Académique Documents
Professionnel Documents
Culture Documents
Diminished Virulence Rabbit Model of Endocarditis: of Mutant of Staphylococcus
Transféré par
Chrisanti Mau MetaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Diminished Virulence Rabbit Model of Endocarditis: of Mutant of Staphylococcus
Transféré par
Chrisanti Mau MetaDroits d'auteur :
Formats disponibles
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.
1172/JCI117530
Diminished Virulence of a sar-lagr- Mutant of Staphylococcus aureus in the
Rabbit Model of Endocarditis
Ambrose L. Cheung,* Kelly J. Eberhardt,* Edward Chung,* Michael R. Yeaman,* Paul M. Sullam,6 Marcelo Ramos,* and
Arnold S. Bayer*
* Laboratory of Bacterial Pathogenesis and Immunology, The Rockefeller University, New York 10021; 1 Division of Infectious Diseases,
Harbor-UCLA Medical Center, the UCLA School of Medicine, Torrance, California 90509; and § The Veterans Affairs Medical Center,
UCSF, San Francisco, California 94121
Abstract has made many currently available antimicrobial agents (includ-
ing fluoroquinolones) ineffective, thereby posing an important
Microbial pathogenicity in Staphylococcus aureus is a com- public health problem (2, 3). Thus, there is an urgent need for
plex process involving a number of virulence genes that are alternative approaches in the treatment of S. aureus infections.
regulated by global regulatory systems including sar and One potentially effective mechanism for dealing with the
agr. To evaluate the roles of these two loci in virulence, we emergence of antibiotic resistance among S. aureus strains is
constructed sar-lagr- mutants of strains RN6390 and the development of safe and effective vaccines. However, ex-
RN450 and compared their phenotypic profiles to the corre- perimental data justifying a direct vaccine strategy are presently
sponding single sar- and agr- mutants and parents. The lacking. An alternative approach is to consider targeting regula-
secretion of all hemolysins was absent in the sar-lagr- mu- tory loci that are involved in the control and expression of
tants while residual 8-hemolysin activity remained in single potential virulence determinants (e.g., hemolysins and fibrino-
agr- mutants. The fibronectin binding capacity was signifi- gen and fibronectin binding proteins) (4-7). From this perspec-
cantly diminished in both single sar- mutants and double tive, an understanding of the genetic control apparatus and their
mutants when compared with parents while the reduction regulatory pathways may allow the design of novel antimicro-
in fibrinogen binding capacity in the double mutants was bial or pharmacologic agents to control this virulent patho-
modest. In the rabbit endocarditis model, there was a sig- gen (8).
nificant decrease in both infectivity rates and intravegeta- Postexponential phase expression in bacteria is generally
tion bacterial densities with the double mutant as compared governed by global regulatory systems in which a common
to the parent (RN6390) at 10'-10' CFU inocula despite com- regulator controls the activities of several unlinked genes (9,
parable levels of early bacteremia among various challenge 10). Many exoproteins normally synthesized and secreted dur-
groups. Notably, fewer bacteria in the double mutant group ing the postexponential phase in S. aureus are virulence factors
adhered to valvular vegetations at 30 min after challenge (4). In contrast, synthesis of a number of surface proteins (e.g.,
(106 CFU) than the parent group. These studies suggest that protein A, coagulase, and fibronectin binding proteins) that
both the sar and agr loci are involved in initial valvular clearly play a role in staphylococcal infections is repressed
adherence, intravegetation persistence and multiplication of postexponentially (4, 9). The regulation of virulence determi-
S. aureus in endocarditis. (J. Clin. Invest. 1994. 94:1815- nants and other exoprotein genes in S. aureus involves at least
1822.) Key words: Staphylococcus aureus * regulation of three global regulatory systems including agr, xpr, and sar (9,
gene expression * endocarditis * bacterial adhesion * staphy- 11, 12). The agr locus controls production of extracellular and
lococcal infections cell wall proteins that appear to play a role in virulence (e.g.,
hemolysins, coagulase, and protein A). The xpr locus, like that
Introduction of agr, also positively regulates exoprotein synthesis (11).
Smeltzer et al. (11) reported that two sets of agr- and xpr-
Septicemia due to Staphylococcus aureus is often the conse- mutants with different genetic backgrounds were less lethal
quence of a local infection that has gained access to the blood- than their corresponding parents in a mouse peritonitis model;
stream (1). Once the bacteria enter the bloodstream, patients however, a very high challenging inoculum (109-1010 CFU)
are at risk of developing endocarditis and other life-threatening was required for lethality in this study.
metastatic complications (1). Although the use of newer antimi- We recently identified by Tn917LTV1 insertional mutagen-
crobial agents has initially controlled some of these infections, esis an additional regulatory locus, designated sar, in S. aureus
the recent emergence of multiple-drug resistance in S. aureus (12). Inactivation of this locus has led to decreased expression
of several exoproteins (hemolysins) and cell wall proteins (fi-
brinogen and fibronectin binding protein) that are potential viru-
M. Ramos's present address is Division of Infectious Diseases FCM, lence determinants in experimental infections (4, 12). In a rabbit
UNICAMP, Campinas, Brazil 13081. endocarditis model, we have previously shown that there
Address correspondence to Ambrose L. Cheung, M.D., The Labora- was a significant decrease in infectivity rates in a sar- mu-
tory of Bacterial Pathogenesis and Immunology, The Rockefeller Uni- tant at 03-_104 CFU inocula when compared to the parental
versity, 1230 York Avenue, New York, NY 10021. strain (13).
Received for publication 3 March 1994 and in revised form July
1994. To evaluate the contributory roles of both sar and agr loci
in staphylococcal virulence, we have constructed sar-/agr- mu-
The Journal of Clinical Investigation, Inc. tants of RN6390 and RN450 and compared their in vitro proper-
Volume 94, November 1994, 1815-1822 ties to the wild-type parent and single isogenic mutants. In
Diminished Virulence of a sar-lagr- Mutant of S. aureus 1815
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
studies with RN6390-related strains in the rabbit endocarditis described (21). PMP was added to washed bacterial suspensions in low
model, there was a significant reduction in infectivity rates protein-binding microtiter plates (Corning Glassworks, Corning, NY)
across a broad range of challenging inocula (103- 106 CFU) to achieve a final PMP concentration of 100 U/ml and a final bacterial
inoculum of 103 CFU/ml. After 2 h of incubation at 370C, 20-pl
-
with the double mutant when compared to the corresponding aliquots were processed for quantitative culture as described previously
sar'/agr' (parent) strain. These results suggest that both loci (21). The percent bacterial survival was calculated for each S. aureus
are involved in the initiation and persistence of S. aureus infec- strain. Bacillus subtilis (ATCC 6633), a highly PMP-susceptible strain,
tion in the fibrin-platelet-endothelial matrix in endocardial vege- served as a positive control for PMP bioactivity while wells containing
tations. the organism plus minimal essential medium (Irvine Scientific, Santa
Ana, CA) alone were used as negative controls (23).
Methods Southern blot hybridization. S. aureus DNA was prepared from
lysostaphin-lysed cells as described (12). Chromosomal DNA was di-
Bacterial strains, plasmids, DNA probes, and phage. The bacterial gested with selected restriction enzymes (New England BioLabs, Bev-
strains, plasmids and DNA probes used in this study are listed in Table erly, MA), transferred onto Hybond N' membrane (Amersharn, Arling-
I. Phage 411 was used as a transducing phage for RN6390 and RN450. ton Heights, IL) and hybridized at 650C with probes labeled with 32p
Media and antibiotics. CYGP, 0.3GL, and Brain-Heart-Infusion by the random-primed method (Ready to Go Labeling Kit; Pharmacia
(Difco Laboratories Inc., Detroit, MI) were used for the growth of Fine Chemicals, Piscataway, NJ) or the nick translation method (Nick
bacteria (12). Antibiotics were used at the following concentrations: Translation Kit; Boehringer Mannheim, Indianapolis, IN). After hybrid-
erythromycin at 10 jig/ml and tetracycline at 5 jig/ml. ization, the membrane was washed with SSPE (10 mM NaH2PO4, 150
Transduction. Phage 411 was used to prepare lysates of strain mM NaCl, 1 mM EDTA, pH 7.4) according to the membrane manufac-
RN6911 (14) which is an agr- mutant of parental strain RN6390 (12). turer's instructions (12) and autoradiographed with an intensifying
The phage lysate was used to infect sar- mutants R and A (15) derived screen at -70'C.
from strains RN6390 and RN450, respectively, at a low multiplicity of Northern blot hybridization. S. aureus RNA was prepared using a
infection (phage to recipient ratio = 1:10). Transductants carrying the modification of the method of Kornblum et al. (24) as previously de-
double mutations were selected on 0.3 GL agar containing both erythro- scribed (15). For Northern blots, equal volumes (7.5 1tl) of RNA samples
mycin and tetracycline. The 411 lysate of RN691 1 was also used to extracted from an equivalent number of bacterial cells were electropho-
infect strain RN450 to obtain tetR transductants carrying the agr mutation resed through a 1.5% agarose-0.66 M formaldehyde gel in MOPS run-
alone. ning buffer as described (15). The intensity of the 23 S and 16 S
Phenotypic characterization. For phenotypic characterization, the ribosomal RNA bands stained with ethidium bromide was verified to
measurements included a; ,3; and 5-hemolysin production on sheep and be equivalent among all samples before transfer. RNA was transferred
rabbit erythrocyte agar as previously described (16), lipase production to Hybond N (Amersham), cross-linked with UV light, allowed to hy-
as measured on 1% Tween 20 agar plates (12), fibronectin binding bridize with a 32P-labeled gel purified DNA probes (25) in 50% for-
capacity as assayed by 251I-labeled fibronectin binding (17), and fibrino- mamide at 420C overnight, washed, and autoradiographed as de-
gen binding capacity as determined by 125I-labeled fibrinogen bind- scribed (15).
ing (18). Animal model of endocarditis. All bacterial strains were grown in
To determine whether the double mutant differed from either the brain-heart infusion broth at 370C overnight with rotation (30 rpm)
parent or single isogenic mutants in other phenotypic traits likely to be supplemented with appropriate antibiotics if necessary. In preliminary
important in endocarditis pathogenesis, we studied the comparative in studies, it was determined that the growth rates of parental and mutant
vitro ability of these strains to: (a) adhere to platelets as measured by strains were almost identical both in the presence and absence of antibi-
flow cytometry (19, 20); and (b) resist the bactericidal action of platelet otics over a 24-h period. In our previous study with a sar- mutant (13),
microbicidal protein (PMP)' (21). we established that incorporation of antibiotics into the overnight growth
For platelet adherence assays, rabbit whole blood was collected media did not influence the ability of the strain to induce experimental
into polypropylene tubes containing sodium citrate (5:1) and 1 pg/ml endocarditis. Overnight cultures were harvested by centrifugation (2,000
prostaglandin E1 (PGEI) (Sigma Chemical Co., St. Louis, MO) to miti- g for 10 min), washed twice in sterile normal saline and resuspended
gate platelet activation. Platelets were isolated by centrifugation (150 g in saline to an OD62Nm of 1.6 (- 1 x 109 CFU/ml). This bacterial
for 10 min) and labeled externally with P2 (2.5 Ag/ml), an FITC-conju- suspension was serially diluted in saline to 103-106 CFU/ml. Each
-
gated monoclonal antibody against CD41 (AMAC, Westbrook, ME), dilution was confirmed by quantitative culture on blood agar plates.
or internally labeled with 5 jiM 5-chloromethylfluorescein diacetate Endocarditis on the aortic valve of New Zealand White rabbits (2-
(CMFDA; Molecular Probes, Eugene, OR). After 1 h of incubation, the 2.5 kg) was induced by a modification of the method of Durack and
platelets were washed and suspended in Tyrode's solution as previously Beeson (26). In brief, rabbits were anesthetized by intramuscular injec-
described (22). To label bacteria, overnight cultures of each strain were tion of ketamine chloride (Aveco Inc., Fort Dodge, IA) at 35 mg/kg
washed in Tris-EDTA-NaCl buffer (0.05 M Tris-HCl with 0.1 M NaCl and xylazine (Mobay Corp., Shawnee, KS) at 1.5 mg/kg. A polyethylene
and 0.02 M EDTA, pH 7.2) and labeled for 3 h with Hoechst 33342 catheter with an internal diameter of 0.86 mm (Becton Dickinson, Cor-
dye (25 1ig/ml; Polysciences, Warrington, PA), a DNA-binding fluoro- keysvilie, MD) was introduced into the left ventricle via the right carotid
chrome (22). Adherence studies were performed at 20°C by mixing
bacteria and platelets in Tyrode's salts solution (Sigma Chemical Co.) artery to produce sterile thrombotic endocarditis. To induce endocarditis,
at a bacterial/platelet ratio of 10:1. One minute after mixing, the suspen- groups of 10-15 animals each were challenged intravenously at 48
-
sions were analyzed using a FACSta?'P" (Becton Dickinson, San Jose, h after catheterization with various inocula of different bacterial strains
CA) cytofluorograph, using predetermined combinations of argon lasers, (103-106 CFU). Catheters remained in place until animals were sacri-
excitatory wavelengths, and appropriate filters (19, 22). The percentage ficed by lethal injection of sodium pentobarbital (100 mg/kg) at 48 h
of bacteria adhering to platelets was determined as previously de- after bacterial challenge. Animals with macroscopic valvular vegetations
scribed (19). and proper catheter placement were analyzed for data in this study. At
For the second assay, PMP was prepared from thrombin-stimulated time of sacrifice, aortic valves and left ventricular vegetations were
platelet-rich plasma and assayed for bioactivity (U/ml) as previously removed, pooled, weighed, homogenized in 0.5 ml of normal saline
and quantitatively cultured in antibiotic-free medium (for all strains),
erythromycin-containing medium (for mutant R and I), tetracycline-
containing medium (for RN6911 and mutant I) or medium containing
1. Abbreviation used in this paper: PMP, platelet microbicidal protein. both antibiotics (double mutant). Animals with negative cultures of
1816 Cheung et al.
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
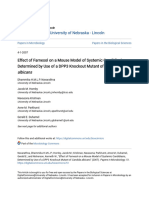
A B specifically, the double sar-lagr- mutants hybridized with tetM
cm c but not the agr probe (Fig. 1, A and B). Notably, two bands
_ < < o
co s I hybridized to the probe that comprises of tetM cloned into
oz E min z 2 = z z : pUC8. The upper band represented hybridization of the pUC8
_x _ probe with the E. coli-derived portion of inserted transposon
Tn917LTV1 while the lower hybridizing band corresponded to
* .
.4
__
that of tetM (Fig. 1 A). This pattern of hybridization of the
double mutants with tetM and agr probes was similar to that
of the agr mutant RN6911 in which the entire agr locus had
been replaced by the tetM gene (Fig. 1, A and B). To verify the
maintenance of the Tn9J7LTV1 insertion in the double mutants,
C a Southern blots of chromosomal DNA from these transductants
C
-Figure 1. Southern blots of digested with EcoRI was performed. Our results indicated that
Za
45z zn
B transductants from sar- mutants a single EcoRI fragment from these double mutants hybridized
a :EEzaa R and A. (A) HindIII chromo- with a 1.5-kb HindIll fragment of Tn917 as a probe (Fig. 1 C).
somal digests probed with
This hybridizing pattern is analogous to that seen with original
pMVN6, a pUC8 plasmid car- sar- mutant 1 ID2, thus suggesting that the location of the
rying a 2.9-kb fragment of tetM;
(B) NcoI chromosomal digests Tn917LTV1 insert in these double mutants was identical to that
probed with a 513-bp PCR frag- of mutant 1I1D2 (Fig. 1 C).
ment of agrA gene; (C) EcoRI We have also transduced the agr- genotype from RN6911
chromosomal digests probed into RN450 to obtain sar+/agr- mutants. At least 10 tetR trans-
with a 1.5-kb HindIII fragment ductants were found one of which (mutant D) is listed in Table
of pLTVlI. I. The results of Southern blot studies of these transductants
with tetM and agr probes were similar to that of RN691 1 (data
not shown), thus implying displacement of the agr locus by the
their undiluted vegetation homogenates were considered to have had no tetM gene in these transductants.
induction of endocarditis at a particular inoculum (13). Northern blot analysis of the sar+/agr- and sar-/agr- mu-
To determine the relative ability of different bacterial strains to tants with both agrA and hid probes confirmed that transcription
adhere to sterile endocardial vegetations early after intravenous chal- of these genes did not occur in these strains (data not shown).
lenge, separate groups of 6-8 catheterized rabbits each were challenged Similarly, no transcripts were detected in the sar-/agr+ and
intravenously at 48 h after catheterization with 104, 105, or 106 CFU of sar-/agr- strains when total cellular RNA immobilized on ny-
either the parent or mutant strains. Thirty minutes after bacterial injec- lon membranes were probed with the sarA gene probe (data
tion, all animals were sacrificed, their hearts opened, and all visible left- not shown).
sided vegetations from each animal excised and pooled. Each vegetation
pool was washed gently with PBS to remove any surface blood and Phenotypic characterization of sar-/agr- mutants in com-
nonadherent bacterial cells, placed in 0.5 ml of PBS, and homogenized parison to sar-/agr+, sar+/agr-, and sar+/agr+ (parent) strains.
in a tissue grinder. To account for any bacterial cells that might have Two sets of mutants were evaluated for phenotypic alterations
adhered to the tissue grinder or tube during homogenization, the appara- (Table II). Compared with parental strain RN6390, no hemoly-
tus were washed with 1 ml of PBS. The vegetation homogenate together sins (a, ,6, or 6) were detected in the corresponding sar-lagr-
with the wash from each individual animal were quantitatively cultured mutant. In contrast, a small but detectable amount of 63-hemoly-
into antibiotic-free as well as antibiotic-selective brain-heart infusion sin was secreted by both single sar- and agr- mutants (mutant
agar based on the infecting strain. R and RN691 1, respectively). In assays with RN450-related
To assure that any observed differences in valvular adherence and strains, the secretion of 6 hemolysin was absent in both sar-/
endocarditis induction rates among strains were not due to a disparity
in the level of early bacteremias, quantitative blood cultures were ob- agr- and sar-lagr+ mutants but present in the sar+lagr- mutant.
tained in a separate study at 1-min post-iv inoculations with each bacte- Northern blot analysis of one set of strains (RN6390, RN691 1,
rial strain at 103-106 CFU. sar- mutant R and double mutant I) with a- and 68-hemolysin
Statistical analysis. The Fisher's exact test was used to compare probes revealed almost complete abolition of these exoprotein
the induction rates of endocarditis among strains. The mean bacterial gene transcripts in the double mutant (Fig. 2, A and B). In
vegetation densities and early adherence were evaluated by the Kruskal- concordance with our previous findings (15) and that of Vandes-
Wallis analysis of variance for nonparametric data with Tukey post-hoc desh et al. (14), a significant downregulation of these transcripts
analysis for multiple comparisons. The Student t test was used to com- in the sar-/agr+ (mutant R) and sar+/agr- (RN691 1) mutants
pare the binding of radiolabeled fibrinogen and fibronectin to different was also observed.
isolates. P valves s 0.05 were considered significant. The effect of these mutations on cell-bound proteins was
also studied. The fibronectin binding capacity was significantly
Results reduced in both double mutants and single sar- mutants (Table
II) when compared with their respective parents. Similar to the
Transduction of the agr- genotype into sar- mutants R and A. results reported by Kornblum et al. (9), the binding of radiola-
Transduction was used to transfer the agr- genotype from agr beled fibronectin was increased in both sar+/agr- mutants. The
mutant RN6911 into sar- mutants R and A derived from ability to bind fibrinogen was diminished in both double mu-
RN6390 and RN450, respectively. Southern blots of chromo- tants, but the magnitude of the reduction, albeit statistically
somal DNA from transductants (mutants AD and I) digested significant, was more substantial in mutant AD than mutant I.
with HindIII, EcoRI, or NcoI revealed a hybridization pattern Consistent with our previous studies (15), there was a modest
consistent with the transfer of the agr- genotype (Fig. 1). More decline in the ability to bind fibrinogen as a result of the sar
Diminished Virulence of a sar-lagr- Mutant of S. aureus 1817
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
Table L Bacterial Strains, Plasmids, and DNA Probes
Types References Comments
Bacterial strains
S. aureus
DB 12 A wild-type blood isolate that is the parent of the original sar- mutant 1D2.
11D2 12 An isogenic mutant of strain DB with a sar::Tn9l7LTV1 mutation
RN6390 40 A prototypic strain that maintains its hemolytic pattern when propagated on sheep erythrocyte agar.
Mutant R 15 An isogenic mutant of RN6390 carrying a sar.:Tn9l7LTVl mutation.
RN6911 14 An isogenic mutant of RN6390 with an agr::getM mutation.
Mutant I This study A derivative of RN6390 carrying both sar::Tn9J7LTV1 and agr::tetM mutations.
RN450 40 A prototypic strain, which is a derivative of NCTC 8325 cured of prophages, secretes /3 hemolysin
but not a hemolysin.
Mutant A 15 An isogenic mutant of RN450 with a sar.:Tn9J7LTVI mutation.
Mutant D This study An isogenic mutant of RN450 with a agr::tetM mutation.
Mutant AD This study A double mutant of RN450 with both sar::Tn917LTVl and agr.:tetM mutations.
E. coli with plasmids
DU5384 41 An E. coli strain carrying a pBR322 plasmid with a 3-kb EcoRI-HindIII fragment of the a-hemolysin
gene to be gel-purified to be used as a probe.
RN6929 42 JM109 containing a pBluescript plasmid with a 2.2-kb Clal fragment of the /3-hemolysin gene which
was gel-purified to be used as a probe.
DNA probes
pLTVl 12, 43 A derivative of Tn9J7 carrying a E. coli replicon. A 1.5-kb Hindu gel-purified fragment was used as
a probe.
pALC4 31 A shuttle plasmid (pSPT181) containing a 732-bp PCR fragment of sarA gene of RN6390.
pMVN6 44 A pUC8 plasmid carrying a 2.9-kb BamHI fragment of tetM.
hld probe This study A 51 1-bp PCR fragment of the RNAIII from nucleotides 1265-1775 based on published sequence
(9).
agrA probe This study A 513-bp PCR fragment of the agrA gene from nucleotides 3830-4342 (9).
Table II. Phenotypic Characterization of RN6390, RN450, and Their Corresponding sar/agr Mutants
Strains
RN6390 RN6911 Mutant R Mutant I RN450 Mutant D Mutant A Mutant AD
Phenotypes* sar+lagr+ sar+/agr7 sar-lagr+ sar-lagr- sar+lagr+ sar+lagr- sar-lagr+ sar-lagr-
a-Hemolysin ++- - - - - - -
,B-Hemolysin ++ + + - + +
6-Hemolysin ++ - - - +--
Lipase + - ++
Fibronectin binding
protein' 3314±35 4059±117 2636±80 2809±60 15221±671 26852±691 4874±192 4909±611
Fibrinogen binding
protein11 5694±70 5427±314 5156±94 5118±118 6139±119 5326±103 3463±449 3094±97
Susceptibility to PMP (%
survival) 0% 0% 0% 1.3%
Platelet binding by FACS
(% bound)1 61.7±39.7 57.3±42 69.1±26.6 72.8±26.1
* + +, strong producer; +, moderate producer; +/-, very weak producer; -, non producer. * The expression of a-hemolysin in these strains was
also confirmed by immunoblots of extracellular proteins probed with affinity purified anti-a-hemolysin antibody (Toxin Technology, Sarasota, FL).
tData are presented as cpm of l"I-fibronectin bound to 109 CFU. Values are given as mean+SEM (n = 4). The reduction in fibronectin binding
of double mutants I and AD in comparison with the respective parental strains is statistically significant (P 5 0.009 and 0.001 for mutant I and
AD, respectively; t test). The augmentation in fibronectin binding as a result of the agr mutation alone (RN6911 and mutant D) was also significant
when compared to the respective parents (P 0.004 and 0.001 for RN6911 and mutant D, respectively; t test). 11 Data are given as cpm of
-
12'I-fibrinogen bound to 109 CFU and are reported as the mean±SEM (n = 4). The binding of radiolabeled fibrinogen to double mutants I and AD
was lower than that of the respective parental strain (P 5 0.023 and 0.0001 for mutants I and AD, respectively; t test). Similarly, sarf mutant A
bound less radiolabeled fibrinogen than parent strain RN450 (P 5 0.008). 1 Platelets labeled with P2 or CMFDA were bound comparably by all
strains.
1818 Cheung et al.
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
A B Table II. The Induction of Endocarditis in a Rabbit Model with
sar+/agr' (RN6390), sarl/agr-, sar-lagr', and sar-lagr- Strains
+tiIX
+ wa
K X Strains
i-
RN6390 RN6911 Mutant R Mutant I
(sar'lagr') (sar'/agr-) (sar-lagr') (sar-lagr-)
103 CFU 9/10 4/10* 0/10* 0/10'
O.
104 CFU 7/10 4/11 1/9* 0/10§
105 CFU 11/11 8/10 10/11 2/7§
106 CFU 9/9 8/11 9/12 4/8§
Figure 2. Northern blots of a- (A) and /B- (B) hemolysin transcripts of At 48 h after catheterization, bacteria were injected directly into the
parental strain (RN6390) and the corresponding sarlagr mutants. The marginal ear veins. All rabbits were sacrificed for morphological exami-
positive control in A is a pBR322 plasmid carrying a 3-kb EcoRI- nation and valvular cultures at 48 h after bacterial challenge. Values
HindII fragment of the a-hemolysin gene (41). In B, the positive control aregiven as numberwithendocarditis/total numberinthegroup. * Statis-
is a pBluescript plasmid with a 2.2-kb Clal fragment of the /3-hemolysin tically significantly when compared with sar'lagr' parental strain (P
gene (42). c 0.03; Fisher Exact test). t When compared with the parental strain,
induction rates are significant with the P values < 0.0001 and 0.015 at
103 and 104 inocula, respectively. § Induction rates are significantly
lower than that of the parental strain at all inocula (P values of 0.00006,
mutation in both strains (see mutants R and A in Table II). 0.0015, 0.002, and 0.03 at 103- 106 CFU, respectively).
However, the effect of the agr mutation alone on fibrinogen
binding capacity was more variable, with one resulting in a
slight reduction (mutant D) while the other one remaining un- plating methods. As shown in Fig. 3, animals challenged with
changed (RN691 1). the double sar-lagr- mutant had significantly lower vegetation
In preliminary studies, it was determined that strain RN6390 densities as compared with the sar'/agr' parent at all challenge
was virulent in the animal endocarditis model (see below). inocula. At the two higher inocula (105- 106), challenge with
Based on this finding, we evaluated other phenotypic traits in the sar-lagr' strain yielded lower bacterial densities in aortic
RN6390 and its corresponding mutants that are likely to be vegetations than the sar'/agr' parent. Although the mean bacte-
important in the pathogenesis of endocarditis, including suscep- rial vegetation densities achievable with the sar+lagr- mutant
tibility to PMP (19) and the ability to bind platelets (27, 28). were lower than the parent at higher challenge inocula (105-
As seen in Table H, all strains were very susceptible to the 106 CFU), they did not reach statistical significance.
killing action of PMP. After exposure to PMP at 100 U/ml for To verify the stability of the sar and agr mutations in mutant
120 min, the mean survival among these strains was between strains after animal passage, chromosomal DNA of sar'lagr+
0 and 1.3%. Likewise, the ability to bind platelets as assayed (parent), sar+/agr-, sar-/agr+, and sar-/agr- colonies isolated
by FACS was similar among all strains studied (Table H).
Animal model of endocarditis. In pilot studies, it was deter-
mined that the ID,0 for parental strain RN6390 was between
103 and 104 CFU. The induction rates for endocarditis with I SD
parent RN6390 and its corresponding mutants were evaluated
* Mean --
at various intravenous challenge inocula between 103 and 106 ....
CFU, thereby encompassing the IDgo for the parental strain 0)
(Table HI). At all four challenge inocula, the sar-lagr- double
n
mutant caused substantially lower induction rates of endocardi-
tis than sar+/agr+ parent (RN6390) (0, 0, 28, and 50% vs 90, T |l
70, 100, and 100% at 103_106 CFU with P < 0.00006, 0.0015, 0)
0.002, and 0.03, respectively). Similar to our previously re- 0
ported data on parent strain DB and its isogenic sar-lagr+ mu-
tant (13), intravenous challenge with the sar-lagr+ mutant R
-3
also resulted in reduced endocarditis induction rates as com- I* * *.
pared with the parent strain at the two lower challenge inocula
(O and 11% vs 90 and 70% at 103-104 CFU with P values N?(X X
NI-> -.X (X"f->Z ..l< x -<,
< 0.0001 and 0.015, respectively). In contrast, the difference x x > x> x
in induction rates between the sar'lagr- (RN6911) strain and
its sar'lagr+ parent (RN6390) was only significant at the lowest
io3 104
Inoculum (cfu)
105 106
inoculum (40 vs 90% at 103 CFU; P _ 0.03). Notably, there
Figure 3. Bacterial vegetation densities of parental strain RN6390 vs
were no statistical differences between the induction rates of
three sarlagr mutants. Values are given as mean values±SD obtained
sar-lagr+ versus sar+/agr- mutants at any challenge inocula. from 8-10 animals in each group (sar'lagr+, sar+/agr-, sar-lagr+, and
The vegetation bacterial densities in different animal groups sar-/agr-). The analyzed statistical data are as follows: 105 CFU +/+
were parallel-plated onto antibiotic-containing as well as antibi- vs -/- (P ' 0.0001), +/+ vs -/+ (P C 0.05); 106 CFU +/+ -/ vs
otic-free media. Our results indicated that there were no differ- - (P - 0.05), +/+ vs -/+ (P - 0.05). * The vegetation cultures in
ences in quantitative recovery of bacteria between these two these animal groups were negative.
Diminished Virulence of a sar-/agr- Mutant of S. aureus 1819
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
determinants controlled by global regulatory systems. The agr
and sar loci in S. aureus are examples of two such regulatory
systems (9, 12). Northern blot analyses suggested that the sar
locus, like that of agr (14), positively regulates exoprotein gene
o 200- .
J - ..........
expression via transcriptional control (15). However, in contrast
0) to agr, the expression of selected cell wall proteins such as
, 150- fibronectin and fibrinogen binding proteins, which have been
shown to be potential adhesins to catheters and endothelium (5,
7, 29, 30), are downregulated in sar- mutants as compared with
, 100 X parent strains (12, 15). We have also established in a previous
study that a sar- mutant was less virulent than the parent at
50- ......... * low challenge inocula (103 and I 04CFU) in the endocarditis
model (13). Because of the counter-regulatory effects of the
0 same set of cell-wall protein genes by sar and agr loci, the
current study was undertaken to examine the in vitro phenotypic
+1/+ +1/- -1+ -I interactions as well as the net in vivo effects of these two loci
in a prototypical endovascular infection model (experimental
Challenge Strains (10-6 inoculum) endocarditis).
Figure 4. Relative bacterial adherence of parent (RN6390) vs three sari Our detailed examination of the phenotypic characterization
agr mutants to aortic valvular vegetations. These are presented as total of sar and agr mutants has yielded interesting insights into the
number of organisms adhering at 30 min post-iv challenge. Values are control of exoprotein expression. As expected from previous
given as mean±SD obtained from 6-8 animals in each bacterial strain studies (9, 15), the secretion of hemolysins was generally de-
group. *The P value in early valvular adherence between sar'Iagr' creased in both sar'lagr- and sar-lagr' mutants derived from
parent and sar-lagr- double mutant is < 0.01. RN6390. Based on our recent observation that the optimal tran-
scription of RNAII, which is the agr regulatory molecule that
from cultured vegetations (at least six each) were digested with controls exoprotein gene transcriptions (9), is dependent on an
EcoRI, NcoI, or HindIII and probed with 32P-labeled agrA, hid, intact sar locus (31), we speculate that the sar locus likely
tetM, Tn917, and sarA probes. In each instance, the colonies regulates exoprotein gene expression via transcriptional control
harvested from the vegetations had hybridization patterns identi- of RNAIH. However, additional phenotypic analysis of the dou-
cal to those of the respective infecting strains isolated before ble sar-/agr- mutant (mutant I in Table I) suggested that the
animal passage (data not shown as they are similar to those in sar locus may also affect exoprotein genes independently of
Fig. 1). These data indicated that the mutations remained stable agr. This is predicated upon the finding that a double mutant
in vivo. does not secrete /3-hemolysin while single isogenic mutants do.
Determination of early adherence to sterile valvular vegeta- Additionally, the mild augmentation of lipase production in
tions. To determine if the above disparities in endocarditis in- sar-lagr+ strain (mutant R) in comparison with the parent
duction rates were attributable to differences in early valvular (RN6390) was completely abolished in the double mutant I.
adherence of bacteria, we sacrificed groups of catheterized ani- Although the fibronectin binding capacity was increased in
mals 30 min after intravenous challenge to determine the total sar+/agr- mutants (RN6911 and mutant D in Table II) as pre-
number of CFU binding to valvular vegetations. At lower inoc- dicted (9), this capacity was reduced in the double mutants
ula (103-105), the number of adherent bacteria for all strains (mutants I and AD) and single sar- mutants (mutants R and A)
was too low to make a meaningful comparison. However, at a as compared to their respective parents. However, the ability to
higher challenge inoculum (106 CFU), the adherence of the bind radiolabeled fibrinogen remained essentially unchanged in
parental strain was significantly higher than that of the double sar'lagr- mutants. In contrast, the fibrinogen-binding capacity
mutant (181±79 vs 18±8 CFU/vegetations, respectively; P of both double mutants decreased to a level similar to that of
< 0.01) (Fig. 4). Notably, the adherence of single mutants was
sar-/agr+ strains, although the magnitude of the decrease was
also less than the parent strain, but these differences did not much smaller in double mutant I. As a single mutation in the
reach statistical significance (e.g., P 0.14 between sar'lagr-
-
sar or agr locus yields opposite effects in cell-wall protein
and sar'lagr' strains). expression, these experimental observations on the double mu-
To confirm that differences in early valve adherence were tants provide two potential insights into regulatory control of
not related to the level of bacteremia following intravenous
challenge, quantitative blood cultures were obtained to assay cell-wall proteins. First, in distinction to the exoprotein control
for comparative bacterial clearance. Because our previous ob- in which the sar and agr loci are possibly elements operating
servations had confirmed that very few colonies (1-5 CFU) under the same regulatory pathway, these data suggested that
could be isolated on blood culture at 30 min post-iv challenge the mechanisms of cell-wall protein control by these two loci
(13), only blood cultures at 1 min post-iv challenge were ob- are likely to be different from that of exoprotein genes. Second,
tained. At each of the tested inoculum (103- 106 CFU), there two theoretical possibilities can account for the cell wall protein
were no significant differences in the mean quantitative counts profile of the double mutant. One possibility is that both sar
of post-challenge bacteremias among various groups at 1 min and agr loci function within the same regulatory pathway in
after intravenous challenge. which the agr and sar gene product(s) act as the repressor and
the activator, respectively, for cell wall protein target sequences.
Discussion In the case of a double mutant, a lack of a repressor (i.e., agr-)
Microbial pathogenicity is a complex process that involves the does not result in an overexpression of cell-wall proteins in the
products of a number of genes many of which are virulence absence of an activator (i.e., sar-) of target sequences. The
1820 Cheung et al.
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
second possibility is that the sar and agr loci control the expres- achieved lower intravegetation bacterial densities than the par-
sion of cell wall proteins via different regulatory mechanisms ent. Whether this reduction in intravegetation bacterial densities
such that a malfunctioning sar locus, irrespective of the func- is due to a diminution in cytotoxin secretion (e.g., hemolysins)
tionality of the agr locus, will lead to downregulation of cell and/or a decline in initial adhesion observed in these mutants
wall proteins. is not resolved in this study. Nevertheless, a-hemolysin, a pore-
As inactivation of both sar and agr loci resulted in reduced forming cellular cytotoxin, has been shown to exhibit lethality
production of extracellular and cell wall proteins that are puta- for platelets and endothelial cells (38, 39), two important com-
tive virulence determinants in S. aureus (5, 7), we evaluated ponents of the vegetation in endocarditis (33). It is conceivable
the net in vivo effect of a double mutant in a rabbit model of that the absence of a hemolysin in a mutant strain may adversely
endocarditis. Our data showed that the double mutant had a affect its ability to persist within vegetation. Because very few,
lower capacity to induce endocarditis as compared to the parent if any, leukocytes are found within infected aortic vegetations
strain at all challenge inocula (Table HI). Moreover, the vegeta- in both experimental and human endocarditis (33), any dissimi-
tion bacterial densities were markedly decreased in the double larity in cellular immune response between the parent vs the
mutant in comparison with the parent strain at all challenge mutant strains is unlikely to explain the observed differences
levels. In contrast, the sar-lagr' mutant induced endocarditis in induction rates or intravegetation bacterial densities. As these
at a significantly lower rate than the parent strain only at the two regulatory loci control the synthesis of a variety of extracel-
two lower challenge inocula (103-104 CFU). This induction lular and cell wall proteins, our data would seem to support the
rate is in concordance with our previous studies in which a multifactorial nature involved in the pathogenesis of S. aureus
different single sar- mutant (strain 1 1D2) had a lower induction endocarditis.
rate than the isogenic parent only at such challenge inocula Considerable efforts have been spent previously to single
(13). Interestingly, the induction rate for sar'/agr- strain dif- out a specific virulence factor in the pathogenesis of S. aureus
fered from the parent only at the 103 CFU inoculum. Although endocarditis and other related intravascular infections. How-
the induction rates for single sar mutant were statistically equiv- ever, these experimental approaches have not been successful
alent to those of the parent strain at the higher challenge inocula in explaining the range of this organism's ability to induce and
(105-106 CFU), the mean vegetation bacterial densities were propagate infections at a challenging inoculum found in similar
substantially lower in the sar mutant group, but not in the agr human infections (4, 7). For example, in experiments involving
mutant group, when compared to the parental group at these inactivation of the fibronectin binding protein alone, the contrib-
inocula (Fig. 3). utory role of this protein in endocarditis virulence is modest at
Differences in the ability of the double mutant and parental best even at a fairly high challenge inoculum (106 CFU) (7).
strains to induce endocarditis as described here may be related Based on these investigations, it appears that S. aureus may have
to potential perturbations in either of the two critical events a wide arsenal of adhesins and toxins to facilitate adherence and
in the pathogenesis of endocarditis: initial adherence to sterile propagation within human valvular tissues. In this study, we
vegetation and/or subsequent intravegetation bacterial propaga- have shown that the sar and agr loci of S. aureus are important
tion (26, 32). Within the matrices of the valvular vegetations, regulatory elements in the expression of virulence determinants
fibrinogen, fibronectin, vitronectin, platelets, and released plate- necessary for the induction and propagation of intravegetation
let proteins (e.g., thrombospondin) are commonly deposited (5, growth in the animal model. Hence, it may be meaningful to
7, 32). In addition, underlying extracellular matrix proteins, target these regulatory loci for the development of novel antimi-
such as collagen and laminin, may also be exposed as a result crobial agents (e.g., designing a synthetic analog to block activa-
of prior endothelial damage (33, 34). During the initial phase tion of virulence factors by the native sar gene products).
of bacteremia, a combination of impaired fibronectin and fi- One of the genes (sarA) within the sar locus has recently
brinogen binding may conceivably contribute to a reduction in been cloned (31). Sequence analysis revealed an open reading
the number of initial adherence events at the vegetation surface frame of 372 bp with a predicted mol wt of 14,718 D. Additional
for the double mutant. Notably, this diminished capacity to studies indicated that this gene is necessary for the optimal
bind fibronectin and fibrinogen in the double mutant is likely transcription of RNAIII (31). In this current study, there is
attributable to a mutation in the sar locus (Table II). This con- additional evidence to suggest that both agr-dependent and agr-
cept is consistent with the experimental observation that at independent pathways are probably operational in the regulation
higher inocula (105_-106 CFU), the bacterial vegetation densities of virulence determinants in S. aureus. To our knowledge, this
achievable by the single sar mutant, but not the single agr is the first study that implicates both regulatory loci (i.e., sar and
mutant, were significantly lower than the parent despite compa- agr) in early bacterial adherence, induction, and intravegetation
rable induction rates (Fig. 3). Nevertheless, the possibility that persistence in the pathogenesis of endocarditis. A detailed un-
additional staphylococcal surface receptors such as those for derstanding of the entire sar locus and its mode of regulation
collagen, laminin and thrombospondin (35-37) may be in- will provide insight into the molecular mechanisms of staphylo-
volved in adhesion cannot be entirely ruled out. Our data also coccal pathogenesis and may uncover specific targets which
seem to minimize the role of platelets in accounting for the may be amenable to therapeutic intervention in S. aureus infec-
differences in early adherence between the parent and mutant tions.
strains since they bound equally well to platelets, and were
highly susceptible to platelet-microbicidal-protein-induced le- Acknowledgments
thality in vitro.
After initial adherence to the vegetation surface, the eventual We thank Patrick Ying, Reva Edelstein, and Wendy Foss for technical
establishment of infection is still dependent on the ability of the assistance; and Steven Projan for performing hemolysin assay and gener-
microorganism to proliferate within the vegetations. As noted in ating helpful discussion.
Fig. 3, both the single sar mutant and the double mutant This work was supported in part by Grants-In-Aid from the Ameri-
Diminished Virulence of a sar-lagr- Mutant of 5. aureus 1821
Downloaded from http://www.jci.org on March 6, 2018. https://doi.org/10.1172/JCI117530
can Heart Association and the New York Heart Association and by with bacteria. VI. contrasting the role of fibrinogen and fibronectin. Am. J. Hema-
Public Health Service grant A130061 (to A. L. Cheung). M. R. Yeaman tol. 9:43-53.
21. Yeaman, M. R., D. C. Norman, and A. S. Bayer. 1992. Staphylococcus
and A. S. Bayer were supported in part by Grants-In-Aid from the aureus susceptibility to thrombin-induced platelet microbicidal protein is indepen-
American Heart Association (Greater Los Angeles Affiliate) and the St. dent of platelet adherence and aggregation in vitro. Infect. Immun. 60:2368-2374.
John Heart Institute (Los Angeles, CA), respectively. P. M. Sullam was 22. Sullam, P. M., D. G. Payan, P. F. Dazin, and F. H. Valone. 1990. Binding
supported by VA Career Development Program and National Institutes of viridans group streptococci to human platelets: a quantitative analysis. Infect.
of Health grant AI-32506. A. L. Cheung is an Investigator of the New Immun. 58:3802-3806.
York Heart Association and is a recipient of an Irma T. Hirshl Career 23. Yeaman, M. R., S. M. Puentes, D. C. Norman, and A. S. Bayer. 1992.
Partial characterization and staphylocidal activity of thrombin-induced platelet
Scientist Award. microbicidal protein. Infect. Immun. 60:1202-1209.
24. Kornblum, J., S. J. Projan, S. L. Moghazeh, and R. Novick. 1988. A rapid
method to quantitate non-labeled RNA species in bacterial cells. Gene. 63:75-
References 85.
25. Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning, a
1. Waldvogel, F. A. 1985. Staphylococcus aureus. In Principles and Practice laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
of Infectious Diseases. G. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett, 26. Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis.
editors. John Wiley & Sons, New York. 1097-1116. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49.
2. Neu, H. C. 1992. The crisis in antibiotic resistance. Science (Wash. DC). 27. Scheld, W. M., J. A. Valone, and M. A. Sande. 1978. Bacterial adherence
257:1064-1072. in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets and
3. Cohen, M. L. 1992. Epidemiology of drug resistance: implications for a fibrin. J. Clin. Invest. 61:1394-1404.
post-antibiotic era. Science (Wash. DC). 257:1050-1055. 28. Herzberg, M. C., K. Gong, and G. D. McMarlane. 1990. Phenotypic
4. Easmon, C. S. F., and C. Adlam. 1983. Staphylococci and Staphylococcal characterization of Streptococcus sanguis virulence factors associated with bacte-
Infections. Academic Press, New York. 705-740. rial endocarditis. Infect. Immun. 58:515-522.
5. Cheung, A. L., M. Krishnan, E. A. Jaffe, and V. A. Fischetti. 1991. Fibrino- 29. Cheung, A. L., and V. A. Fischetti. 1990. The role of fibrinogen in
gen acts as a bridging molecule in the adherence of Staphylococcus aureus to staphylococcal adherence to catheters in vitro. J. Infect. Dis. 161:1177-1186.
cultured human endothelial cells. J. Clin. Invest. 87:2236-2245. 30. Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F.
6. Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrin-
Schumacher Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrin- ogen, and laminin act as mediators of adherence of clinical staphylococcal isolates
ogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701.
to foreign material. J. Infect. Dis. 158:693-701. 31. Cheung, A. L., and S. J. Projan. 1994. Cloning and sequencing of sarA:
7. Kuypers, J. M., and R. A. Proctor. 1989. Reduced adherence to traumatized a gene required for the expression of agr. J. Bacteriol. 176:4168-4172.
rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. 32. Scheld, W. M., R. W. Strunk, G. Balian, and R. A. Calderone. 1985.
Infect. Immun. 57:2306-2312. Microbial adhesion to fibronectin in vitro correlates with production of endocardi-
8. DeRita, V. J., and J. J. Mekalanos. 1989. Genetic regulation of bacterial tis in rabbits. Proc. Soc. Exp. Biol. Med. 180:474-482.
virulence. Annu. Rev. Genet. 23:455-482. 33. Durack, D. T., and P. B. Beeson. 1980. Pathogenesis of infective endocar-
9. Kornblum, J., B. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. ditis. In Infective Endocarditis. S. H. Rahimtoola, editor. Grune and Stratton, Inc.,
Agr: A polycistronic locus regulating exoprotein synthesis in Staphylococcus New York. 1-53.
aureus. In Molecular Biology of the Staphylococci. R. P. Novick, editor. VCH 34. Jaffe, E. 1987. Cell biology of endothelial cells. Hum. Pathol. 18:234-
Publishers, New York. 373-402. 239.
10. Nixon, B. T., C. W. Ronson, and R. M. Ausubel. 1986. Two component 35. Lopes, J. D., M. D. Reis, and R. R. Bretani. 1985. Presence of laminin
regulatory systems responsive to environmental stimuli share strongly conserved receptors in Staphylococcus aureus. Science (Wash. DC). 229:275-277.
domains with the nitrogen assimilation regulatory genes ntrB and btrC. Proc. 36. Patti, J. M., T. Bremell, D. Krajewska-Paetrasik, A. Adelnour, A. Tarkow-
Natl. Acad. Sci. USA. 83:7850-7854. ski, C. Ryden, and M. Hook. The Staphylococcus aureus collagen adhesin is a
11. Smeltzer, M. S., M. E. Hart, and J. J. Iandolo. 1993. Phenotypic character- virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161.
ization of xpr, a global regulator of extracellular virulence factors in Staphylococ- 37. Herrmann, M., S. J. Suchard, L. A. Boxer, F. A. Waldvogel, and P. D.
cus aureus. Infect. Immun. 61:919-925. Lew. 1991. Thrombospondin binds to Staphylococcus aureus and promotes staph-
12. Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. ylococcal adhesion to surfaces. Infect. Immun. 59:279-288.
Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by 38. Bhakdi, S., M. Muhly, U. Mannhardt, F. Hugo, K. Klapettek, C. Muller-
a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA. 89:6462-6466. Eckhardt, and L. Roka. 1988. Staphylococcal a-toxin promotes blood coagulation
13. Cheung, A. L., M. Yeaman, and A. S. Bayer. 1994. The role of the sar via attack on human platelets. J. Exp. Med. 168:527-542.
locus of Staphylococcus aureus in the induction of endocarditis in rabbits. Infect. 39. Suttorp, N., T. Hessz, W. Seeger, A. Wilke, R. Koob, F. Lutz, and D.
Immun. 62:1719-1725. Drenckhahn. 1988. Bacterial exotoxins and endothelial permeability for water and
14. Vandenesch, F., J. Kornblum, and R. P. Novick. 1991. A temporal signal, albumin in vitro. Am. J. Physiol. 255:C368.
independent of agr, is required for hla but not spa transcription in Staphylococcus 40. Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol.
aureus. J. Bacteriol. 173:6313-6320. 204:587-636.
15. Cheung, A. L., and P. Ying. 1994. Regulation of a and fi hemolysins by 41. O'Reilly, M., J. C. S. de Azavedo, S. Kennedy, and T. J. Foster. 1986.
the sar locus of Staphylococcus aureus. J. Bacteriol. 176:580-585. Inactivation of the alpha-hemolysin of Staphylococcus aureus 8325-4 by site
16. Rescei, P., B. Kreiswirth, M. O'Reilly, P. Schlievert, A. Gruss, and R. P. directed mutagenesis and studies on the expression of its haemolysins. Microb.
Novick. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus Pathog. 1:125-138.
by agr. Mol. & Gen. Genet. 202:58-61. 42. Projan, S. J., J. Kornblum, B. Kreiswirth, S. Moghazeh, W. Eisner, and
17. Froman, G., L. Switalski, P. Speziale, and M. Hook. 1987. Isolation and R. P. Novick. 1989. The 63 hemolysin gene of Staphylococcus aureus. Nucleic
characterization of a fibronectin receptor from Staphylococcus aureus. J. Biol. Acids Res. 17:3305.
Chem. 262:6564-6571. 43. Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagene-
18. Lantz, M., R. D. Allen, P. Bounelis, L. M. Switalski, and M. Hook. 1990. sis of Listeria monocytogenes with a novel Tn917 derivative that allows direct
Bacteroides gingivalis and Bacteroides intermedius recognize different sites on cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744.
human fibrinogen. J. Bacteriol. 172:716-726. 44. Nesin, M., P. Svec, J. R. Lupski, G. N. Godson, B. Kreiswirth, J. Korn-
19. Yeaman, M. R., P. M. Sullam, P. F. Dazin, D. C. Norman, and A. S. blum, and S. J. Projan. 1990. Cloning and nucleotide sequencing of a chromosom-
Bayer. 1992. Characterization of Staphylococcus aureus-platelet binding by quan- ally encoded tetracycline resistance determinant, tetA(M), from a pathogenic,
titative flow cytometric analysis. J. Infect. Dis. 166:65-73. methicillin resistant strain of Staphylococcus aureus. Antimicrob. Agents Chemo-
20. Clawson, C. C., J. G. White, and M. C. Herzberg. 1980. Platelet interaction ther. 34:2273-2276.
1822 Cheung et at.
Vous aimerez peut-être aussi
- Mechanisms of Eukaryotic DNA RecombinationD'EverandMechanisms of Eukaryotic DNA RecombinationMax E GottesmanPas encore d'évaluation
- High Prevalence of Methicillin-Resistant: Staphylococcus Haemolyticus Isolated From Commensals in Healthy AdultsDocument8 pagesHigh Prevalence of Methicillin-Resistant: Staphylococcus Haemolyticus Isolated From Commensals in Healthy AdultsElok NurfaiqohPas encore d'évaluation
- Bacillus Anthracis Produces Membrane-Derived Vesicles Containing Biologically Active ToxinsDocument6 pagesBacillus Anthracis Produces Membrane-Derived Vesicles Containing Biologically Active ToxinsGuhan KAPas encore d'évaluation
- Identification of A Staphylococcal Complement Inhibitor With Broad Host Specificity in Equid Staphylococcus Aureus StrainsDocument10 pagesIdentification of A Staphylococcal Complement Inhibitor With Broad Host Specificity in Equid Staphylococcus Aureus StrainsChlo14Pas encore d'évaluation
- 2007 Effects of Sex, Parity, and Sequence Variation On Seroreactivity To Candidate Pregnancy Malaria Vaccine AntigensDocument10 pages2007 Effects of Sex, Parity, and Sequence Variation On Seroreactivity To Candidate Pregnancy Malaria Vaccine AntigenssethawudPas encore d'évaluation
- Ec 00174-07Document8 pagesEc 00174-07dj1223549Pas encore d'évaluation
- PNAS 1999 Tan 2408 13Document6 pagesPNAS 1999 Tan 2408 13chai236Pas encore d'évaluation
- Articulo 7 Mig OkDocument11 pagesArticulo 7 Mig Okoscarbio20090% (1)
- The Genetics of ImmunityDocument4 pagesThe Genetics of ImmunityichaPas encore d'évaluation
- Khan 2014Document9 pagesKhan 2014M.Febrian BachtiarPas encore d'évaluation
- 49Document8 pages49Angel SousouPas encore d'évaluation
- Patfis Molecular PDFDocument8 pagesPatfis Molecular PDFHardi HutabaratPas encore d'évaluation
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins As Target For Pan-Coronavirus InhibitorsDocument15 pagesThe SARS-Coronavirus-Host Interactome: Identification of Cyclophilins As Target For Pan-Coronavirus Inhibitorsfaris daboolPas encore d'évaluation
- Evaluation of Antibiotic Susceptibilities of Ehrlichia Canis, Ehrlichia Chaffeensis, and Anaplasma Phagocytophilum by Real-Time PCRDocument7 pagesEvaluation of Antibiotic Susceptibilities of Ehrlichia Canis, Ehrlichia Chaffeensis, and Anaplasma Phagocytophilum by Real-Time PCRyudhi arjentiniaPas encore d'évaluation
- Bacterial Control of Host Gene ExpressioDocument14 pagesBacterial Control of Host Gene ExpressioJose Leonel Fajardo RapaloPas encore d'évaluation
- Literature Review On KlebsiellaDocument4 pagesLiterature Review On Klebsiellaafmztwoalwbteq100% (1)
- Molecular Characterization of Clinical Multidrug-Resistant Klebsiella Pneumoniae IsolatesDocument5 pagesMolecular Characterization of Clinical Multidrug-Resistant Klebsiella Pneumoniae IsolatesSamman zakaPas encore d'évaluation
- Sortase A Contributes To Pneumococcal Nasopharyngeal Colonization in The Chinchilla ModelDocument4 pagesSortase A Contributes To Pneumococcal Nasopharyngeal Colonization in The Chinchilla ModelRin ChanPas encore d'évaluation
- 2012 SPN MaldiDocument6 pages2012 SPN MaldiSergey SidorenkoPas encore d'évaluation
- Molecular Epidemiologic Analysis of Enterococcus FaecalisDocument8 pagesMolecular Epidemiologic Analysis of Enterococcus FaecalisoinkPas encore d'évaluation
- Apoptosis in Natural Rabies Virus Infection in DogsDocument5 pagesApoptosis in Natural Rabies Virus Infection in DogspapiipiiPas encore d'évaluation
- Antimicrobial Resistance and Molecular Characterization of Gene Cassettes From Class 1 Integrons in Pseudomonas Aeruginosa S PDFDocument7 pagesAntimicrobial Resistance and Molecular Characterization of Gene Cassettes From Class 1 Integrons in Pseudomonas Aeruginosa S PDFValentina RondonPas encore d'évaluation
- Journal of Virology-2010-Graham-3134.fullDocument13 pagesJournal of Virology-2010-Graham-3134.fullJohn WilliamsonPas encore d'évaluation
- Arti IngleDocument6 pagesArti Ingleshadow darkPas encore d'évaluation
- Articulo 1 InmunogenomicaDocument6 pagesArticulo 1 InmunogenomicaMichelle PoulainPas encore d'évaluation
- Emergence of Ciprofloxacin Heteroresistance in Foodborne Salmonella Enterica Serovar AgonaDocument7 pagesEmergence of Ciprofloxacin Heteroresistance in Foodborne Salmonella Enterica Serovar AgonaAdul BenchawanPas encore d'évaluation
- FulltextDocument12 pagesFulltextakshadaranalkar27Pas encore d'évaluation
- 5.parish KDPD Mice 2003Document7 pages5.parish KDPD Mice 2003Juan Camilo Ocampo MartinezPas encore d'évaluation
- Typing African Relapsing Fever Spirochetes: Julie Christine Scott, David Julian Maurice Wright, and Sally Jane CutlerDocument8 pagesTyping African Relapsing Fever Spirochetes: Julie Christine Scott, David Julian Maurice Wright, and Sally Jane Cutlerjesus hazeem Contreras VallejoPas encore d'évaluation
- Appl. Environ. Microbiol. 2001 Steidle 5761 70Document10 pagesAppl. Environ. Microbiol. 2001 Steidle 5761 70Iulia IonPas encore d'évaluation
- Klebsiella Pneumoniae ThesisDocument6 pagesKlebsiella Pneumoniae Thesisaprillaceyjackson100% (1)
- Jem 1953359Document7 pagesJem 195335990Agva Dwi FatikaPas encore d'évaluation
- BF01309668Document13 pagesBF01309668pafspontesPas encore d'évaluation
- Interaction of Bunyamwera Orthobunyavirus Nss Protein With Mediator Protein Med8: A Mechanism For Inhibiting The Interferon ResponseDocument9 pagesInteraction of Bunyamwera Orthobunyavirus Nss Protein With Mediator Protein Med8: A Mechanism For Inhibiting The Interferon ResponseJUAN OSWALDO CONCHA CASAVERDEPas encore d'évaluation
- Aem 02081-12Document5 pagesAem 02081-12Dylan TeránPas encore d'évaluation
- Molecular Characterization of A 28 Kda Surface Antigen Gene Family of The Tribe EhrlichiaeDocument8 pagesMolecular Characterization of A 28 Kda Surface Antigen Gene Family of The Tribe EhrlichiaeDIANA PATRICIA MOLANO PEREZPas encore d'évaluation
- Full TextfDocument5 pagesFull TextfVALDINEI JUNIO BRITO VILELAPas encore d'évaluation
- Candida and Candidiasis 2023 Abstract BookDocument209 pagesCandida and Candidiasis 2023 Abstract BooknohemiPas encore d'évaluation
- Thesis Paper On PneumoniaDocument7 pagesThesis Paper On Pneumoniagbwwdfq5100% (1)
- Arqma Quimica PhytoptoaDocument4 pagesArqma Quimica PhytoptoaOmar CabezasPas encore d'évaluation
- Epitope Mapping of Trans-Sialidase From Trypanosoma Cruzi RevealsDocument7 pagesEpitope Mapping of Trans-Sialidase From Trypanosoma Cruzi RevealsmclimacoPas encore d'évaluation
- J Immunol 2007 Rydström 5789 801Document14 pagesJ Immunol 2007 Rydström 5789 801susana1616Pas encore d'évaluation
- Molecular Analysis of Antimicrobial Resistance Mechanisms in N-GonoDocument11 pagesMolecular Analysis of Antimicrobial Resistance Mechanisms in N-GonoMulatuPas encore d'évaluation
- Methicillin-Resistant StaphylococcusDocument5 pagesMethicillin-Resistant StaphylococcusAriane Barcellos Dos SantosPas encore d'évaluation
- Cortes Lara, 2021Document7 pagesCortes Lara, 2021Gabriela Chichón de la FuentePas encore d'évaluation
- Dex3 and Survivin-2B: Two Novel Splice Variants of The ApoptosisDocument6 pagesDex3 and Survivin-2B: Two Novel Splice Variants of The ApoptosisNona NonicaaPas encore d'évaluation
- Campylobacter Jejuni Biofilms Up-Regulated in The Absence of The Stringent Response Utilize A Calcofluor White-Reactive PolysaccharideDocument11 pagesCampylobacter Jejuni Biofilms Up-Regulated in The Absence of The Stringent Response Utilize A Calcofluor White-Reactive PolysaccharideMichaelPas encore d'évaluation
- Fungi: Allergic Bronchopulmonary AspergillosisDocument18 pagesFungi: Allergic Bronchopulmonary AspergillosisChristian Andrés CasasPas encore d'évaluation
- Alterations in Macrophage-Produced CytokinesDocument8 pagesAlterations in Macrophage-Produced CytokinessanielPas encore d'évaluation
- 4 Tomova 2015Document9 pages4 Tomova 2015Joel SolisPas encore d'évaluation
- Suraprasit 1Document9 pagesSuraprasit 1Denis ChandraPas encore d'évaluation
- Mast Cells in Periapical Lesions: Potential Role in Their PathogenesisDocument6 pagesMast Cells in Periapical Lesions: Potential Role in Their Pathogenesiskaren marcela rodriguez solanoPas encore d'évaluation
- Random Distribution of Mixed Species Malaria Infections in Papua New GuineaDocument7 pagesRandom Distribution of Mixed Species Malaria Infections in Papua New GuineaAwliyana Risla PutriPas encore d'évaluation
- Epidermidis Carrying Biofilm Formation GenesDocument5 pagesEpidermidis Carrying Biofilm Formation GenesLini MaliqisnayantiPas encore d'évaluation
- Neisseria 1Document16 pagesNeisseria 1RaffaharianggaraPas encore d'évaluation
- Dks 002Document9 pagesDks 002RizkyPas encore d'évaluation
- Veterinary Immunology and ImmunopathologyDocument7 pagesVeterinary Immunology and Immunopathologyjunior5787Pas encore d'évaluation
- Aem 69 8 4806-4813 2003Document8 pagesAem 69 8 4806-4813 2003chafeb febiPas encore d'évaluation
- Antimicrobial Agents and Chemotherapy-2015-Pöppel-2508.fullDocument7 pagesAntimicrobial Agents and Chemotherapy-2015-Pöppel-2508.fullEEDIEB Prof. Millton Marques CurvoPas encore d'évaluation
- Ampicillin Sodium - Sulbactam Sodium Drug StudyDocument1 pageAmpicillin Sodium - Sulbactam Sodium Drug StudyMelissa Marie Custodio100% (3)
- Annotated Bibliography FinalDocument2 pagesAnnotated Bibliography FinalAndrew LoppPas encore d'évaluation
- Adventist University of The PhilippinesDocument2 pagesAdventist University of The PhilippinesWinona ShenaniPas encore d'évaluation
- USPI - Bicillin C-R - Penicillin G Benzathine, Penicillin GDocument11 pagesUSPI - Bicillin C-R - Penicillin G Benzathine, Penicillin GRoberto Alfredo SáenzPas encore d'évaluation
- 5000 Q&aDocument60 pages5000 Q&aaboaasy96% (25)
- Peningkatan Penggunaan Antibiotik Secara Bijak Melalui Intervensi Alih Pengetahuan Untuk Merubah Persepsi Masyarakat Di Kabupaten BanyumasDocument8 pagesPeningkatan Penggunaan Antibiotik Secara Bijak Melalui Intervensi Alih Pengetahuan Untuk Merubah Persepsi Masyarakat Di Kabupaten BanyumasasmanPas encore d'évaluation
- AntibioticsDocument7 pagesAntibioticsRahmania Eka SagitaPas encore d'évaluation
- Antibiotic EssentialsDocument6 pagesAntibiotic EssentialsAna MariaPas encore d'évaluation
- DR - Kima PresentationDocument49 pagesDR - Kima Presentationdvo aizawlPas encore d'évaluation
- Meso 2021Document2 pagesMeso 2021Happy Serevia AdistyPas encore d'évaluation
- PharmaDocument275 pagesPharmarahul sharma0% (1)
- 2013 34 438 Rani S. Gereige and Pablo Marcelo Laufer: Pediatrics in ReviewDocument21 pages2013 34 438 Rani S. Gereige and Pablo Marcelo Laufer: Pediatrics in ReviewAlvaro Andres Flores JimenezPas encore d'évaluation
- Research ProposalDocument6 pagesResearch ProposalJerimiah Miranda100% (1)
- Final EditedDocument31 pagesFinal Editedlaehaaaa40% (5)
- Classification of AntibioticsDocument14 pagesClassification of Antibioticsdoc_abdullahPas encore d'évaluation
- Cefepime MaxipimeDocument2 pagesCefepime MaxipimeKristi Wray100% (1)
- DR - Dash Profile Nutra Now 14 PDFDocument19 pagesDR - Dash Profile Nutra Now 14 PDFChintalaRaoPas encore d'évaluation
- Questions Evafos Avneeshh0427 18052023125059290Document3 pagesQuestions Evafos Avneeshh0427 18052023125059290Manoj TambePas encore d'évaluation
- Mankind FinDocument12 pagesMankind FinHitesh BhadiyadaraPas encore d'évaluation
- Examples of Antibiotic Sensitivity Testing Methods - Antimicrobial ResistanceDocument3 pagesExamples of Antibiotic Sensitivity Testing Methods - Antimicrobial ResistanceGuranda SilvianPas encore d'évaluation
- Tendencia - Antibiotic Resistance of Bacteria From Shrimp PondsDocument12 pagesTendencia - Antibiotic Resistance of Bacteria From Shrimp PondsDet GuillermoPas encore d'évaluation
- Chapter 22 The Organic Chemistry of Drugs: Discovery and DesignDocument18 pagesChapter 22 The Organic Chemistry of Drugs: Discovery and Designtyron9520Pas encore d'évaluation
- Amoxicillin PDFDocument6 pagesAmoxicillin PDFNaveen KumarPas encore d'évaluation
- Updated IDSA - ATS Guidelines On Management of Adults With HAP and VAPDocument11 pagesUpdated IDSA - ATS Guidelines On Management of Adults With HAP and VAPr dwiandiniPas encore d'évaluation
- BUANADocument53 pagesBUANAIntanRatnadii Ni PutuPas encore d'évaluation
- In Vitro Study On The Effects of Temperature and PH On Antimicrobial Activity of Aqueous Garlic ExtractDocument48 pagesIn Vitro Study On The Effects of Temperature and PH On Antimicrobial Activity of Aqueous Garlic ExtractThomas Sykes-BarnettPas encore d'évaluation
- Bacteriophage TherapyDocument5 pagesBacteriophage TherapyMário FernandesPas encore d'évaluation
- Natural Products As Pharmaceuticals and Sources of LeadDocument15 pagesNatural Products As Pharmaceuticals and Sources of LeadQudsia RehmanPas encore d'évaluation
- Антимикробиал чбDocument71 pagesАнтимикробиал чбSangeeta SharmaPas encore d'évaluation
- Jurnal Reading OsteomyelitisDocument11 pagesJurnal Reading Osteomyelitisfidela_ffPas encore d'évaluation
- Do You Believe in Magic?: The Sense and Nonsense of Alternative MedicineD'EverandDo You Believe in Magic?: The Sense and Nonsense of Alternative MedicinePas encore d'évaluation
- The Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsD'EverandThe Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsÉvaluation : 4.5 sur 5 étoiles4.5/5 (6)
- Summary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisD'EverandSummary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisÉvaluation : 4 sur 5 étoiles4/5 (9)
- Uncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicD'EverandUncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicPas encore d'évaluation
- The Gut-Immune Connection: How Understanding the Connection Between Food and Immunity Can Help Us Regain Our HealthD'EverandThe Gut-Immune Connection: How Understanding the Connection Between Food and Immunity Can Help Us Regain Our HealthPas encore d'évaluation
- Fatal Conveniences: The Toxic Products and Harmful Habits That Are Making You Sick—and the Simple Changes That Will Save Your HealthD'EverandFatal Conveniences: The Toxic Products and Harmful Habits That Are Making You Sick—and the Simple Changes That Will Save Your HealthÉvaluation : 4 sur 5 étoiles4/5 (7)
- There Are No Accidents: The Deadly Rise of Injury and Disaster—Who Profits and Who Pays the PriceD'EverandThere Are No Accidents: The Deadly Rise of Injury and Disaster—Who Profits and Who Pays the PriceÉvaluation : 4.5 sur 5 étoiles4.5/5 (15)
- Deaths of Despair and the Future of CapitalismD'EverandDeaths of Despair and the Future of CapitalismÉvaluation : 4.5 sur 5 étoiles4.5/5 (30)
- The Truth about Wuhan: How I Uncovered the Biggest Lie in HistoryD'EverandThe Truth about Wuhan: How I Uncovered the Biggest Lie in HistoryÉvaluation : 4 sur 5 étoiles4/5 (6)
- Sickening: How Big Pharma Broke American Health Care and How We Can Repair ItD'EverandSickening: How Big Pharma Broke American Health Care and How We Can Repair ItÉvaluation : 4 sur 5 étoiles4/5 (9)
- The Hair Color Mix Book: More Than 150 Recipes for Salon-Perfect Color at HomeD'EverandThe Hair Color Mix Book: More Than 150 Recipes for Salon-Perfect Color at HomeÉvaluation : 3.5 sur 5 étoiles3.5/5 (7)
- The Inescapable Immune Escape PandemicD'EverandThe Inescapable Immune Escape PandemicÉvaluation : 5 sur 5 étoiles5/5 (1)
- Epidemics and Society: From the Black Death to the PresentD'EverandEpidemics and Society: From the Black Death to the PresentÉvaluation : 4.5 sur 5 étoiles4.5/5 (9)
- Vaccines Did Not Cause Rachel's Autism: My Journey as a Vaccine Scientist, Pediatrician, and Autism DadD'EverandVaccines Did Not Cause Rachel's Autism: My Journey as a Vaccine Scientist, Pediatrician, and Autism DadÉvaluation : 4.5 sur 5 étoiles4.5/5 (3)
- Profiles of the Vaccine-Injured: "A Lifetime Price to Pay"D'EverandProfiles of the Vaccine-Injured: "A Lifetime Price to Pay"Évaluation : 3.5 sur 5 étoiles3.5/5 (3)
- War on Ivermectin: The Medicine that Saved Millions and Could Have Ended the PandemicD'EverandWar on Ivermectin: The Medicine that Saved Millions and Could Have Ended the PandemicÉvaluation : 4 sur 5 étoiles4/5 (7)
- Make America Healthy Again: How Bad Behavior and Big Government Caused a Trillion-Dollar CrisisD'EverandMake America Healthy Again: How Bad Behavior and Big Government Caused a Trillion-Dollar CrisisPas encore d'évaluation
- Summary: The Real Anthony Fauci: Bill Gates, Big Pharma, and the Global War on Democracy and Public Health by Robert F. Kennedy Jr: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: The Real Anthony Fauci: Bill Gates, Big Pharma, and the Global War on Democracy and Public Health by Robert F. Kennedy Jr: Key Takeaways, Summary & Analysis IncludedPas encore d'évaluation
- Environmental Health and Occupational Health & SafetyD'EverandEnvironmental Health and Occupational Health & SafetyÉvaluation : 3.5 sur 5 étoiles3.5/5 (9)
- Community-Acquired Pneumonia: Strategies for ManagementD'EverandCommunity-Acquired Pneumonia: Strategies for ManagementAntoni TorresÉvaluation : 4.5 sur 5 étoiles4.5/5 (2)
- The Wuhan Cover-Up: And the Terrifying Bioweapons Arms RaceD'EverandThe Wuhan Cover-Up: And the Terrifying Bioweapons Arms RacePas encore d'évaluation
- The Atlas of Disease: Mapping Deadly Epidemics and Contagion from the Plague to the CoronavirusD'EverandThe Atlas of Disease: Mapping Deadly Epidemics and Contagion from the Plague to the CoronavirusÉvaluation : 4.5 sur 5 étoiles4.5/5 (10)
- "Cause Unknown": The Epidemic of Sudden Deaths in 2021 & 2022D'Everand"Cause Unknown": The Epidemic of Sudden Deaths in 2021 & 2022Évaluation : 4.5 sur 5 étoiles4.5/5 (19)
- Clean: Overcoming Addiction and Ending America’s Greatest TragedyD'EverandClean: Overcoming Addiction and Ending America’s Greatest TragedyÉvaluation : 4 sur 5 étoiles4/5 (18)