Académique Documents
Professionnel Documents
Culture Documents
Halogenoalkanes, Nucleophilic Substitution, Elimination Reactions, Uses and CFC Problems PDF
Transféré par
Grace KamauTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Halogenoalkanes, Nucleophilic Substitution, Elimination Reactions, Uses and CFC Problems PDF
Transféré par
Grace KamauDroits d'auteur :
Formats disponibles
Halogenoalkanes 1
HALOALKANES (HALOGENOALKANES)
Structure Contain the functional group C-X where X is a halogen (F, Cl, Br or I)
Types Haloalkanes - halogen is attached to an aliphatic skeleton - alkyl group
Haloarenes - halogen is attached directly to a benzene (aromatic) ring
Classification Classified according to what is attached to the functional group.
H H C C
H C X C C X C C X C C X
H H H C
methyl primary secondary tertiary
1° 2° 3°
Names Based on the original alkane with a prefix indicating halogens and their position.
Knockhardy Publishing
CH3CH2CH2Cl 1-chloropropane CH2ClCHClCH3 1,2-dichloropropane
CH3CHClCH3 2-chloropropane CH3CBr(CH3)CH3 2-bromo-2-methylpropane
Q.1 Draw and name all the structural isomers of C3H6Br2, C4H9Cl and C5H11Br.
Q.2 Classify the structural isomers of C4H9Cl and C5H11Br as 1°, 2° or 3°.
Physical properties
Boiling
points • boiling point increases with mass
• for isomeric compounds the greater the branching, the lower the boiling point
Solubility • haloalkanes are soluble in organic solvents but insoluble in water - they
are not polar enough and don’t exhibit hydrogen bonding.
© KNOCKHARDY PUBLISHING 2015
2 Halogenoalkanes
NUCLEOPHILIC SUBSTITUTION REACTIONS
Theory • halogens have a greater electronegativity than carbon δ+ δ−
• a dipole is induced in the C-X bond and it becomes polar C Br
• the carbon is thus open to attack by nucleophiles polarity in a C-Br bond
Nucleophiles • examples are OH¯, CN¯, NH3 and H2O
• possess at least one LONE PAIR of electrons
• are attracted to the slightly positive (electron deficient) carbon
Basic
mechanism • the nucleophile uses its lone pair to provide the electrons for a new bond
• as carbon can only have 8 electrons in its outer shell a halide ion is displaced
• the result is substitution following attack by a nucleophile
• the mechanism is therefore known as - NUCLEOPHILIC SUBSTITUTION
Nu:-
Knockhardy Publishing
H H H H
-
: :
H C C Br H C C Nu + : Br:
H H H H
Rate of
reaction • the rate of reaction depends on the strength not the polarity of the C-X bond
C-I 238 kJmol-1
........... least polar
276 kJmol-1 WEAKEST BOND
C-Br ...........
338 EASIEST TO BREAK
C-Cl ........... kJmol-1 FASTEST REACTION
484 kJmol-1 most polar
C-F ...........
Practical
investigation The time taken for a precipitate of silver halide is measured. The faster the
precipitate forms, the faster the hydrolysis and the weaker the C-X bond.
• warm equal amounts of each haloalkane in a water bath
• add a solution of ethanol, water and aqueous silver nitrate to each
• record the time it takes for a precipitate to appear
AgCl - white AgBr - cream AgI - yellow (AgF is soluble)
© KNOCKHARDY PUBLISHING 2015
Halogenoalkanes 3
NaOH Reagent AQUEOUS sodium (or potassium) hydroxide
Conditions Reflux in aqueous solution (SOLVENT IS IMPORTANT)
Product Alcohol
Nucleophile hydroxide ion (OH¯)
Equation e.g. C2H5Br(l) + NaOH(aq) ——> C2H5OH(l) + NaBr(aq)
Mechanism
-
: :
HO: H H H H
-
: :
: :
H C C Br H C C OH + : Br:
H H H H
WARNING It is important to quote the solvent when answering questions.
Elimination takes place when ethanol is the solvent.
This reaction (and the one with water) is sometimes known as HYDROLYSIS
Knockhardy Publishing
H2O • A similar reaction to the above but SLOWER because...
a) the liquids are immiscible - less chance of molecules colliding
b) water is a poor nucleophile
• faster reaction in an alcohol/water mixture; miscible = more collisions
Equation e.g. C2H5Br(l) + H2O(l) ——> C2H5OH(aq/alc) + HBr(aq)
AgNO3 Used to identify the halide in a haloalkane - see above
(aq/alc)
Q.3 Write equations for the reactions of hot, aqueous NaOH with...
a) CH3CH2CH2 Br
b) CH3CHBrCH2CH3
c) (CH3)3CBr
Advanced This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular
work process. An alternative method involves the initial breaking of the C-X bond to form a
carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then
attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric
hindrance to the attack and a more stable tertiary, 3°, carbocation intermediate is formed.
© KNOCKHARDY PUBLISHING 2015
4 Halogenoalkanes
KCN Reagent Aqueous, alcoholic potassium (or sodium) cyanide
Conditions Reflux in aqueous, alcoholic solution
Product Nitrile (cyanide)
Nucleophile cyanide ion (CN¯)
Equation C2H5Br + KCN(aq/alc) ——> C2H5CN + KBr
Mechanism
N C :- H H H H
-
: :
H C C Br H C C CN + : Br:
H H H H
Importance extends the carbon chain by one carbon atom as the CN
group can then be converted to carboxylic acids or amines
Hydrolysis C2H5CN + 2H2O ——> C2H5COOH + NH3
Reduction C2H5CN + 4[H] ——> C2H5CH2NH2
Knockhardy Publishing
NH3 Reagent Aqueous, alcoholic ammonia (in EXCESS)
Conditions Reflux in aqueous, alcoholic solution under pressure
Product Amine (or its salt due to a reaction with the acid produced)
Nucleophile Ammonia (NH3)
Equation C2H5Br + NH3 (aq/alc) ——> C2H5NH2 + HBr
HBr + NH3(aq/alc) ——> NH4Br
C2H5Br + 2NH3(aq/alc) ——> C2H5NH2 + NH4Br
Mechanism
H
H N:
H H H H H H
H H
+ -
: :
H C C Br H C C N H + : Br: H C C N H2 + HBr
H
H H H H H H
Why the excess ammonia?
The second ammonia molecule ensures the removal of HBr which would lead to
the formation of a salt.
A large excess of ammonia ensures further substitution doesn’t take place
© KNOCKHARDY PUBLISHING 2015
Halogenoalkanes 5
Problem The amine produced is also a nucleophile (lone pair on the N) and can attack
another molecule of haloalkane to produce a 2° amine. This in turn is a
nucleophile and can react further producing a 3° amine and, eventually an ionic
quarternary ammonium salt.
C2H5NH2 + C2H5Br —> HBr + (C2H5)2NH diethylamine, a 2° amine
(C2H5)2NH + C2H5Br —> HBr + (C2H5)3N triethylamine, a 3° amine
(C2H5)3N + C2H5Br —> (C2H5)4N+ Br¯ tetraethylammonium bromide,
(a 4° salt)
OTHER REACTIONS OF HALOALKANES
Friedel Crafts
alkylation substitutes an alkyl (e.g. methyl, ethyl) group onto a benzene ring
reagents a haloalkane (RX) and anhydrous aluminium chloride AlCl3
conditions room temperature; dry inert solvent (ether)
Knockhardy Publishing
mechanism electrophilic substitution
electrophile a carbocation ion R+ (e.g. CH3+)
AlCl3
equation C2H5Br + C6H6 ——> C6H5C2H5 + HBr
see notes on benzene
Preparation of haloalkanes - Summary
(details can be found in other sections)
From
alkanes CH4 + Cl2 —> CH3Cl + HCl Free radical substitution / UV light
alkenes C2H4 + HBr —> C2H5Br Electrophilic addition / no catalyst or UV
alcohols C2H5OH + HBr —> C2H5Br + H2O Protonation of alcohol with acid catalyst
© KNOCKHARDY PUBLISHING 2015
6 Halogenoalkanes
USES OF HALOALKANES
Synthetic The reactivity of the C-X bond means that haloalkanes play an important part in
synthetic organic chemistry. The halogen can be replaced by a variety of groups
via a nucleophilic substitution mechanism.
During the manufacture of ibuprofen, substitution of a bromine atom takes place.
Monomers chloroethene CH2 = CHCl tetrafluoroethene CF2 = CF2
Polymers poly(chloroethene) PVC —(CH2 — CHCl)n— packaging
poly(tetrafluoroethene) PTFE —(CF2 — CF2)n— non-stick surfaces
CFC’s dichlorofluoromethane CHFCl2 refrigerant
trichlorofluoromethane CF3Cl aerosol propellant
blowing agent
bromochlorodifluoromethane CBrClF2 fire extinguishers
CCl2FCClF2 dry cleaning solvent
Knockhardy Publishing
degreasing agent
All the above were chosen because of their... • low reactivity
• volatility
• non-toxicity
PROBLEMS WITH CFC’s
Ozone layer • CFC’s have been blamed for environmental damage by thinning the ozone layer
• Ozone absorbs a lot of harmful UV radiation
• CFC’s break up in the atmosphere to form free radicals
CF2Cl2 ——> •CF2Cl + •Cl
• free radicals catalyse ozone decomposition e.g. •Cl + O3 ——> •ClO + O2
•ClO + O ——> •Cl + O2
• overall 2O3 ——> 3O2
Solution • CFC’s were designed by chemists to help people
• chemists now synthesise alternatives to CFC’s to protect the environment
such as hydrocarbons and HCFC’s
• CO2 can be use as an alternative blowing agent
• this will allow the reversal of the ozone layer problem
© KNOCKHARDY PUBLISHING 2015
Halogenoalkanes 7
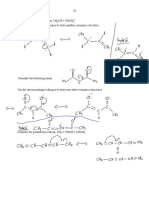
ELIMINATION REACTIONS OF HALOALKANES
Problem The products of reactions between haloalkanes and OH¯ are influenced by the
solvent. Both mechanisms take place simultaneously but the choice of solvent
favours one route.
Solvent Product Action of OH¯ Mechanism
WATER ALCOHOL NUCLEOPHILE SUBSTITUTION
ALCOHOL ALKENE BASE ELIMINATION
Reaction Reagent Alcoholic sodium (or potassium) hydroxide
Conditions Reflux in alcoholic solution
Product Alkene
Mechanism Elimination
Equation C3H7Br + NaOH(alc) ——> C3H6 + H2O + NaBr
Mechanism
Knockhardy Publishing
HO:-
: :
H H H H
δ+ δ− -
:
CH3 C C Br C C + H2O + : Br:
:
CH3 H
H H
• the OH¯ ion acts as a base and picks up a proton
• the proton comes from a carbon atom next to the one bonded to the halogen
• the electron pair left moves to form a second bond between the carbon atoms
• the halide ion is displaced
• overall there is ELIMINATION of HBr.
Q.4 What organic products are formed when concurrent substitution and elimination
takes place with CH3CHBrCH3 ?
Complication The OH¯ removes a proton from a carbon atom adjacent the C bearing the
halogen. If there had been another carbon atom on the other side of the C-X, its
hydrogen(s) would also be open to attack. If the haloalkane is unsymmetrical
(e.g. 2-bromobutane) a mixture of isomeric alkene products is obtained.
Q.5 What organic products do you get with alcoholic NaOH and CH3CHBrCH2CH3 ?
Explain your answers with a mechanism.
© KNOCKHARDY PUBLISHING 2015
Vous aimerez peut-être aussi
- Haloalkanes PDFDocument6 pagesHaloalkanes PDFthc8477Pas encore d'évaluation
- Lesson 8 Reactions of HalogenoalkanesDocument15 pagesLesson 8 Reactions of Halogenoalkanesdela2Pas encore d'évaluation
- 4alkyl Halides and AlcoholsDocument85 pages4alkyl Halides and AlcoholssharmimiameerasanadyPas encore d'évaluation
- Alkyl HalideDocument54 pagesAlkyl HalideNelvianaPas encore d'évaluation
- Substitution Elimination (Alkilhalides) Bab 5 FessendenDocument113 pagesSubstitution Elimination (Alkilhalides) Bab 5 Fessendenahmad jamalPas encore d'évaluation
- Alkyl Halides & Aryl Halides-01 - TheoryDocument32 pagesAlkyl Halides & Aryl Halides-01 - TheoryRaju SinghPas encore d'évaluation
- chm207 chp5Document92 pageschm207 chp5Muhd Mirza HizamiPas encore d'évaluation
- 14.0 Haloalkanes - 02Document4 pages14.0 Haloalkanes - 02Mumtaz BarhiyaPas encore d'évaluation
- Chapter 6: Organohalogens: Alkyl Halide Vinyl Halide Aryl HalideDocument13 pagesChapter 6: Organohalogens: Alkyl Halide Vinyl Halide Aryl HalidecikguhafidzuddinPas encore d'évaluation
- Lectures Ch4 Ns PDFDocument85 pagesLectures Ch4 Ns PDFPatricio VillarrealPas encore d'évaluation
- Alkyl Halides - Elimination ReactionsDocument25 pagesAlkyl Halides - Elimination ReactionsWidya FatmawatiPas encore d'évaluation
- 6.1.2 Revision Guide CarbonylsDocument3 pages6.1.2 Revision Guide Carbonylsannabel.turner1412Pas encore d'évaluation
- 6 8 Acyl Chlorides and Acid AnhydridesDocument5 pages6 8 Acyl Chlorides and Acid AnhydridesPedro Moreno de SouzaPas encore d'évaluation
- Draw Out Some Suitable Structures Which Fit The Molecular Formula C HDocument4 pagesDraw Out Some Suitable Structures Which Fit The Molecular Formula C HSean s ChipangaPas encore d'évaluation
- Alkil HalidaDocument58 pagesAlkil Halidadellany ayuninditaPas encore d'évaluation
- The Chemistry of HaloalkanesDocument42 pagesThe Chemistry of HaloalkanesMervinboPas encore d'évaluation
- CHAPTER 6 Alkyl Halides and Aryl HalidesDocument150 pagesCHAPTER 6 Alkyl Halides and Aryl HalidesexpertwritersPas encore d'évaluation
- Organic Reaction: Addition Substitution Elimination RearrangementDocument39 pagesOrganic Reaction: Addition Substitution Elimination RearrangementJulJayaPas encore d'évaluation
- Halo Alkanes & HaloarenesDocument30 pagesHalo Alkanes & HaloarenesEarPas encore d'évaluation
- Chapter 8 SlidesDocument63 pagesChapter 8 SlidespoojaPas encore d'évaluation
- 5 Alkyl HalideDocument53 pages5 Alkyl Haliderusnah chungPas encore d'évaluation
- Ch8 Elimination ReactionDocument25 pagesCh8 Elimination Reactionsitiaisyah harizamrryPas encore d'évaluation
- Haloalkanes Haloarnes NotesDocument44 pagesHaloalkanes Haloarnes Noteshareharanbt22Pas encore d'évaluation
- BPS 2110 F15 MetabolismDocument19 pagesBPS 2110 F15 MetabolismSumayah Al-SamiPas encore d'évaluation
- Naming HalogenoalkanesDocument13 pagesNaming HalogenoalkanesAsma AkterPas encore d'évaluation
- Carbocations & CarbanionDocument41 pagesCarbocations & CarbanionMuttu MPas encore d'évaluation
- Organic Chemistry Ii CHM 301: (Chapter 4)Document14 pagesOrganic Chemistry Ii CHM 301: (Chapter 4)WAN NUR AISYAH WAN AZIZANPas encore d'évaluation
- AlkyneDocument11 pagesAlkyneAnil KumarPas encore d'évaluation
- L5 BiologyDocument26 pagesL5 Biologyسلطان العكاديPas encore d'évaluation
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document22 pagesPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak ModiPas encore d'évaluation
- Chapter 6 Nucleophilic Substitution On Saturated CarbonsDocument46 pagesChapter 6 Nucleophilic Substitution On Saturated CarbonsNUR HAZWANI BINTI MOHAMAD SANI / UPMPas encore d'évaluation
- Chapter 8: Chemistry of Alkynes (C H) Bonding & HybridizationDocument11 pagesChapter 8: Chemistry of Alkynes (C H) Bonding & HybridizationimPERFECTme09Pas encore d'évaluation
- SN and E 2019 PDFDocument108 pagesSN and E 2019 PDFDwi ShabrinaPas encore d'évaluation
- Organic Chemistry: CollegeDocument34 pagesOrganic Chemistry: CollegeArwa AhmedPas encore d'évaluation
- Amino Acid and BiochemistryDocument10 pagesAmino Acid and BiochemistryUNKNOWNPas encore d'évaluation
- Chapter 5 Elimination Reaction - 2016Document19 pagesChapter 5 Elimination Reaction - 2016Syuhadah NoordinPas encore d'évaluation
- Reactions of Ether: Do Not ReactDocument10 pagesReactions of Ether: Do Not ReactcikguhafidzuddinPas encore d'évaluation
- Aldehydes and KetonesDocument7 pagesAldehydes and KetonesA LEVEL TOPPas encore d'évaluation
- Haloalkanes and HaloarenesDocument16 pagesHaloalkanes and Haloarenesnadeemmessi30Pas encore d'évaluation
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document21 pagesPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak Modi100% (1)
- 2016 Lect6a Substitution Elimination (Alkilhalides)Document144 pages2016 Lect6a Substitution Elimination (Alkilhalides)Bryan SuryapranataPas encore d'évaluation
- Aldehid Keton 08Document48 pagesAldehid Keton 08Eva IndriyaniPas encore d'évaluation
- Alkyl Halides & Aryl Halides: Victor GrignardDocument50 pagesAlkyl Halides & Aryl Halides: Victor GrignardsarahPas encore d'évaluation
- Aldehydes and KetonesDocument19 pagesAldehydes and KetonesVaibhav TarkasbandPas encore d'évaluation
- JB CI 13.1 HalogenoalkanesDocument6 pagesJB CI 13.1 HalogenoalkanesOCRChemistrySaltersPas encore d'évaluation
- Chapter 11Document17 pagesChapter 11abubakarabubakarbah563Pas encore d'évaluation
- Alcohols Phenols Carboxylic AcidsDocument5 pagesAlcohols Phenols Carboxylic AcidsAnanya AryaPas encore d'évaluation
- Carbonyl Compounds Aldehydes and Ketones2Document6 pagesCarbonyl Compounds Aldehydes and Ketones2Sachitra WijethungaPas encore d'évaluation
- Halogenalkanes: Unit 2 Chemistry C. Bailey PolackDocument23 pagesHalogenalkanes: Unit 2 Chemistry C. Bailey PolackBritney PattersonPas encore d'évaluation
- Halogenalkanes: Unit 2 Chemistry C. Bailey PolackDocument23 pagesHalogenalkanes: Unit 2 Chemistry C. Bailey PolackBritney PattersonPas encore d'évaluation
- Organic Chemistry MinDocument18 pagesOrganic Chemistry Mina711789322Pas encore d'évaluation
- Alkene Preparation and ReactionsDocument46 pagesAlkene Preparation and Reactionsnlprofessional07Pas encore d'évaluation
- CHAPTER 4-Carboxylic Acids DerivativesDocument20 pagesCHAPTER 4-Carboxylic Acids Derivativesshahera rosdiPas encore d'évaluation
- Consider The Following Anion CH CH CHCHDocument13 pagesConsider The Following Anion CH CH CHCHbobPas encore d'évaluation
- Things To Remember Only Alc Phe 2022-23Document17 pagesThings To Remember Only Alc Phe 2022-23poornaPas encore d'évaluation
- Hydrocarbon Alkenes 2Document25 pagesHydrocarbon Alkenes 2Raju SinghPas encore d'évaluation
- Alkyl Halides CHM457Document59 pagesAlkyl Halides CHM457AIMAN IMAN SHAIFUDDINPas encore d'évaluation
- Topic 6 - Alkyl Halide and Carbonyl Compounds Organic Compounds Containing A HalogenDocument11 pagesTopic 6 - Alkyl Halide and Carbonyl Compounds Organic Compounds Containing A HalogenamitPas encore d'évaluation
- Schaum's Easy Outline of Organic Chemistry, Second EditionD'EverandSchaum's Easy Outline of Organic Chemistry, Second EditionÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Bio ChemistryDocument12 pagesBio ChemistryGrace KamauPas encore d'évaluation
- 13 English Mark SchemeDocument12 pages13 English Mark SchemeGrace KamauPas encore d'évaluation
- Haloalkanes MSDocument14 pagesHaloalkanes MSGrace KamauPas encore d'évaluation
- Cambridge Chemistry SpecificationDocument99 pagesCambridge Chemistry SpecificationarmanPas encore d'évaluation
- A2 Hydrocarbons: Zahid Iqbal Warraich 0333-4200541Document7 pagesA2 Hydrocarbons: Zahid Iqbal Warraich 0333-4200541Grace KamauPas encore d'évaluation
- June 2015 (v5) QP - Paper 3 CIE Chemistry A-Level PDFDocument16 pagesJune 2015 (v5) QP - Paper 3 CIE Chemistry A-Level PDFGrace KamauPas encore d'évaluation
- June 2015 (v4) QP - Paper 3 CIE Chemistry A-Level PDFDocument12 pagesJune 2015 (v4) QP - Paper 3 CIE Chemistry A-Level PDFGrace KamauPas encore d'évaluation
- June 2015 (v2) QP - Paper 3 CIE Chemistry A-LevelDocument12 pagesJune 2015 (v2) QP - Paper 3 CIE Chemistry A-LevelGrace KamauPas encore d'évaluation
- Alternative Approaches To Slum UpgradingDocument11 pagesAlternative Approaches To Slum UpgradingGrace KamauPas encore d'évaluation
- June 2015 (v3) QP - Paper 3 CIE Chemistry A-Level PDFDocument12 pagesJune 2015 (v3) QP - Paper 3 CIE Chemistry A-Level PDFGrace KamauPas encore d'évaluation
- KiberaDocument3 pagesKiberaGrace KamauPas encore d'évaluation
- Case Study of A Drainage BasinDocument8 pagesCase Study of A Drainage BasinGrace KamauPas encore d'évaluation
- 2016 Specimen Paper 2 InsertDocument2 pages2016 Specimen Paper 2 InsertGrace KamauPas encore d'évaluation
- Sibelius 7 - Project 1 LyricsDocument1 pageSibelius 7 - Project 1 LyricsKieren ShepherdPas encore d'évaluation
- BioDocument2 pagesBioGrace KamauPas encore d'évaluation
- AerospaceDocument8 pagesAerospaceGrace KamauPas encore d'évaluation
- Emma JonesDocument3 pagesEmma JonesGrace KamauPas encore d'évaluation
- String Quartet - Violoncello PDFDocument2 pagesString Quartet - Violoncello PDFhancockstreetPas encore d'évaluation
- RevisionChecklistIGCSEICE0417 PDFDocument42 pagesRevisionChecklistIGCSEICE0417 PDFGrace KamauPas encore d'évaluation
- Cuarteto de Cuerda - Violín IIDocument2 pagesCuarteto de Cuerda - Violín IIChimoy LuisPas encore d'évaluation
- InductionDocument3 pagesInductionGrace KamauPas encore d'évaluation
- Task 3Document2 pagesTask 3Grace KamauPas encore d'évaluation
- TASK4 CurrencyDocument5 pagesTASK4 CurrencyGrace KamauPas encore d'évaluation
- 2015 Case Studies BOOKLET With DetailDocument28 pages2015 Case Studies BOOKLET With DetailGrace KamauPas encore d'évaluation
- Ict ExtraworkDocument3 pagesIct ExtraworkGrace KamauPas encore d'évaluation
- CSEC Chemistry June 2013 P1 PDFDocument9 pagesCSEC Chemistry June 2013 P1 PDFJeff LamboPas encore d'évaluation
- Heat Treatment ProcessesDocument7 pagesHeat Treatment Processessonu100% (1)
- prEN ISO 15614-5 2002 (E)Document27 pagesprEN ISO 15614-5 2002 (E)Mohammad LavasaniPas encore d'évaluation
- 3.1 1P SL CutoutDocument1 page3.1 1P SL CutoutSalman AlyazouriPas encore d'évaluation
- Loctite 648™: Technical Data SheetDocument4 pagesLoctite 648™: Technical Data SheetEriksonPas encore d'évaluation
- 19 - Weld Fit Up Inspection Employee TrainingDocument10 pages19 - Weld Fit Up Inspection Employee TrainingManoj KumarPas encore d'évaluation
- Instructions Bluestar & Optyma: Importer Autoryzowany PrzedstawicielDocument28 pagesInstructions Bluestar & Optyma: Importer Autoryzowany PrzedstawicielJaime EnriquePas encore d'évaluation
- Stoichiometric Ratios For BiogasDocument2 pagesStoichiometric Ratios For BiogasGovind RaoPas encore d'évaluation
- 8 The Table Shows Information About The Effect of Adding Sodium Hydroxide SolutionDocument1 page8 The Table Shows Information About The Effect of Adding Sodium Hydroxide SolutionAshrafPas encore d'évaluation
- Shrink Fitting - Liquefied NitrogenDocument3 pagesShrink Fitting - Liquefied NitrogenRicky WCKPas encore d'évaluation
- Kemasan ShanghaiDocument16 pagesKemasan ShanghaiadibaPas encore d'évaluation
- AdhesionDocument4 pagesAdhesionved100% (1)
- Response of Ferrocement Confinement On Behavior of Square RC Short ColumnDocument10 pagesResponse of Ferrocement Confinement On Behavior of Square RC Short ColumnPANKAJ TAMBAKHEPas encore d'évaluation
- The Hong Kong Polytechnic University Department of Building Environment and Energy EngineeringDocument24 pagesThe Hong Kong Polytechnic University Department of Building Environment and Energy Engineeringtsz hin chengPas encore d'évaluation
- Biodeseal Raw MaterialDocument13 pagesBiodeseal Raw MaterialJay MaradiyaPas encore d'évaluation
- Test Chemistry Class 10 Fifth Chapter To Last FBISEDocument2 pagesTest Chemistry Class 10 Fifth Chapter To Last FBISESaadia AsgharPas encore d'évaluation
- Hot Weather ConcretingDocument4 pagesHot Weather ConcretingyurendraPas encore d'évaluation
- 9 A, 9 B, 9 C 1.3mcm RWR Bid Boq-9Document10 pages9 A, 9 B, 9 C 1.3mcm RWR Bid Boq-9Ajaykumar MistryPas encore d'évaluation
- 4.2 Bonding, Structure and The Properties of MatterDocument6 pages4.2 Bonding, Structure and The Properties of MatterEashwar RajakumarPas encore d'évaluation
- Nozzle CloggingDocument17 pagesNozzle CloggingRonald Rayme VenturaPas encore d'évaluation
- Schunk Carbon Technology Carbon Slide Bearings enDocument11 pagesSchunk Carbon Technology Carbon Slide Bearings enamr yosryPas encore d'évaluation
- What Is Tungsten DisulfideDocument3 pagesWhat Is Tungsten DisulfidePrathyusha RamadurgamPas encore d'évaluation
- Welding Electrode SelectionDocument1 pageWelding Electrode SelectionNadeemPas encore d'évaluation
- Balistik Bak 3Document9 pagesBalistik Bak 3KübraPas encore d'évaluation
- Vibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyDocument9 pagesVibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyMelin YohanaPas encore d'évaluation
- Lecture 3-5Document23 pagesLecture 3-5pritishPas encore d'évaluation
- Dean StarkDocument15 pagesDean Starkfaizuanismail100% (1)
- Iridium Platinum AlloysDocument9 pagesIridium Platinum Alloysnguyen9si9nguyenPas encore d'évaluation
- EAC GAZETTE No 20 of 25th July 2022Document41 pagesEAC GAZETTE No 20 of 25th July 2022Puneet GandhiPas encore d'évaluation
- Basics Industrial Heat TreatmentDocument16 pagesBasics Industrial Heat TreatmenttbmariPas encore d'évaluation