Académique Documents
Professionnel Documents
Culture Documents
Standardreductionpotentials PDF
Transféré par
Badrus Syamsi0 évaluation0% ont trouvé ce document utile (0 vote)
21 vues1 pageThis document lists standard reduction potentials (E0) for various half-cell reactions involving oxidation and reduction. It provides the half-reactions, E0 values in volts, and indicates that ionic concentrations are at 1M and temperature is 250C. The most positive E0 values belong to oxidizing agents like F2, MnO4-, and Cl2 which readily gain electrons. The most negative E0 values belong to reactive metals like Li, Ca, and Ba that readily lose electrons to be reduced.
Description originale:
Titre original
standardreductionpotentials.pdf
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentThis document lists standard reduction potentials (E0) for various half-cell reactions involving oxidation and reduction. It provides the half-reactions, E0 values in volts, and indicates that ionic concentrations are at 1M and temperature is 250C. The most positive E0 values belong to oxidizing agents like F2, MnO4-, and Cl2 which readily gain electrons. The most negative E0 values belong to reactive metals like Li, Ca, and Ba that readily lose electrons to be reduced.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
21 vues1 pageStandardreductionpotentials PDF
Transféré par
Badrus SyamsiThis document lists standard reduction potentials (E0) for various half-cell reactions involving oxidation and reduction. It provides the half-reactions, E0 values in volts, and indicates that ionic concentrations are at 1M and temperature is 250C. The most positive E0 values belong to oxidizing agents like F2, MnO4-, and Cl2 which readily gain electrons. The most negative E0 values belong to reactive metals like Li, Ca, and Ba that readily lose electrons to be reduced.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 1
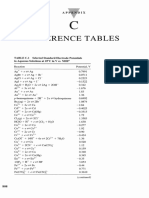
Standard Reduction Potentials of Half-Cells
(Ionic concentrations are at 1M in water @ 250 C)
Oxidizing Agents Reducing Agents E0 (Volts)
F2(g) + 2e- → 2F-(aq) +2.87
PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- → PbSO4(s) + 2H2O(A) +1.69
MnO4-(aq) + 8H+(aq) + 5e- → Mn2+(aq) + 4H2O(A) +1.51
Au3+(aq) + 3e- → Au(s) +1.50
ClO4-(aq) + 8H+(aq) + 8e- → Cl-(aq) + 4H2O(A) +1.39
Cl2(g) + 2e- → 2Cl-(aq) +1.36
Cr2O72-(aq) + 14H+(aq) + 6e- → 2Cr3+(aq) + 7H2O(A) +1.33
2HNO2(aq) + 4H+(aq) + 4e- → N2O(g) + 3H2O(A) +1.30
O2(g) + 4H+(aq) + 4e- → 2H2O(A) +1.23
+
MnO2(s) + 4H (aq) + 2e -
→ 2+
Mn (aq) + 2H2O(A) +1.22
Br2 (aq) + 2e -
→ -
2Br (aq) +1.07
Hg2+(aq) + 2e- → Hg(A) +0.85
ClO-(aq) + H2O(A) + 2e- → Cl-(aq) + 2OH-(aq) +0.84
Ag (aq) + e + -
→ Ag(s) +0.80
-
NO3 (aq) + 2H+(aq) + e- → NO2(g) + H2O(A) +0.80
Fe3+(aq) + e- → Fe2+(aq) +0.77
O2(g) + 2H+(aq) + 2e- → H2O2(A) +0.70
I2(s) + 2e- → 2I-(aq) +0.54
O2(g) + 2H2O(A) + 4e- → 4OH-(aq) +0.40
Cu2+(aq) + 2e- → Cu(s) +0.34
SO42-(aq) + 4H+(aq) + 2e- → H2SO3 (aq) + H2O(A) +0.17
Sn4+(aq) + 2e- → Sn2+(aq) +0.15
+
S(s) + 2H (aq) + 2e -
→ H2S (aq) +0.14
AgBr(s) + e -
→ Ag(s) + Br (aq) -
+0.07
+
2H (aq) + 2e -
→ H2(g) 0.00
2+
Pb (aq) + 2e -
→ Pb(s) -0.13
2+
Sn (aq) + 2e -
→ Sn(s) -0.14
AgI(s) + e -
→ -
Ag(s) + I (aq) -0.15
2+
Ni (aq) + 2e -
→ Ni(s) -0.26
2+
Co (aq) + 2e -
→ Co(s) -0.28
PbSO4(s) + 2e -
→ Pb(s) + SO4 (aq)2-
-0.36
+
Se(s) + 2H (aq) + 2e -
→ H2Se (aq) -0.40
2+
Cd (aq) + 2e -
→ Cd(s) -0.40
3+
Cr (aq) + e -
→ 2+
Cr (aq) -0.41
2+
Fe (aq) + 2e -
→ Fe(s) -0.45
-
NO2 (aq) + H2O(A) + e -
→ NO(g) + 2OH (aq) -
-0.46
Ag2S(s) + 2e- → 2Ag(s) + S2-(aq) -0.69
2+
Zn (aq) + 2e -
→ Zn(s) -0.76
2H2O(A) + 2e -
→ H2(g) + 2OH (aq) -
-0.83
Cr2+(aq) + 2e- → Cr(s) -0.91
Se(s) + 2e -
→ 2-
Se (aq) -0.92
SO42-(aq) + H2O(A) + 2e- → SO32-(aq) + 2OH-(aq) -0.93
Al3+(aq) + 3e- → Al(s) -1.66
2+
Mg (aq) + 2e -
→ Mg(s) -2.37
+
Na (aq) + e -
→ Na(s) -2.71
2+
Ca (aq) + 2e -
→ Ca(s) -2.87
2+
Ba (aq) + 2e -
→ Ba(s) -2.91
+
Li (aq) + e -
→ Li(s) -3.04
Vous aimerez peut-être aussi
- Aisi 4140 Alloy Steel (Uns g41400)Document4 pagesAisi 4140 Alloy Steel (Uns g41400)UmerPas encore d'évaluation
- 22 - The Nuclear AtomDocument10 pages22 - The Nuclear AtomEdgardo LeysaPas encore d'évaluation
- Copper Nickel Pipe Din86089 Eemua145Document1 pageCopper Nickel Pipe Din86089 Eemua145Araby GamalPas encore d'évaluation
- The Metallurgy of The Common Metals.Document650 pagesThe Metallurgy of The Common Metals.Richard.nlPas encore d'évaluation
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhPas encore d'évaluation
- STANDARD ELECTRODE POTENTIALS LISTDocument3 pagesSTANDARD ELECTRODE POTENTIALS LISTVishal PamnaniPas encore d'évaluation
- DebateDocument3 pagesDebatebbangeles1Pas encore d'évaluation
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaPas encore d'évaluation
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewPas encore d'évaluation
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaPas encore d'évaluation
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188Pas encore d'évaluation
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZPas encore d'évaluation
- Standard Reduction Potentials at 298KDocument3 pagesStandard Reduction Potentials at 298KjaverfrivPas encore d'évaluation
- Appendix EDocument1 pageAppendix EYrhon IbanezPas encore d'évaluation
- Standard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Document2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Mus'abIdriesAbuzetunPas encore d'évaluation
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoePas encore d'évaluation
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAcePas encore d'évaluation
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukePas encore d'évaluation
- Chemistry Databook WDocument24 pagesChemistry Databook Wdaemperor216Pas encore d'évaluation
- Redox Reaction Definition and Key ConceptsDocument68 pagesRedox Reaction Definition and Key ConceptsPHƯƠNG ĐẶNG YẾNPas encore d'évaluation
- Elements, Compounds and Molecules ExplainedDocument20 pagesElements, Compounds and Molecules ExplainedPevin De silvaPas encore d'évaluation
- Chemistry: Written Examination 2Document3 pagesChemistry: Written Examination 2Mohamed MawasPas encore d'évaluation
- E ValuesDocument1 pageE ValuesShania LoveresPas encore d'évaluation
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsunePas encore d'évaluation
- Standard Reduction PotentialsDocument3 pagesStandard Reduction Potentialscarina_yii9690Pas encore d'évaluation
- Standard Calulation For Potentials AcrossDocument2 pagesStandard Calulation For Potentials Acrossmurugan_kribhcoPas encore d'évaluation
- Standard potentials at 298 K tableDocument3 pagesStandard potentials at 298 K tabledavidPas encore d'évaluation
- Elc STD PotentialsDocument1 pageElc STD PotentialsArchita VPas encore d'évaluation
- HalfCellPoten TableDocument1 pageHalfCellPoten TableJeisson David Sanchez PInzonPas encore d'évaluation
- UNIT 2 Electrochemistry FinalDocument26 pagesUNIT 2 Electrochemistry FinalA HPas encore d'évaluation
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 pagesStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezPas encore d'évaluation
- UNIT 2 Electrochemistry FinalDocument25 pagesUNIT 2 Electrochemistry FinalPisces SandPas encore d'évaluation
- Reaksi Redox dan Potensial Standar Elektroda Logam dengan Iodin dan AirDocument2 pagesReaksi Redox dan Potensial Standar Elektroda Logam dengan Iodin dan Airherna watiPas encore d'évaluation
- SRP Table Chem DataDocument1 pageSRP Table Chem Dataapi-222503660Pas encore d'évaluation
- Nguyên tố Dạng oxi hoá +ne Dạng khử E, VDocument12 pagesNguyên tố Dạng oxi hoá +ne Dạng khử E, VNhat KhanhPas encore d'évaluation
- EMF SeriesDocument5 pagesEMF Seriesmike rosaPas encore d'évaluation
- Chem 2Document8 pagesChem 22021302095Pas encore d'évaluation
- Cations LabDocument4 pagesCations LabKayshalee BlackburnPas encore d'évaluation
- ElectrodeDocument2 pagesElectrodeThatcher PanchoPas encore d'évaluation
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimPas encore d'évaluation
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiPas encore d'évaluation
- Kims CopiesDocument17 pagesKims Copieszafarchem_iqbalPas encore d'évaluation
- Electrolysis of Aqueoues SolutionDocument1 pageElectrolysis of Aqueoues SolutionSharonPas encore d'évaluation
- High School Science - Redox ReactionsDocument12 pagesHigh School Science - Redox ReactionsPort of Long BeachPas encore d'évaluation
- Appendix 1: The Periodic Table of The ElementsDocument7 pagesAppendix 1: The Periodic Table of The ElementshassanPas encore d'évaluation
- Silo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsDocument40 pagesSilo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsAkash BhoiPas encore d'évaluation
- 3b Balancing Equations CGPDocument1 page3b Balancing Equations CGPKarina LeungPas encore d'évaluation
- Chemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryDocument31 pagesChemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryLakshmi SinghPas encore d'évaluation
- A2 Extension1 Electrochemistry and RedoxDocument10 pagesA2 Extension1 Electrochemistry and RedoxDavid MathewsPas encore d'évaluation
- Electrochemistry Revision-3 OnlineDocument10 pagesElectrochemistry Revision-3 Onlinetumimogotsi14Pas encore d'évaluation
- Sap 5Document22 pagesSap 5reza noviyantiPas encore d'évaluation
- Chem16 Experiment Chemical ChangesDocument1 pageChem16 Experiment Chemical ChangesDiyanikaPas encore d'évaluation
- ImageDocument2 pagesImageWinterSwiftZPas encore d'évaluation
- Chapter 5 Answers Practice Examples: ReductionDocument7 pagesChapter 5 Answers Practice Examples: ReductionEmre Enes EdizPas encore d'évaluation
- Zimsec JUNE2020MS3Document12 pagesZimsec JUNE2020MS3Tichafara Paul ShumbaPas encore d'évaluation
- 78 128Document51 pages78 128Anonymous qKeDFDPas encore d'évaluation
- E41ad 9d85Document18 pagesE41ad 9d85sayyed bassir ajellehPas encore d'évaluation
- CH 11 - Writing - Chemical - Equation - 1 - AnsDocument2 pagesCH 11 - Writing - Chemical - Equation - 1 - AnsOlivia LinPas encore d'évaluation
- 02 Neutralization Reactions Problem Set 2Document2 pages02 Neutralization Reactions Problem Set 2Jonghyun (Justin) YangPas encore d'évaluation
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsCamiloPas encore d'évaluation
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioPas encore d'évaluation
- Tabla Potencial Reduccion PDFDocument13 pagesTabla Potencial Reduccion PDFFóxel ArgPas encore d'évaluation
- As Equations: Lithium Monoxide (Contains O) Sodium Peroxide (Contains O) Potassium Superoxide (Contains O)Document4 pagesAs Equations: Lithium Monoxide (Contains O) Sodium Peroxide (Contains O) Potassium Superoxide (Contains O)arcmikePas encore d'évaluation
- Physics NoDocument14 pagesPhysics NosofiajamePas encore d'évaluation
- Sesi 7 Hukum KeplerDocument2 pagesSesi 7 Hukum KeplerBadrus SyamsiPas encore d'évaluation
- Ionones Acidity RankingDocument2 pagesIonones Acidity RankingBadrus SyamsiPas encore d'évaluation
- Ionones Acidity RankingDocument2 pagesIonones Acidity RankingBadrus SyamsiPas encore d'évaluation
- Olimpiade 23022020Document5 pagesOlimpiade 23022020Badrus SyamsiPas encore d'évaluation
- BornHaberProb2011 PDFDocument2 pagesBornHaberProb2011 PDFCicy IrnaPas encore d'évaluation
- 2.2. How Bonding and Structure Are Related To The Properties of SubstancesDocument3 pages2.2. How Bonding and Structure Are Related To The Properties of SubstancesBadrus SyamsiPas encore d'évaluation
- Ringkasan Broker-20200218Document6 pagesRingkasan Broker-20200218Badrus SyamsiPas encore d'évaluation
- Utul Ugm 2015.1Document3 pagesUtul Ugm 2015.1Badrus SyamsiPas encore d'évaluation
- Company Profile Tribhakti InspektamaDocument12 pagesCompany Profile Tribhakti InspektamaBadrus SyamsiPas encore d'évaluation
- Naskah Soal UN Kimia SMA 2014 Paket 1Document15 pagesNaskah Soal UN Kimia SMA 2014 Paket 1Katiman, S.PdPas encore d'évaluation
- Food Dfood Dyes Rainbow of RisksDocument68 pagesFood Dfood Dyes Rainbow of Riskscajunkid01Pas encore d'évaluation
- Bahaya Zat Warna Sintesis PDFDocument68 pagesBahaya Zat Warna Sintesis PDFBadrus SyamsiPas encore d'évaluation
- 10 1016@j Apsusc 2016 08 078Document7 pages10 1016@j Apsusc 2016 08 078Badrus SyamsiPas encore d'évaluation
- 99 Ways To Improve Your English PDFDocument81 pages99 Ways To Improve Your English PDFHakan Citlak100% (1)
- Atomic Absorption Determination of Zinc and Copper in A MultivitaminDocument7 pagesAtomic Absorption Determination of Zinc and Copper in A Multivitaminlkomninos2221Pas encore d'évaluation
- Extraction of MetalsDocument2 pagesExtraction of Metalsdan964Pas encore d'évaluation
- Group 17 - Inorganic ChemistryDocument7 pagesGroup 17 - Inorganic ChemistryDefaults rulezPas encore d'évaluation
- Hellagrolip NutrActive Brochure enDocument2 pagesHellagrolip NutrActive Brochure enBashir A. SialPas encore d'évaluation
- 2019 Specimen Paper 2Document18 pages2019 Specimen Paper 2Susanna NgPas encore d'évaluation
- CHE122 Engineering Chemistry Laboratory 16842::Dr. Tanay Pramanik 0.0 0.0 2.0 1.0 1:discipline Knowledge, 2:skill EnhancementDocument5 pagesCHE122 Engineering Chemistry Laboratory 16842::Dr. Tanay Pramanik 0.0 0.0 2.0 1.0 1:discipline Knowledge, 2:skill EnhancementSandeep KakranPas encore d'évaluation
- Calcium and Magnesium in Water: Standard Test Methods ForDocument9 pagesCalcium and Magnesium in Water: Standard Test Methods ForasmybablooPas encore d'évaluation
- Chem MoleDocument29 pagesChem Mole叶子临Pas encore d'évaluation
- Analysis of Nitric Acid in The Presence of Hydrofluoric AcidDocument4 pagesAnalysis of Nitric Acid in The Presence of Hydrofluoric Acidmohsen_267Pas encore d'évaluation
- Chlorine Dioxide As A DisinfectantDocument2 pagesChlorine Dioxide As A DisinfectantWill XiaPas encore d'évaluation
- How Is Helium Made - HowStuffWorksDocument3 pagesHow Is Helium Made - HowStuffWorksait oubella marouanePas encore d'évaluation
- LECTURE Naming CompoundsDocument63 pagesLECTURE Naming CompoundsCheri BulahanPas encore d'évaluation
- Chemical Reactions Equations Chapter-Wise Important Questions Class 10 Science - LearnCBSE - in PDFDocument23 pagesChemical Reactions Equations Chapter-Wise Important Questions Class 10 Science - LearnCBSE - in PDFmsu chennaiPas encore d'évaluation
- Metales Pesados - Met. Magnesio BN12000001456Document1 pageMetales Pesados - Met. Magnesio BN12000001456Alexis F.G.Pas encore d'évaluation
- Phosphate Rock Processing and Fertilizers Production at Al-Qaim Fertilizers Complex, IraqDocument12 pagesPhosphate Rock Processing and Fertilizers Production at Al-Qaim Fertilizers Complex, IraqEndah SaraswatiPas encore d'évaluation
- Sdfine ChemicalDocument262 pagesSdfine Chemicalchvar80Pas encore d'évaluation
- BIO - How Do You Tell The Difference Between Organic and Inorganic CompoundsDocument2 pagesBIO - How Do You Tell The Difference Between Organic and Inorganic CompoundshafsatutuPas encore d'évaluation
- Everything You Need to Know About Water PurificationDocument26 pagesEverything You Need to Know About Water PurificationRatulPas encore d'évaluation
- Dogs & CatsDocument2 pagesDogs & CatsavdpoortPas encore d'évaluation
- Advances and Challenges in Metal Sulfides - Selenides For Next-Generation Rechargeable Sodium Ion Batteries PDFDocument24 pagesAdvances and Challenges in Metal Sulfides - Selenides For Next-Generation Rechargeable Sodium Ion Batteries PDFLE Thi LyPas encore d'évaluation
- Separate Chemistry: Higher Tier in BoldDocument16 pagesSeparate Chemistry: Higher Tier in BoldzipperPas encore d'évaluation
- Wispeco Aluminium ProfilesDocument22 pagesWispeco Aluminium ProfilesPatrick153Pas encore d'évaluation
- TOPAS UserGuide V1 3 20151026a PDFDocument126 pagesTOPAS UserGuide V1 3 20151026a PDFRosa LourençoPas encore d'évaluation
- LAS No. 4 Formation of Elements Heavier Than IronDocument2 pagesLAS No. 4 Formation of Elements Heavier Than IronWarren OlemberioPas encore d'évaluation
- SCH3U Unit 1 - Chapter 1 - Section 1.3Document10 pagesSCH3U Unit 1 - Chapter 1 - Section 1.3Wenyin DaiPas encore d'évaluation
- JEE (Main + Advanced) : NURTURE COURSE: Classroom Contact ProgrammeDocument10 pagesJEE (Main + Advanced) : NURTURE COURSE: Classroom Contact ProgrammeYashSGPas encore d'évaluation
- ConclusionDocument3 pagesConclusionAbdul HakeemPas encore d'évaluation