Académique Documents
Professionnel Documents
Culture Documents
Equivalent Conductivity of Electrolytes in Aqueous Solution: Petr Vany Sek
Transféré par
antonioTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Equivalent Conductivity of Electrolytes in Aqueous Solution: Petr Vany Sek
Transféré par
antonioDroits d'auteur :
Formats disponibles
Equivalent Conductivity of Electrolytes In Aqueous Solution
Petr Vanýsek
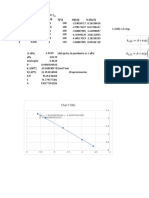
This table gives the equivalent (molar) conductivity Λ at 25 °C For a solution at 25 °C and both cation and anion with charge
for some common electrolytes in aqueous solution at concentra- |1|, the constants are A = 60.20 and B = 0.229. Λ° can be found
tions up to 0.1 mol/L. The units of Λ are 10–4 m2 S mol–1. from the next table, “Ionic Conductivity and Diffusion at Infinite
For very dilute solutions, the equivalent conductivity for any elec- Dilution.” The equation is reliable for c < 0.001 mol/L; with higher
trolyte of concentration c can be approximately calculated using the concentration the error increases.
Debye–Hückel–Onsager equation, which can be written for a sym-
metrical (equal charge on cation and anion) electrolyte as
Λ = Λ° – (A + BΛ°)c1/2
Concentration (mol/L)
Compound Infinite dilution 0.0005 0.001 0.005 0.01 0.02 0.05 0.1
Λ° Λ (10–4 m2 S mol–1)
AgNO3 133.29 131.29 130.45 127.14 124.70 121.35 115.18 109.09
1/2BaCl2 139.91 135.89 134.27 127.96 123.88 119.03 111.42 105.14
1/2CaCl2 135.77 131.86 130.30 124.19 120.30 115.59 108.42 102.41
1/2Ca(OH)2 258 — — 233 226 214 — —
CuSO4 133.6 121.6 115.20 94.02 83.08 72.16 59.02 50.55
HCl 425.95 422.53 421.15 415.59 411.80 407.04 398.89 391.13
KBr 151.9 149.8 148.9 146.02 143.36 140.41 135.61 131.32
KCl 149.79 147.74 146.88 143.48 141.20 138.27 133.30 128.90
KClO4 139.97 138.69 137.80 134.09 131.39 127.86 121.56 115.14
1/3K3Fe(CN)6 174.5 166.4 163.1 150.7 — — — —
1/4K4Fe(CN)6 184 — 167.16 146.02 134.76 122.76 107.65 97.82
KHCO3 117.94 116.04 115.28 112.18 110.03 107.17 — —
KI 150.31 148.2 143.32 144.30 142.11 139.38 134.90 131.05
KIO4 127.86 125.74 124.88 121.18 118.45 114.08 106.67 98.2
KNO3 144.89 142.70 141.77 138.41 132.75 132.34 126.25 120.34
KMnO4 134.8 132.7 131.9 — 126.5 — — 113
KOH 271.5 — 234 230 228 — 219 213
KReO4 128.20 126.03 125.12 121.31 118.49 114.49 106.40 97.40
1/3LaCl3 145.9 139.6 137.0 127.5 121.8 115.3 106.2 99.1
LiCl 114.97 113.09 112.34 109.35 107.27 104.60 100.06 95.81
LiClO4 105.93 104.13 103.39 100.52 98.56 96.13 92.15 88.52
1/2MgCl2 129.34 125.55 124.15 118.25 114.49 109.99 103.03 97.05
NH4Cl 149.6 147.5 146.7 143.9 141.21 138.25 133.22 128.69
NaCl 126.39 124.44 123.68 120.59 118.45 115.70 111.01 106.69
NaClO4 117.42 115.58 114.82 111.70 109.54 106.91 102.35 98.38
NaI 126.88 125.30 124.19 121.19 119.18 116.64 112.73 108.73
NaOOCCH3 91.0 89.2 88.5 85.68 83.72 81.20 76.88 72.76
NaOH 247.7 245.5 244.6 240.7 237.9 — — —
Na picrate 80.45 78.7 78.6 75.7 73.7 — 66.3 61.8
1/2Na2SO4 129.8 125.68 124.09 117.09 112.38 106.73 97.70 89.94
1/2SrCl2 135.73 131.84 130.27 124.18 120.23 115.48 108.20 102.14
ZnSO4 132.7 121.3 114.47 95.44 84.87 74.20 61.17 52.61
5-76
Vous aimerez peut-être aussi
- 010 - Permit To Work Procedure Feb 2013Document61 pages010 - Permit To Work Procedure Feb 2013vdeviv100% (1)
- Body FluidsDocument10 pagesBody FluidsShashwat Jindal100% (1)
- Example Calculations of Chlorine DosageDocument5 pagesExample Calculations of Chlorine DosageMuammar QadafiPas encore d'évaluation
- 01 PDFDocument51 pages01 PDFantonioPas encore d'évaluation
- Reactor DesignDocument31 pagesReactor DesignMortada OthmanPas encore d'évaluation
- Wolfson Eup3 Ch15 Test BankDocument16 pagesWolfson Eup3 Ch15 Test Bankifghelpdesk100% (1)
- STD 154 PDFDocument55 pagesSTD 154 PDFDiwakar Nigam100% (1)
- Physical Properties of Sucrose SolutionDocument23 pagesPhysical Properties of Sucrose Solutionbùi tuấn tùngPas encore d'évaluation
- CaCl2 TableDocument1 pageCaCl2 TablelatnrythmzPas encore d'évaluation
- 2019 MFG Industries India 667Document6 pages2019 MFG Industries India 667Uday kumarPas encore d'évaluation
- Debit Banjir BulunganDocument54 pagesDebit Banjir BulunganNendi SubaktiPas encore d'évaluation
- 13 3-8 CSG TallyDocument7 pages13 3-8 CSG TallyGPCPas encore d'évaluation
- SECOP R600a CompressorsDocument8 pagesSECOP R600a Compressorshermit44535Pas encore d'évaluation
- Cod No TK/KG WT/KG Cost/kg Spec/kg ML TK/LTR WT/MLDocument6 pagesCod No TK/KG WT/KG Cost/kg Spec/kg ML TK/LTR WT/MLFazlul HoquePas encore d'évaluation
- Brix Wine ChartDocument4 pagesBrix Wine ChartreddyyyvPas encore d'évaluation
- SL - No 1 2 3 4 5 6 7 8 9 Oxides MPD-11 TPD-06 VPD-3 Chpd-01 NSKD - 06 VKPD - 03 CHPD - 03 NSKD - 07 Kopd - 10Document2 pagesSL - No 1 2 3 4 5 6 7 8 9 Oxides MPD-11 TPD-06 VPD-3 Chpd-01 NSKD - 06 VKPD - 03 CHPD - 03 NSKD - 07 Kopd - 10vanithaPas encore d'évaluation
- DBQ Workshop WritingDocument2 pagesDBQ Workshop WritingAshwin ChandraPas encore d'évaluation
- Van Der Waals CoefficientsDocument1 pageVan Der Waals CoefficientsHaden GonzalezPas encore d'évaluation
- Thermistor Resistance TableDocument1 pageThermistor Resistance TablePin KisPas encore d'évaluation
- Equation Sheet Exam2Document2 pagesEquation Sheet Exam2Ashwin ChandraPas encore d'évaluation
- External Top Chord DL LL WL WR DL+LL DL+WRDocument4 pagesExternal Top Chord DL LL WL WR DL+LL DL+WRAnonymous P1iMibPas encore d'évaluation
- Atomic and Nuclear Properties of Materials: Z A hZ/Ai De/dx - X (G/CM) (CM) (G/CM) (G/') 1) ×10Document2 pagesAtomic and Nuclear Properties of Materials: Z A hZ/Ai De/dx - X (G/CM) (CM) (G/CM) (G/') 1) ×10MohammadPas encore d'évaluation
- APPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CDocument7 pagesAPPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CJenny ZevallosPas encore d'évaluation
- CRC Handbook KivonatDocument12 pagesCRC Handbook KivonatPresslly Gebol LopezPas encore d'évaluation
- Tech. DataDocument15 pagesTech. DataNavnit VishnoiPas encore d'évaluation
- This Is Dumb 2Document3 pagesThis Is Dumb 2Jacob ForscythePas encore d'évaluation
- WWTP & STP DesignDocument16 pagesWWTP & STP DesignJanzzen CrudaPas encore d'évaluation
- Ce - Notes NSCP 2015Document142 pagesCe - Notes NSCP 2015Justin Kurt TesiornaPas encore d'évaluation
- Eq - KPI W25Document27 pagesEq - KPI W25Ho dinh TranPas encore d'évaluation
- Tugas BesarDocument8 pagesTugas BesarFajar SatriaPas encore d'évaluation
- Formulario - Q1022.800-2020-LastDocument1 pageFormulario - Q1022.800-2020-LastAna Pau CerecedoPas encore d'évaluation
- 2.5 Mass Spectrometry: 2 Summary TablesDocument27 pages2.5 Mass Spectrometry: 2 Summary TablesJoz Mercado TinocoPas encore d'évaluation
- HRU Unit Base Working FinalDocument8 pagesHRU Unit Base Working FinalManish GautamPas encore d'évaluation
- 180724-27 WsocDocument67 pages180724-27 WsocOsqi C. BlancoPas encore d'évaluation
- Flotation Tests (NH 4) 2SO4 and NaHSDocument6 pagesFlotation Tests (NH 4) 2SO4 and NaHSJerry TshimonaPas encore d'évaluation
- Name: Student ID Number: Section Number:: Version A KeyDocument2 pagesName: Student ID Number: Section Number:: Version A KeyAileen LiangPas encore d'évaluation
- Thermal Conductivity (SI)Document6 pagesThermal Conductivity (SI)Juan Antonio SánchezPas encore d'évaluation
- Balances Metalurgicos JohelDocument3 pagesBalances Metalurgicos JohelJohel Bautista HernandezPas encore d'évaluation
- Cabeza Conc Cobre Conc Plomo Conc Zinc Relave: para Tres ProductosDocument3 pagesCabeza Conc Cobre Conc Plomo Conc Zinc Relave: para Tres ProductosJohel Bautista HernandezPas encore d'évaluation
- Bab 4Document8 pagesBab 4Attaya SalsabillaPas encore d'évaluation
- Copia de Fao-PmonDocument8 pagesCopia de Fao-PmonCarlos MuPas encore d'évaluation
- Periodic Table AP ChemDocument1 pagePeriodic Table AP ChemJoshua KimPas encore d'évaluation
- 1 Appendix A. Properties of The Elements: HHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHDocument4 pages1 Appendix A. Properties of The Elements: HHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHtarek moahmoud khalifaPas encore d'évaluation
- Naocl Examples PDFDocument5 pagesNaocl Examples PDFBasu RayalaPas encore d'évaluation
- Water % Used For Cement TestDocument1 pageWater % Used For Cement Testसागर फुकटPas encore d'évaluation
- ) of Pure Sucrose Solutions) of Impure Sucrose Solutions: DS DSDocument23 pages) of Pure Sucrose Solutions) of Impure Sucrose Solutions: DS DSSamuel GermatusPas encore d'évaluation
- Diagram T-X-YDocument5 pagesDiagram T-X-YNafilah Insan BestariPas encore d'évaluation
- Reducciones de Energia Eolica y Solar en El SEN Diciembre 2022Document20 pagesReducciones de Energia Eolica y Solar en El SEN Diciembre 2022Diego Marquez PadillaPas encore d'évaluation
- Hidrograf Banjir Metode Nakayasu: Daerah Aliran Sungai LimbotoDocument6 pagesHidrograf Banjir Metode Nakayasu: Daerah Aliran Sungai LimbotoBarry LabdulPas encore d'évaluation
- RTD Reference Tables: Return To MenuDocument22 pagesRTD Reference Tables: Return To Menuعبدالحميد عبدالغفار الدرديريPas encore d'évaluation
- Initial Concentrati On, C Half-Life, T (Gmol L) : Chart TitleDocument4 pagesInitial Concentrati On, C Half-Life, T (Gmol L) : Chart TitleCamila TorresPas encore d'évaluation
- Acetone - Methanol, ChloroformDocument6 pagesAcetone - Methanol, ChloroformAlejandra InsuastyPas encore d'évaluation
- Cálculos TriaxialDocument46 pagesCálculos TriaxialJose Andres Duitama ParraPas encore d'évaluation
- Calculo Hidraulico - Red de A.P.Document2 pagesCalculo Hidraulico - Red de A.P.Alonso Juarez MayangaPas encore d'évaluation
- BM Gullhosp1Document22 pagesBM Gullhosp1Aly Arquillano JrPas encore d'évaluation
- Nocal IDocument2 pagesNocal IIsabel España MPas encore d'évaluation
- CHM2000 General Chemistry: Faculty of Agriculture and Food ScienceDocument2 pagesCHM2000 General Chemistry: Faculty of Agriculture and Food ScienceLEE PEI XIAN / UPMPas encore d'évaluation
- Data ICP PART 1Document3 pagesData ICP PART 1FahmiRamdanPas encore d'évaluation
- Periodic Table of Chemical ElementsDocument1 pagePeriodic Table of Chemical ElementsMihaiPas encore d'évaluation
- Aluminum Cost DataDocument18 pagesAluminum Cost DataArchana RaghavendraPas encore d'évaluation
- Chemistry SL P1Document11 pagesChemistry SL P1Juan Fernando Velasco ForeroPas encore d'évaluation
- Calcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WaterDocument6 pagesCalcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WateraliPas encore d'évaluation
- Tank DimDocument5 pagesTank Dimiqramoyale022Pas encore d'évaluation
- All About MachineDocument7 pagesAll About MachineTulus PrasetyoPas encore d'évaluation
- Viper-Skin Technical Binder (KR - 7.6.10)Document47 pagesViper-Skin Technical Binder (KR - 7.6.10)antonioPas encore d'évaluation
- t2 PDFDocument1 paget2 PDFantonioPas encore d'évaluation
- 05 29 92Document26 pages05 29 92antonioPas encore d'évaluation
- Units For Magnetic Properties: Quantity Symbol Gaussian & Cgs Emu Conversion Factor, C Si & Rationalized MksDocument1 pageUnits For Magnetic Properties: Quantity Symbol Gaussian & Cgs Emu Conversion Factor, C Si & Rationalized MksantonioPas encore d'évaluation
- t2 PDFDocument1 paget2 PDFantonioPas encore d'évaluation
- Common Spurious Signals Observed in Mass Spectrometers ReferenceDocument1 pageCommon Spurious Signals Observed in Mass Spectrometers ReferenceantonioPas encore d'évaluation
- Properties of Amino Acids: M T, PK, PKDocument2 pagesProperties of Amino Acids: M T, PK, PKantonioPas encore d'évaluation
- Fracturamiento de Rocas Mediante Tecnolo PDFDocument16 pagesFracturamiento de Rocas Mediante Tecnolo PDFantonioPas encore d'évaluation
- Index Of Refraction Of Air: N = Λ P (1 + P (60 .1 - 0 .972T) × 10Document1 pageIndex Of Refraction Of Air: N = Λ P (1 + P (60 .1 - 0 .972T) × 10antonioPas encore d'évaluation
- Percentage Points, Student'S T-Distribution: Normal Probability FunctionDocument1 pagePercentage Points, Student'S T-Distribution: Normal Probability FunctionantonioPas encore d'évaluation
- 12 20 86Document9 pages12 20 86antonioPas encore d'évaluation
- Melting, Boiling, Triple, and Critical Points of The ElementsDocument3 pagesMelting, Boiling, Triple, and Critical Points of The ElementsantonioPas encore d'évaluation
- 12 01 91Document4 pages12 01 91antonioPas encore d'évaluation
- Preface: HandbookDocument3 pagesPreface: HandbookantonioPas encore d'évaluation
- Electrical Conductivity Of Water Reference: Conductivity In Μs/Cm At The Indicated PressureDocument1 pageElectrical Conductivity Of Water Reference: Conductivity In Μs/Cm At The Indicated PressureantonioPas encore d'évaluation
- Cryoscopic Constants For Calculation of Freezing Point DepressionDocument1 pageCryoscopic Constants For Calculation of Freezing Point DepressionantonioPas encore d'évaluation
- Solubility of Hydrocarbons in Seawater: S/PPM (Mass) 10 MDocument2 pagesSolubility of Hydrocarbons in Seawater: S/PPM (Mass) 10 MantonioPas encore d'évaluation
- Nomenclature For Organic Polymers: Robert B. Fox and Edward S. WilksDocument4 pagesNomenclature For Organic Polymers: Robert B. Fox and Edward S. WilksantonioPas encore d'évaluation
- 08 60 93Document8 pages08 60 93antonioPas encore d'évaluation
- Tables Relocated or Removed From CRC Handbook of Chemistry and Physics, 71st Through 93rd EditionsDocument2 pagesTables Relocated or Removed From CRC Handbook of Chemistry and Physics, 71st Through 93rd EditionsantonioPas encore d'évaluation
- Electron Stopping Powers: Cedric J. PowellDocument2 pagesElectron Stopping Powers: Cedric J. PowellantonioPas encore d'évaluation
- The Factorial Function The Gamma FunctionDocument2 pagesThe Factorial Function The Gamma FunctionantonioPas encore d'évaluation
- Refractive Index and Transmittance of Representative Glasses ReferencesDocument1 pageRefractive Index and Transmittance of Representative Glasses ReferencesantonioPas encore d'évaluation
- Practical PH Measurements On Natural Waters: A. K. Covington and W. DavisonDocument2 pagesPractical PH Measurements On Natural Waters: A. K. Covington and W. DavisonantonioPas encore d'évaluation
- Infrared Correlation ChartsDocument5 pagesInfrared Correlation ChartsantonioPas encore d'évaluation
- Moment of Inertia For Various Bodies of MassDocument1 pageMoment of Inertia For Various Bodies of MassantonioPas encore d'évaluation
- Nomenclature For Chemical Compounds: Macromolecular ChemistryDocument1 pageNomenclature For Chemical Compounds: Macromolecular ChemistryantonioPas encore d'évaluation
- Codata Key Values For Thermodynamics: H° Column For An Element Indicates The ReferDocument3 pagesCodata Key Values For Thermodynamics: H° Column For An Element Indicates The ReferantonioPas encore d'évaluation
- Enthalpy of Dilution of Acids: H, The Negative of TheDocument1 pageEnthalpy of Dilution of Acids: H, The Negative of TheantonioPas encore d'évaluation
- Worksheet - Experiment 9 MilkDocument2 pagesWorksheet - Experiment 9 MilkYuraPas encore d'évaluation
- Waqia Karbala Tareekh Ke Aine Me - Urdu BookDocument86 pagesWaqia Karbala Tareekh Ke Aine Me - Urdu BookABBAS ZAIDIPas encore d'évaluation
- Synthetic PolymerDocument7 pagesSynthetic PolymerJunyipp Chai50% (2)
- Tallentex Class 10 # 2022-23Document20 pagesTallentex Class 10 # 2022-23Sreeja SujithPas encore d'évaluation
- SPT2021 Butadiene AAMDocument29 pagesSPT2021 Butadiene AAMTasneem MPas encore d'évaluation
- Zero Door SealsDocument120 pagesZero Door SealsChen Yaohui VictorPas encore d'évaluation
- Guidelines For The Establishment of Pharmaceutical Retail BusinessDocument19 pagesGuidelines For The Establishment of Pharmaceutical Retail BusinessFranc100% (1)
- Catalogue Product M-Plus FilterDocument40 pagesCatalogue Product M-Plus FilterAdrian Samuel ThenochPas encore d'évaluation
- O-Rings and SealsDocument10 pagesO-Rings and SealsManuel CarvalloPas encore d'évaluation
- Chemical Raw Material PDFDocument4 pagesChemical Raw Material PDFA MahmoodPas encore d'évaluation
- Safety Data Sheet: - Made Under Licence of European Label System® Msds Software From Infodyne - HTTPDocument5 pagesSafety Data Sheet: - Made Under Licence of European Label System® Msds Software From Infodyne - HTTPManish KumarPas encore d'évaluation
- Material Data Sheet NC 259 Sn100c Solder Paste Rev 1Document3 pagesMaterial Data Sheet NC 259 Sn100c Solder Paste Rev 1vkmsPas encore d'évaluation
- PagesDocument1 pagePagesEnedis Pimentel0% (1)
- Chemical Equilibrium (Reversible Reactions)Document22 pagesChemical Equilibrium (Reversible Reactions)Anthony AbesadoPas encore d'évaluation
- Ecomax Front Loading DishwashersDocument4 pagesEcomax Front Loading Dishwashersrichard9982Pas encore d'évaluation
- CLS Aipmt 16 17 XI Che Study Package 3 SET 2 Chapter 10Document18 pagesCLS Aipmt 16 17 XI Che Study Package 3 SET 2 Chapter 10kalloli67% (3)
- Yellow Passion Fruits Headspace Werkhoff1998Document18 pagesYellow Passion Fruits Headspace Werkhoff1998mapollo2000Pas encore d'évaluation
- Tablas TermodinámicasDocument5 pagesTablas TermodinámicasSarahí CabreraPas encore d'évaluation
- Chapter 10Document42 pagesChapter 10Teddy Matthew AudleyPas encore d'évaluation
- Measurements of Surface TensionDocument11 pagesMeasurements of Surface TensionHema ParasuramanPas encore d'évaluation
- Gliesse Plant PresentationDocument20 pagesGliesse Plant PresentationNeel Gliesse PharmaPas encore d'évaluation
- AHMSW 8 Fiche de PhaseDocument12 pagesAHMSW 8 Fiche de PhaseBOUBAKER LOGBIPas encore d'évaluation
- bw-G1030 USDocument24 pagesbw-G1030 USEuojrPas encore d'évaluation
- BHDT Fertilizer Engl Neu Online PDFDocument8 pagesBHDT Fertilizer Engl Neu Online PDFRAVINDR.KPas encore d'évaluation