Académique Documents
Professionnel Documents
Culture Documents
Mass Spectrometry Common Mass Spectra Fragments 2016
Transféré par
Aglaete AraújoCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Mass Spectrometry Common Mass Spectra Fragments 2016
Transféré par
Aglaete AraújoDroits d'auteur :
Formats disponibles
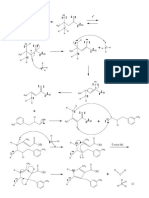
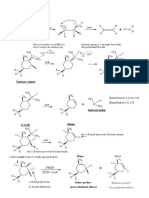
A GUIDE TO INTERPRETING MASS SPECTRA
Mass spectrometry is an analytical technique that allows us to measure the masses of atoms and molecules. The most important peak in a mass spectrum
is the molecular ion peak, which can be used to determine the mass of the molecule, but fragment ions can also provide information on chemical structure.
2 3 H H H
H
H
H
H Cl
H
H H
ELEC
1 TRO
M
H C H C C N C O C 2 peaks seen due to the 35Cl & C C C
AG H
37
Cl isotopes, in a 3:1 ratio due
N H H H H H to their natural abundance. H
H
ET
15 29 30 31 35/37 41
3:1
4
H H H H O H H O H H H H H
H H
H C C C H C C N C C N C
Cl C H C C C C
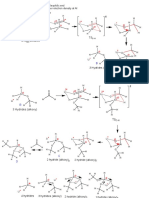
HOW MASS SPECTROMETRY WORKS H
H H H H H H H H H H H H
5 43 43 44 44 49/51 57
3:1
H H O H H H H O H H H H H H H H
The sample is introduced to the mass spectrometer. H
1 Only very small samples are required. A heater is often
present to vapourise the sample. H C C C N C C C H C O C H C C O C H C C C C C
H
H H H H H H H H H H H H H H
An electron gun ionises molecules in the sample by
2 knocking out electrons, producing positive ions. Some
molecules break into smaller ions & fragments.
57 58 59 59 71
H H
H H H O Br H H H H H H H
The positive ions generated are passed through an C C
3 electric field which accelerates them into a magnetic
field generated by an electromagnet.
H C C C C H C C
2 peaks seen due to the 79Br & H C C C C C C Br C
C C Br isotopes, in a 1:1 ratio due
81
H H H to their natural abundance. H H H H H H H
H H
As the positive ions pass through the magnetic field,
71 77 79/81 85 93/95
4 they are deflected. Lighter ions are deflected more
than heavier ions, as are those with higher charges.
1:1 1:1
Above are shown a selection of common fragment ions seen in mass spectra, along with their masses. Note that
The positive ions hit a charged plate & accept electrons, the structures shown are general representations, and it can also be possible for isomeric structures (those with the
5 creating a signal. The more ions that hit, the greater the
signal. The output is a complex stick diagram.
same constituent atoms, but a different structure) to cause the peaks in spectra. There are also many more fragments
possible than those shown, but knowledge of these should suffice to interpret spectra of most simple molecules.
© COMPOUND INTEREST 2015 - WWW.COMPOUNDCHEM.COM | Twitter: @compoundchem | Facebook: www.facebook.com/compoundchem
C This graphic is shared under a Creative Commons Attribution-NonCommercial-NoDerivatives licence. BY NC ND
Vous aimerez peut-être aussi
- Wagon WheelDocument2 pagesWagon WheeltievPas encore d'évaluation
- Mercruiser Service Manual #14 Alpha I Gen II Outdrives 1991-NewerDocument715 pagesMercruiser Service Manual #14 Alpha I Gen II Outdrives 1991-NewerM5Melo100% (10)
- Capricho Árabe - Arr. MarcinDocument4 pagesCapricho Árabe - Arr. MarcinRan TanPlanPas encore d'évaluation
- Aerospace MaterialsDocument68 pagesAerospace MaterialsAykut Üçtepe100% (1)
- Production ManagementDocument81 pagesProduction ManagementrrathorePas encore d'évaluation
- Isomerism: Definition-Structural Isomers: Same Molecular Formula Different Structures (Or Structural Formulae)Document13 pagesIsomerism: Definition-Structural Isomers: Same Molecular Formula Different Structures (Or Structural Formulae)Pedro Moreno de Souza100% (1)
- ASHRAE Fundamentals 2005 - SI Units - Extract of Tables PDFDocument40 pagesASHRAE Fundamentals 2005 - SI Units - Extract of Tables PDFSufian SarwarPas encore d'évaluation
- ULPTK Triplets WorkoutDocument14 pagesULPTK Triplets WorkoutMateusz WodzickiPas encore d'évaluation
- C 1252 PDFDocument5 pagesC 1252 PDFAgatha ShirleyPas encore d'évaluation
- Alkenes 1 QPDocument7 pagesAlkenes 1 QPHoaXPas encore d'évaluation
- Alkenes 1 QPDocument10 pagesAlkenes 1 QPanimecuPas encore d'évaluation
- Alkanes 4 QPDocument14 pagesAlkanes 4 QPBethPas encore d'évaluation
- Alkenes 2 QPDocument12 pagesAlkenes 2 QPemanPas encore d'évaluation
- Unit9 Organic Chemistry2Document1 pageUnit9 Organic Chemistry2uditi kalraPas encore d'évaluation
- TABORADA - Act8 (Ketones and Aldehydes)Document4 pagesTABORADA - Act8 (Ketones and Aldehydes)Justin Habaña TaboradaPas encore d'évaluation
- 3 StereochemisryDocument24 pages3 StereochemisryAlina TilekPas encore d'évaluation
- Nuevas Sustancias Ingles WebDocument126 pagesNuevas Sustancias Ingles WebMargari MargasPas encore d'évaluation
- Ibu Gram CantikDocument2 pagesIbu Gram CantikgramatolinaPas encore d'évaluation
- Alkenes 1 QPDocument7 pagesAlkenes 1 QPDewei LohPas encore d'évaluation
- CHZCHZ: Resonating ResonantingDocument5 pagesCHZCHZ: Resonating ResonantingPavneet GrewalPas encore d'évaluation
- Holi4 PDFDocument1 pageHoli4 PDFKAREN ROSAS GARCIAPas encore d'évaluation
- HOLI4Document1 pageHOLI4KAREN ROSAS GARCIAPas encore d'évaluation
- TCP - TH22Document1 pageTCP - TH22prasanth kutyPas encore d'évaluation
- Isomers PDFDocument2 pagesIsomers PDFGapor examPas encore d'évaluation
- CarbProtFat CompareDocument3 pagesCarbProtFat ComparePauline FrascaPas encore d'évaluation
- Let Invariant Rationale Discovery Inspire Graph Contrastive LearningDocument14 pagesLet Invariant Rationale Discovery Inspire Graph Contrastive LearningSicong CaoPas encore d'évaluation
- Alcanos - 4° SecDocument1 pageAlcanos - 4° SecSergio Curay OjedaPas encore d'évaluation
- Jaw Stabilization #1: Bryan Davis EOTW001Document2 pagesJaw Stabilization #1: Bryan Davis EOTW001Philippe KinnaerPas encore d'évaluation
- TCP - TH21Document1 pageTCP - TH21prasanth kutyPas encore d'évaluation
- Temple H H H H H H: Towards GopalapatnamDocument1 pageTemple H H H H H H: Towards GopalapatnamMahesh VamsiPas encore d'évaluation
- Ejercicios Axial EcuatorialDocument1 pageEjercicios Axial EcuatorialJulio Cesar Boada MartinezPas encore d'évaluation
- TCP - TH19Document1 pageTCP - TH19prasanth kutyPas encore d'évaluation
- 1,3 CycloDocument1 page1,3 CycloIsmail ZitouniPas encore d'évaluation
- Homework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundDocument8 pagesHomework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundPrachi KaushikPas encore d'évaluation
- Estrutura QuimicaDocument3 pagesEstrutura QuimicaAlveiro cPas encore d'évaluation
- DR Viossy - Moonlight Sonata 3Rd Movement JWDocument16 pagesDR Viossy - Moonlight Sonata 3Rd Movement JWGilvan NovaisPas encore d'évaluation
- Chemsheets AS 1077 Petroleum and AlkanesDocument4 pagesChemsheets AS 1077 Petroleum and Alkanescharlesma123Pas encore d'évaluation
- Journal Pre-ProofDocument53 pagesJournal Pre-ProofAndres Fernando Silvestre SuarezPas encore d'évaluation
- Mekanisme PDFDocument1 pageMekanisme PDFSoy Viranda KusumaPas encore d'évaluation
- Hino Nacional Brasileiro (Arranjo Fabio Lima) (HD)Document7 pagesHino Nacional Brasileiro (Arranjo Fabio Lima) (HD)Dudu BarrettoPas encore d'évaluation
- Croquis Sotano 1Document1 pageCroquis Sotano 1KHENYO LINO QUILLCA SILVERAPas encore d'évaluation
- Butano: C H CH CH CH CHDocument3 pagesButano: C H CH CH CH CHAlveiro cPas encore d'évaluation
- Game of Thrones - Main Theme: WWW - Fingerstyle-Guitare - FR Julien MenagerDocument4 pagesGame of Thrones - Main Theme: WWW - Fingerstyle-Guitare - FR Julien MenagerJEAN PIERRE PICARDPas encore d'évaluation
- Fabrizio de Andre - Un GiudiceDocument3 pagesFabrizio de Andre - Un GiudiceacobacchoPas encore d'évaluation
- Fabrizio de Andre - Un GiudiceDocument3 pagesFabrizio de Andre - Un GiudicetelevisionePas encore d'évaluation
- Schematic Diagram Typical Fire Alarm: Main FacpDocument1 pageSchematic Diagram Typical Fire Alarm: Main FacpFathy RamadanPas encore d'évaluation
- A3 ReactionsDocument3 pagesA3 ReactionshaPas encore d'évaluation
- Cope EliminationDocument2 pagesCope EliminationArt Julius D. HallazgoPas encore d'évaluation
- Nomenclature Isomerism Worksheet AnswersDocument3 pagesNomenclature Isomerism Worksheet AnswersYee MeiPas encore d'évaluation
- The Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463Document133 pagesThe Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463bann tvPas encore d'évaluation
- Chapter 4Document20 pagesChapter 4Simran Saiinii0% (1)
- 8thdec2022 Asymmetric Synthesis Using Chiral ReagentDocument14 pages8thdec2022 Asymmetric Synthesis Using Chiral ReagentPrince MeliodasPas encore d'évaluation
- Chemistry Heinemann U4 Workbook Solutions, 2 (3rd Edition)Document20 pagesChemistry Heinemann U4 Workbook Solutions, 2 (3rd Edition)Tiana Pedulla100% (1)
- DrawingsDocument1 pageDrawingsJAGTAP UTKARSH ASHOKRAOPas encore d'évaluation
- Written NotesDocument7 pagesWritten NotesShehryar IftikharPas encore d'évaluation
- Methylene (CH2) : C C CCDocument2 pagesMethylene (CH2) : C C CCItachi UchihaPas encore d'évaluation
- X910 - ALBiology - Assignment - 01 V2Document14 pagesX910 - ALBiology - Assignment - 01 V2Mikila GittensPas encore d'évaluation
- Ex Iii: Ligados Guilherme AlvarengaDocument1 pageEx Iii: Ligados Guilherme AlvarengaGuilherme AlvarengaPas encore d'évaluation
- 132 PDFDocument5 pages132 PDFClaryPas encore d'évaluation
- Natalia FinalDocument3 pagesNatalia FinalcdmazoffPas encore d'évaluation
- Synthesizing A Biofuel ActivityDocument7 pagesSynthesizing A Biofuel ActivityShay BrokenshaPas encore d'évaluation
- HalogenalkaneDocument4 pagesHalogenalkanePutri Nur SyafieqahPas encore d'évaluation
- Plano para Montaje en Obra: H H H HDocument1 pagePlano para Montaje en Obra: H H H Hdavid edgar ramos huallpaPas encore d'évaluation
- Sungha Jung FightDocument5 pagesSungha Jung FightAman LaiPas encore d'évaluation
- Hayward Super II Pump Model SP3005X7 ManualDocument14 pagesHayward Super II Pump Model SP3005X7 ManualhsmerkelPas encore d'évaluation
- Standard Terms & Conditions of Sale Pre-Engineered BuildingsDocument18 pagesStandard Terms & Conditions of Sale Pre-Engineered BuildingsHongducBuiPas encore d'évaluation
- Computer Netwroks 15CS52: Venugopala Rao A S Assistant Professor (Senior) Dept of CSE, SMVITM BantakalDocument12 pagesComputer Netwroks 15CS52: Venugopala Rao A S Assistant Professor (Senior) Dept of CSE, SMVITM BantakalVenugopal RaoPas encore d'évaluation
- Graco Pumps Catalog 300435EN MDocument76 pagesGraco Pumps Catalog 300435EN MAlbu MihaiPas encore d'évaluation
- Karcher K - 791 - MDocument12 pagesKarcher K - 791 - MJoão Paulo FernandesPas encore d'évaluation
- MyNotes ConcreteDocument18 pagesMyNotes ConcreteKarl Si AkoPas encore d'évaluation
- 9.16. Prepare A Plot of Work Per Pound Mole Versus The Pressue Ratio PDocument6 pages9.16. Prepare A Plot of Work Per Pound Mole Versus The Pressue Ratio PttussenoPas encore d'évaluation
- Manual Kls MartinDocument27 pagesManual Kls MartinChris AliPas encore d'évaluation
- 10SQ050 PDFDocument3 pages10SQ050 PDFprojects eastlinkPas encore d'évaluation
- CT Terminology LexiconDocument12 pagesCT Terminology LexiconjtbushbergPas encore d'évaluation
- Technical LetteringDocument12 pagesTechnical LetteringMaverick Timbol50% (2)
- Taipei 102Document2 pagesTaipei 102militansinaPas encore d'évaluation
- Mix Design Practice For Bituminous MixDocument49 pagesMix Design Practice For Bituminous MixAshwani KesharwaniPas encore d'évaluation
- InventoryOverview HN (08.05.2023)Document15 pagesInventoryOverview HN (08.05.2023)Văn Thế NamPas encore d'évaluation
- Powered by QFD OnlineDocument1 pagePowered by QFD OnlineNiswa RochimPas encore d'évaluation
- Scenario of Shale Gas Exploration in IndiaDocument24 pagesScenario of Shale Gas Exploration in IndiaManish TiwariPas encore d'évaluation
- Watertight Doors On Ships: Types, Maintenance & SOLAS RegulationsDocument16 pagesWatertight Doors On Ships: Types, Maintenance & SOLAS Regulationsgeorgesaguna100% (1)
- Brickwork ApparatusDocument4 pagesBrickwork ApparatusRoslie AbdullahPas encore d'évaluation
- Java SampleExamQuestionsDocument18 pagesJava SampleExamQuestionshmasryPas encore d'évaluation
- Bomba de Vácuo Sotorbilt 4mrDocument12 pagesBomba de Vácuo Sotorbilt 4mrWormInchPas encore d'évaluation
- SAX Brochure - Web ReadyDocument4 pagesSAX Brochure - Web ReadyEng-Ahmad Abo-AledousPas encore d'évaluation
- XRD ProcedureDocument2 pagesXRD Procedurepullo123Pas encore d'évaluation
- Getting Started With Java: Atul PrakashDocument20 pagesGetting Started With Java: Atul PrakashOwsozeroPas encore d'évaluation
- D-Link DI-524 ManualDocument92 pagesD-Link DI-524 ManualhadzicinetPas encore d'évaluation
- Manufacturing Layout Analysis - Comparing Flexsim With Excel SpreadsheetsDocument2 pagesManufacturing Layout Analysis - Comparing Flexsim With Excel Spreadsheetsmano7428Pas encore d'évaluation