
Inverse (1000) (600) (100)
Wall Resistance 0.000030 0.00027 «(0.00049
Inverse (32,000) (3760) (2030)
Total Resistance 0.00303 0.01227, (0.10050
Inverse (330) (1) (10)
THE CONTROLLING COEFFICIENT
* Frequently one of the Two Film Coefficients determines the
Value of the Overall Coefficient:
Outside Coefficient, h, = 75 75 150
Inside Coefficient, hi. 1000 3000 1000
R, = 0.01333 0.01333 0.00667
Rig = 0.00100 0.00033 0.00100
Twit le +f. = 0.00070 0.00070 0.00070
Ry = 0.01503 0.01436 0.00837
U= 665 69.68 1195
Improvement = Base +46% = +80%
* Hence h, is the ‘Controlling Coefficient”, and Efforts to
Improve Exchanger Performance Should Concentrate on
This Side of the Exchanger.
A. TEMPERATURE DROPS ACROSS THE RESISTANCES
+ Temperature Drop Across Each of the Resistances is Directly
Proportional to Each Resistance.
* For Example, If T, = 200 and T, = 80, Then Total Temperature
Drop = 120°F, and:
Temperature
Drop 0.01333
R, = 0.01333 776 = 9.01333
Rig = 0.00500 29.1 aad
fw = 0.00030 17
To + fo = 0.00200 1
Ry = 0.02063" 120°F
B. TEMPERATURE DROPS ACROSS THE RESISTANCES
A Useful Concept is Heat Fiux = (2) = Bt
Q=U+A+ (12-1) =U A+ aT
Then aT =
= (2) = Fux. Resistance
Q AT
a
U-A
120
In Previous Slide, If A = 4000 Ft? Then p= Ry = 002063
= 5817 —BW. and AT Across Ry = 5817-0.01333 = 75°F
?
as Shown on That Slide,
BACK TO BASICS
+ We've Looked at Basic Theory, and Discussed Q = Up + A + AT.
In Refinery Work We Usually Know Elther Q or A, and Need to.
Calculate the Other Value.
How Do We Do It?
+ Either Question Requires Calculating Ug and AT.
+ We'll Talk About U, Later, First Let's Discuss AT, The
‘Temperature Driving Force.
+ Note that Capital Letter T Denotes the Hot Stream, While Lower
Case t Denotes the Cold Stream:
Ty = Hotin —T,= Hot Out
ty) =Coldin ty = Cold Out
310
FLOW PATTERNS AND TEMPERATURE DRIVING FORCE
A. Cocurrent (Parallel) Flow
FLOW PATTERNS AND TEMPERATURE DRIVING FORCE
8. Countercurrent Flow
i Double Pipe Exch.
4 h-hh
It, Cross When
bot
FLOW PATTERNS AND TEMPERATURE DRIVING FORCE
Mixed Flow
FLOW PATTERNS AND TEMPERATURE DRIVING FORCE
©. Transverse Flow :
y”" we"
Tete" a,
este ay
7
TEMPERATURE DRIVING FORCE
* From the Preceeding Slides, It is Clear That Some Sort of
‘Average Driving Force Must Be Used in Design Calculations.
What is This Average?
* The Average Is Called ““The Effective Mean Temperature
Difference”, or MTD,.
* For True Countercurrent and True Cocurrent Flow, the
Effective Driving Force Equals the Log Mean Average of
the Two Extreme (Largest and Smallest) Deltas,
ATT,
aT, = LMTD = ATS
ATs
This is Precisely True Only When the Heat Release Curves
are Straight Lines. Otherwise it is an Approximation.
TEMPERATURE DRIVING FORCE
What About Mixed Flow: Shell and Tube Exchangers?
‘The Complex Flow in These Units was Analyzed Mathematically
Many Years Ago, Resulting in Rigorous Equations for a
Correction Factor, F,. This is Multiplied by the LMTD to Give the
Correct MTD,.
MTD, = F, * LMTD
Equations are Valid Only When Heat Release Curves are Lin
Similar Relations are Available for Transverse Flow (Air Fin
Coolers, For Example).
a6
CALCULATION OF F,
* Depends on the Number of Shells in Series
(“Shell Passes”)
* The More Shells One Has in Series, the Closer
F, Approaches 1.0
* Typically the Minimum Acceptable Value of
is 08
+ What Exactly Do We Mean by “Shells in
Series” or “‘Sheli Passes"?
a7
CALCULATION OF F,—SHELL PASSES
ONE PASS
%
4 :
th
7
Ta
te
Max 257,
CALCULATION OF F,—SHELL PASSES
‘THREE PASSES
Max t,>>T,
a0
CALCULATION OF F,,
* Complex Equations Simplified to Charts
* See TEMA Section 7, Pages 112-127
or Exxon DP IX-D, ppS3-pp58
* Applicable Only to Linear Heat Curves
Min. for Design
1.0
“Temperature Efficiency”
320
CALCULATION OF F,,
F, (1 Shell) = <0.8 (Unreadable on Chart)-Unacceptable
F, (2 Shells) = 0.95 Use Two Shells,
oat
CALCULATION OF F,,
* Since This Technique is Applicable Only to
the Case of Straight-Line Heat Release, How
Do We Estimate Number of Shells and MTD,
for Other Cases?
NON-LINEAR HEAT RELEASE—MTD,
‘SUGGESTION FOR COMPLEX CASES.
SUCH AS REFORMER FEED/EFFLUENT
—Plot Tvs. Enthalpy
— Step Off to Get Minimum Number of Shelts
— Calculate MTD, for Each Shell (Discuss Later)
‘Temperature
Need Minimum ot 5 Shells
NON-LINEAR HEAT RELEASE—MTD,
SUGGESTION FOR CONDENSERS
—Plot the Condensing Curve
Assume Cold Side is Line:
Draw in Cold Side Flow Pattern
It Two Shells, Assume Equal Duties
‘TOP SHELL BOTTOM SHELL
Oesuperheatinge}-—————condenaing +} Sub--
a
MTD, FOR CONDENSERS (Cont’d.)
* Calculate the LMTD for Each Zone, Assuming That the Cold
‘Temperature in Each Zone Is the Average of the Inlet/Outlet
Cold Temperatures of the Shell in Which the Zone Occurs
(See Graph)
* Then Weight the Overall MTD, as Follows:
Qtotal
MTD, (Weighted) = Gone1 _Gzonea — Guanes |, Gzoned
tmTo, * LMTo, * LMTD, * LMTD,
a2
HEAT TRANSFER COEFFICIENTS
+ Film Coefficients are Relatively Easy to Estimate:
They are a Function of
+ Reynolds Number _
C,
+ Prandtl Number Shi
‘See Addendum 4.01
+ Similarly, Pressure Drop is a Function of Reynolds
Number and Length of Flow Path. ?
26
HEAT TRANSFER COEFFICIENTS (Cont’d.)
* The Handouts Just Examined are Suitable ONLY for Estimates
of Coefficients.
‘* For Detailed Coefficients on Which to Base the Purchase of
an Exchanger, Detailed Computer Calculations are Necessary.
* Detailed Computer Calculations Examine the Effects of Many
Other Parameters, Particularly Shell-Side Effects Such as
Channelling and Baffle Leakage.
az7
EXCHANGER DESIGN PROCEDURE
First Need to Know:
* Permissible Tube Sizes—Diameter, Gauge, Length.
(Frequently Set by Refinery Maintenance Department)
The Appropriate Tube Material for the Service
The Allowable System Pressure Drops for Each Stream.
EXCHANGER DESIGN PROCEDURE (Cont’d.)
(1) Assume an Overall Heat Transfer Coefficient (U,) and
Calculate Area,
(2) Using the Required Tube Size and Length, Calculate
the Number of Tubes,
(3) Using a “Reasonable” Tube-Side Velocity (2-15 Ft/s),
Calculate the Tube Side Flow Area Required:
_ fs
.
(4) Determine the EVEN Number of Tube Passes Which
Will Most Closely Approximate the Needed Flow Area
329
Vous aimerez peut-être aussi
- Process Control FundamentalsDocument59 pagesProcess Control Fundamentalsaanouar77Pas encore d'évaluation
- Section 10 - CompressorsDocument69 pagesSection 10 - CompressorsChakerZagroubaPas encore d'évaluation
- Section 08 - DrumsDocument66 pagesSection 08 - Drumsaldoacss_148400122Pas encore d'évaluation
- DCs of Water Tank PlatformDocument16 pagesDCs of Water Tank PlatformChakerZagroubaPas encore d'évaluation
- Design of Pressure Vessel (Int & Ext)Document394 pagesDesign of Pressure Vessel (Int & Ext)api-3824026100% (8)
- Section 06 - PumpsDocument28 pagesSection 06 - Pumpssaadashfaq100% (1)
- Basic Data Sources and Distillation FundamentalsDocument27 pagesBasic Data Sources and Distillation FundamentalsChakerZagroubaPas encore d'évaluation
- Vertical Coalescer Separators For API-1581 Category C Type SDocument2 pagesVertical Coalescer Separators For API-1581 Category C Type SGaluh AjengPas encore d'évaluation
- PVElite Manual PDFDocument738 pagesPVElite Manual PDFVictor Jesus Aragon Peñarrieta100% (3)
- PSV For Centrifugal PumpsDocument2 pagesPSV For Centrifugal PumpsChakerZagroubaPas encore d'évaluation
- Density ChangeDocument3 pagesDensity ChangeChakerZagroubaPas encore d'évaluation
- Distillation The TheoryDocument133 pagesDistillation The TheoryFrank Quijada100% (1)
- 54 Tank Test MeasurementsDocument5 pages54 Tank Test MeasurementsChakerZagroubaPas encore d'évaluation
- ADG 003B Design Model Estimation Common FactorDocument13 pagesADG 003B Design Model Estimation Common FactorChakerZagroubaPas encore d'évaluation
- CRF250 Shim CalcDocument2 pagesCRF250 Shim CalcChakerZagroubaPas encore d'évaluation
- Total Process Engineering ManualDocument260 pagesTotal Process Engineering Manualmusabammadkhan86% (7)
- 1252106057bit HydraulicsTFADocument2 pages1252106057bit HydraulicsTFAChakerZagroubaPas encore d'évaluation
- Scrubber Design (Packed Column)Document10 pagesScrubber Design (Packed Column)alinaveed198367% (3)
- Storage Tank Normal Venting Capacity Calculations PDFDocument1 pageStorage Tank Normal Venting Capacity Calculations PDFNathan MoralesPas encore d'évaluation
- Blind Flange Design Calculations - by Abdel Halim GalalaDocument6 pagesBlind Flange Design Calculations - by Abdel Halim GalalaNirmalraj Manoharan67% (3)
- Instruction Flowchart For Using The 2Phaserelief0000.Xls Worksheet (User Manual Is Also Available)Document14 pagesInstruction Flowchart For Using The 2Phaserelief0000.Xls Worksheet (User Manual Is Also Available)ChakerZagroubaPas encore d'évaluation
- Relief Valve Version 1Document37 pagesRelief Valve Version 1ChakerZagrouba100% (2)
- Rotary Vacuum FilterDocument2 pagesRotary Vacuum FilterWade ColemanPas encore d'évaluation
- Process CalculationsDocument225 pagesProcess CalculationsIsabel Justiniano Olivera86% (22)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)