Académique Documents
Professionnel Documents
Culture Documents
Thermophysical Properties of Refrigerants: Intel Technology India Pvt. LTD
Transféré par
Felipe Cáceres S.Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Thermophysical Properties of Refrigerants: Intel Technology India Pvt. LTD
Transféré par
Felipe Cáceres S.Droits d'auteur :
Formats disponibles
A5
Thermophysical
Properties of
Nitin Goel Refrigerants
Intel Technology India Pvt. Ltd.
R-22 (Chlorodifluoromethane)
R-134a (1,1,12-Tetrafluoroethane)
R-404A [R-125/143a/134a (44/52/4)]
R-407C [R-32/125/134a (23/25/52)
R-410A [R-32/125 (50/50)]
Ammonia/Water
Water/Lithium bromide
The Montreal Protocol, signed in 1987 and later amended in 1990, 1992, 1997, and 1999 controls the
production of ozone-depleting substances including refrigerants containing chlorine and/or bromine
production chloro-fluoro-carbons (CFC). Pursuent to this treaty, refrigerants such as R-11 and R-12,
ceased to exist in 1996 although continued use from existing stocks is permitted. In addition,
hydrofluorocarbon (HCFCs) (such as R-22 and R-123) are being phased out, with complete cessation
of production by January 1, 2030.
These refrigerants are being replaced by HFC refrigerants which have zero ozone depletion potential.
Common HFC refrigerants are R-32, R-125, 134a, and R-143a and their mixtures, such as, R-404A,
R-407C, and R-410A.
This appendix gives thermophysical properties of these HFC refrigerants and ammonia water and
water–lithium bromide mixtures which are used in absorption refrigeration systems. Properties of R-22
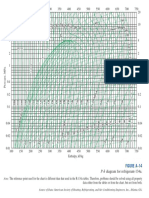
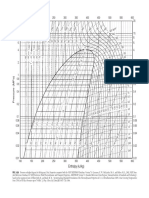
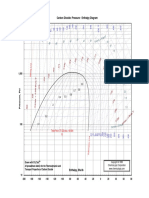
are given to serve as a reference (Figure A5.1 through Figure A5.8).
A5-1
q 2007 by Taylor & Francis Group, LLC
A5-2
100 150 200 250 300 350 400 450 500 550 600
20 20
50
400
00
0 0 600
50
00
500
00
50
700 3
130
90 80 0 kg/m
12
11
10
10
12
11
10 n = 30 10
8 8
200
90
80
70
10
20
30
40
50
60
6 150 6
1500
1450
1400
1350
4 100 4
80
T = −6.0°C 80
70
−40
−30
−70
−50
−10
−20
−80
60
0
60
2 50 2
40
40
30
30
1 1
20 20
0.8 0.8
Pressure, MPa
10 15
0.6 0.6
Handbook of Energy Efficiency and Renewable Energy
0
10
0.4 0.4
−10 8.0
T = 180°C
6.0
T = −20°C
190
200
170
160
150
140
130
0.2 0.2
120
110
100
4.0
90
−30
d Vapor
80
70
60
50
40
30
id
3.0
20
10
−10
qu
−20
0
−40
d li
0.1 0.1
2.0
Saturate
0.4
0.8
0.9
ate
0.6
0.5
0.1
0.2
0.3
0.7
0.08 0.08
1.5
tur
−50
x=
0.06 0.06
Sa
K)
1.0
g·
0.04 0.04
/(k
−60 0.80
kJ
0.70
0.80
0.90
1.00
1.10
1.60
1.70
1.80
1.20
0
1.40
1.50
1.30
20
40
20
0.60
1.9
2.0
2.1
2.
2.
2.
=
0.02 −70 0.02
s
0.40
0.30
−80
0.01 0.01
100 150 200 250 300 350 400 450 500 550 600
Properties computed with: NIST REFPROP Enthalpy, kJ/kg Based on formulation of Kamei et al. (1995)
version 7.0
FIGURE A5.1 Pressure–enthalpy diagram for refrigerant R-22.
q 2007 by Taylor & Francis Group, LLC
Thermophysical Properties of Refrigerants
100 150 200 250 300 350 400 450 500 550 600
20 20
00
00
50
00
00
3
50
50
0 0 700 600 m
10 90 80 0 kg/
13
11
12
12
10
11
10 ρ=50 10
400
8 300
8
100
30
40
50
60
70
90
10
20
80
6 200 6
1350
1450
1400
150
4 4
T=–40˚C
90 100
–60
–50
–30
–20
–10
80 80
0
2 70 60 2
60
50 40
1 40 30 1
0.8 0.8
Pressure, MPa
30 20
0.6 20 0.6
15
0.4 10 0.4
10
0 8.0
6.0
T= 160˚C
0.2 T=–10˚C 0.2
180
160
150
140
130
120
110
100
90
4.0
80
70
60
–20
50
40
30
20
10
3.0
0
0.1 0.1
ro
0.08 –30 0.08
Vap
id
iqu
2.0
0.4
0.1
0.2
0.3
0.8
0.5
0.6
0.7
0.9
dl
0.06 0.06
d
ate
rate
1.5
x=
–40
tur
Sa
Satu
0.04 0.04
)
1.0
·K
kg
–50 0.80
J/(
0K
0.80
0.90
1.20
1.00
1.10
1.30
0.70
1.40
0
1.50
1.60
0
0.60
1.7
1.8
1.9
2.0
2.1
2.3
2.2
0.02 0.02
s=
0
–60
2.4
0.40
0.01 0.01
100 150 200 250 300 350 400 450 500 550 600
Properties computed with: NIST REFPROP Enthalpy, kJ/kg Based on formulation of Tsiner-Roth and Beehr (1994)
A5-3
version 7.0
FIGURE A5.2 Pressure–enthalpy diagram for refrigerant R-134a.
q 2007 by Taylor & Francis Group, LLC
A5-4
100 150 200 250 300 350 400 450 500 550 600
10.0 100.0
18
0
8.0 14 15
6
01 0.00 020 80.0
24
0.0 30
0
00 .00 0.00
0
.0
23
0. 2
0
13
1
01
0
. 0.004
5
0
01
0 0
01
22
60.0
010
00
6.0
0.0
0.0050
0.0
0.0
DuPont Fluorochemicals
0.0
0.0060
Suva® 404A (R–404A) m'/kg 40.0
4.0 Pressure–Enthalpy Diagram = 0.0080
Volume
60
0.010
(SI Units) 60
50
50 0.015
40
2.0 20.0
30
40 0.020
0.00095
0.00085
0.00080
0.00090
0.00075
30
20
0.030
20
1.0 10.0
10
0.040
Temperature = −50°C
10 8.0
−70
−60
−40
−30
−20
−10
0
0.8 0.050
0.6 Temperature = 0°C 0.060 6.0
Pressure (MPa)
Pressure (bar)
−10 0.080
0.4 4.0
0.10
Handbook of Energy Efficiency and Renewable Energy
−20
0.15
id
−30
iqu
2.0
r
vapo
0.2 0.20
dl
0.5
0.4
0.6
0.9
0.1
0.7
0.8
210
0.2
Temperature = 140°C
ate
200
190
0.3
180
170
150
160
rated
130
tur
−40
120
110
=
100
Sa
90
0.30
80
lity
70
60
Satu
50
40
1.0
qua
0.1
30
20
0.40
10
0
−50
−10
−20
0.8
−30
0.08
−40
0.06 0.6
0.70
−60
0.04 0.4
1.0
.k
−70
kg
kj/
1.5
.20
0.02 2.0 0.2
=2
0
py
0
1.35
1.40
1.45
1.50
1.55
5
5
5
0.60
0.65
0.70
0.75
0.80
0.85
0.90
0.85
1.00
1.05
1.60
1.65
1.70
1.75
1.80
1.10
1.15
1.20
1.25
1.30
0
1.85
1.90
1.95
2.1
2.1
2.3
2.3
2.2
2.0
45
2.0
50
40
tro
2.
2.
2.
En
0.01 0.1
100 150 200 250 300 350 400 450 500 550 600
Enthalpy (kJ/kg)
FIGURE A5.3 Pressure–enthalpy diagram for refrigerant R-404A.
q 2007 by Taylor & Francis Group, LLC
Thermophysical Properties of Refrigerants
100 150 200 250 300 350 400 450 500 550 600
10.0 100.0
4 6 8
3 01 01 01
01 0.0
0
0.0 0.0
0
8.0 25 80.0
12
0.00
01
22
11
0
085
0.
5
30
00
0
0
009
00
0 0.00
0.0
02
009
21
0.
15
0.00
0.
0.0 0
0.004
0
00
0.0
6.0 60.0
80
20
0.0
Dupont fluorochemicals 0.0050
0.
c.p.
0
70
19
o 0.0060
Suva® 407C (R–407C) 3 g
4.0 80 m /k 40.0
Pressure–Enthalpy diagram e = 0.00
60
70 Volum 0.010
3.0 (SI units) 30.0
50
60
40
0.015
50 20.0
2.0
0.00070
0.020
30
0.00075
0.00080
40
20
30 0.030
10
1.0 10.0
20 0.040
0.8 0.050
0 10
0.060 8.0
0.6
0.080 6.0
Pressure (MPa)
0.4 0.10 4.0
0.3 3.0
0.15
id
0.2 0.20 2.0
iqu
dl
r
vapo
0.1
ate
0.2
.3
0.4
0.30
tur
0.5
0.6
=0
0.7
0.8
rated
0.9
Sa
ality
0.1 0.40 1.0
Satu
Qu
0.08 0.50 0.8
0.06 0.70 0.6
180
160
170
Temperature = 120°C
140
150
130
110
100
90
80
70
1.0
60
0.04 0.4
50
40
30
20
10
–10
0
–20
–30
0.03 0.3
–40
g.K
–50
1.5
J\k
5k
0.02 2.0 0.2
2.3
=
py
2.00
2.15
2.05
2.10
0.90
0
0.95
0.
0.85
1.90
1.50
1.45
1.95
1.60
1.65
0.60
0.65
0.70
0.75
0.80
1.00
1.55
1.75
5
1.40
1.70
1.10
1.15
1.05
1.25
1.80
1.85
1.20
1.30
1.35
tro
55
5
0
2.2
60
2.2
2.3
2.4
2.4
2.5
2.
En
65
2.
2.
0.01 0.1
100 150 200 250 300 350 400 450 500 550 600
A5-5
Enthalpy (kJ/kg)
FIGURE A5.4 Pressure–enthalpy diagram for refrigerant R-407C.
q 2007 by Taylor & Francis Group, LLC
A5-6
Enthalpy (kJ/kg)
100 150 200 250 300 350 400 450 500 550 600
6 6
0.004.005 0.008
0
4 ®
60 0.006 0.010 4
Dupont fluorochemicals
50
0.015
Suva® 410A (R–410A)
40
Pressure–Enthalpy diagram 0.020 2
2 30
(SI units)
20 0.030
10 0.040
1 1
0.050
0.8 Temperature = 0°C 0.8
0.060
50°C
180
170
160
150
0.6
140
0.6
130
120
110
100
Temperature =
90
80
70
0.10
60
40
30
0.4 0.4
20
10
0
Handbook of Energy Efficiency and Renewable Energy
0.15
Pressure (MPa)
Pressure (MPa)
0.20
0.2 0.2
0.30
0.40
d vapor
0.1
0.50πY/k
0.1 g
id
Volume =
0.5
liqu
0.08 0.60 0.08
lity =
Saturate
ted
0.8
0.9
0.7
0.6
0.1
0.3
0.4
0.2
0.06 0.80 0.06
ura
Qua
Sat
1.0
0.04 0.04
1.5
0.03
2.0
K
0.02
/kg.
0.02
8 kJ
3.0
2.0
1.6
1.4
1.5
1.7
1.3
2.2
0.5
0.6
0.7
0.8
0.9
1.0
1.2
2.1
1.1
= 1.
2.3
2.5
1.9
2.6
2.4
4.0
opy
0.01 0.01
2.8
2.7
5.0
Entr
0.008 6.0 0.008
0.006 0.006
8.0
100 150 200 250 300 350 400 450 500 550 600
Enthalpy (kJ/kg)
FIGURE A5.5 Pressure–enthalpy diagram for refrigerant R-410A.
q 2007 by Taylor & Francis Group, LLC
Thermophysical Properties of Refrigerants
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
10
210 20 22 25 28 31 34 38 43 48 5 5 6 9 00 Saturation pressure, kPa 210
18 00 00 00 00 00 00
00 00 00 300 800 300 6800 730 780 83 890 400 0
00 0 0 00 0 Enthalpy of saturated vapor, kJ/kg vapor
200 16 200
Vapor composition, kg NH1/kg vapor
1400
190 5 Enthalpy of saturated liquid, kJ/kg liquid
13 0 190
0
11 0
180 50 180
10
00 700
170 90 170
0.300
00
50
80 0
0
0.50
0.60
80
85
0.7
0.7
0 0
0.
90
0.
160 70
0 0. 160
0
60 600 92
150 0 0 0. 150
50 170 40 600
450 0.9
140
00
400 500 00 140
16
18
35 0 60 1000
130 0 0.9 500 0
30 130
0 8900 9400
25
120 0 00 120
22 400 80 7800 8300
5 15 0.9
110 20 400 6800 7300 110
0
90 6300
100 15 50 0.9 5800
100
12 0 14
300 5300
5 300 4800
90 10 90
Temperature, °C
5
0 0 .99
75 4300
80 0 3800 80
1 40 97
50 200 0.9 200 3400
70 3100 70
40
2800
60 30 2500 60
99
50 100 0.9 2200
50 20 100 13 2000 50
1800
40 1600
10 1450 40
0 1300
30 0 1150 30
1000
1300 900
20 800 20
−100 −100 700
10 600 10
500
0 450
1275 400 0
−200 −200 350
−10 300
−10
225 250
−20 −300 1250 200
−20
175
−300 150
−30 125 −30
−400 1225 100
−40 75
−40
−400
50
−50 40 −50
30
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Ammonia in saturated liquid, kg (ammonia)/kg (liquid)
A5-7
FIGURE A5.6 Enthalpy–concentration diagram for ammonia/water solutions prepared by Kwang Kim and Keith Herold, Centre for Environmental Energy Engineering,
University of Maryland at Colleage Park.
q 2007 by Taylor & Francis Group, LLC
A5-8 Handbook of Energy Efficiency and Renewable Energy
500
18
0
17
0
450
16
0
15
0
14
0
400
13
0
12
0
11
350
0
10
0°
C
90
300
Enthalpy, kJ/kg solution
80
250
70
60
200
50
150
40
100 30
20
50
10
0
0 10 20 30 40 50 60 70
Lithium bromide concentration, mass%
Equations Concentration range 40 < x < 70% LiBr Temperature range 15 < t < 165°C
h = Σ04 AnX7 + rΣ04 BnX n + r2Σ04 CpX n in kJ/kg, where t = °C and X = %LiBr
A0 = −2024.33 B0 = 18.2829 C0 = −3.7008214 E-2
A1 = 163.309 B1 = −1.1691757 C1 = 2.8877666 E-3
A2 = −4.88161 B2 = 3.248041E-2 C2 = −8.1313015 E-5
A3 = 6.302948 E-2 B3 = −4.034184 E-4 C3 = 9.9116628 E-7
A4 = −2.913705 E-4 B4 = 1.8520569 E-6 C4 = −4.4441207 E-9
FIGURE A5.7 Enthalpy–concentration diagram for water/lithium bromide solutions.
q 2007 by Taylor & Francis Group, LLC
Thermophysical Properties of Refrigerants
Equations 120 200
3 3
1. t = S0B0X"+t'S0A0X" Solution temperature, °C
3 3
2. t ′ = (t –S0B0X" )/S0A0X" Refrigerant temperature, °C 110 150
3. log P = C+DIT' + E/T 2 P = kPa T ′= K
-2E
4. Γ = 100
D+|D2−4E(C−log P)|0.5 100
A0 = −2.007 55 B0 = 124.937 C = 7.05
A1 = 0.169 76 B1 = −7 710.49 D = −1596.49
A2 = −3.133 362 E-03 B2 = 0.152 286 D = −104 095.5 90
A3 = 1.976 68 E-05 B3 = −7.950 90 E-04
Range: −15 < t ' < 110°C
0
50
C
80
%
5 < t < 175°C
30
e,
Saturation pressure (P), kPa
45 < X < 70% LiBr 40
ur
40
t
ra
pe
em
70
%
30
50
tt
an
er
rig
20
ef
60
%
R
60
50
%
10
70
40
5
30 4
3
20
2
10 1
0
10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180
A5-9
Solution temperature,°C
FIGURE A5.8 Equilibrium chart for aqueous lithium bromide solutions reprinted by permission of Carrier Corp.
q 2007 by Taylor & Francis Group, LLC
Vous aimerez peut-être aussi
- Database Management Systems: Understanding and Applying Database TechnologyD'EverandDatabase Management Systems: Understanding and Applying Database TechnologyÉvaluation : 4 sur 5 étoiles4/5 (8)
- (Colored) Refrigerant 134a P-H Diagram (SI Units)Document1 page(Colored) Refrigerant 134a P-H Diagram (SI Units)Mc Jason LauretePas encore d'évaluation
- (Colored) Refrigerant 134a P-H Diagram (SI Units) PDFDocument1 page(Colored) Refrigerant 134a P-H Diagram (SI Units) PDFMc Jason LauretePas encore d'évaluation
- Gea Hilge TP Centrifugal Pumps Us 170792Document6 pagesGea Hilge TP Centrifugal Pumps Us 170792German TchiliPas encore d'évaluation
- Kpi Information: TCH Traffic (Erl)Document25 pagesKpi Information: TCH Traffic (Erl)taji arselanPas encore d'évaluation
- LF CL Brochure 2019Document4 pagesLF CL Brochure 2019indra putraPas encore d'évaluation
- 88HST (12.5) Pump Curve PDFDocument1 page88HST (12.5) Pump Curve PDFDaylis GonzalezPas encore d'évaluation
- Mapa de Comunidades NativasDocument1 pageMapa de Comunidades NativasLizeth Licas camposPas encore d'évaluation
- SPT 22022Document2 pagesSPT 22022BUMAPas encore d'évaluation
- Proposed (2) Two Storey With Roof Deck Mrs. Marilyn Chua Marianne S. AbordeDocument3 pagesProposed (2) Two Storey With Roof Deck Mrs. Marilyn Chua Marianne S. AbordeMarianne Solito-Aborde100% (1)
- Description: 0529EN March 2016Document2 pagesDescription: 0529EN March 2016Cristian NicolescuPas encore d'évaluation
- Detail Siku Landasan: Penutup Atap TrimdecDocument1 pageDetail Siku Landasan: Penutup Atap TrimdecMuhammad FikryPas encore d'évaluation
- Peta Sequence Elev 20-25Document1 pagePeta Sequence Elev 20-25haltimberdikariPas encore d'évaluation
- Update - BV - Detail Fasad - 08 - 12 - 2022-1Document1 pageUpdate - BV - Detail Fasad - 08 - 12 - 2022-1Benny SuryadiPas encore d'évaluation
- Tie Beam and Pile Cap LayoutDocument1 pageTie Beam and Pile Cap Layoutlayaljamal2Pas encore d'évaluation
- Vỏ Dưới - HGT MQY 3600-1Document1 pageVỏ Dưới - HGT MQY 3600-1Tung Nguyen XuanPas encore d'évaluation
- Canopy For HT Pane - 20-10Document2 pagesCanopy For HT Pane - 20-10ARUN RAWATPas encore d'évaluation
- Residencial America 1Document1 pageResidencial America 1Marcelo Miguel GonzalezPas encore d'évaluation
- VolcanDocument1 pageVolcanRaul RosasPas encore d'évaluation
- Rifki-Model DenahDocument1 pageRifki-Model DenahrifkicimakPas encore d'évaluation
- Baja 2Document1 pageBaja 2Reki AliansyahPas encore d'évaluation
- Peta UTS Penyaliran (+aliran Alami Dan Catchment)Document1 pagePeta UTS Penyaliran (+aliran Alami Dan Catchment)Abie BadhurahmanPas encore d'évaluation
- 1 10Document10 pages1 10aleksandr.mamrenkoPas encore d'évaluation
- Frame: Drilled 6,3 Drilled 6,3 0,05 B BDocument1 pageFrame: Drilled 6,3 Drilled 6,3 0,05 B BEnes YerişenoğluPas encore d'évaluation
- Housing Type CDocument4 pagesHousing Type CMasri NurdinPas encore d'évaluation
- (1,1,1,2-Tetrafluoroethane) Reference State: H 200.0 KJ/KG, S 1.00 KJ/ (KG K) For Saturated Liquid at 0 °CDocument1 page(1,1,1,2-Tetrafluoroethane) Reference State: H 200.0 KJ/KG, S 1.00 KJ/ (KG K) For Saturated Liquid at 0 °CdanisenicoledavidPas encore d'évaluation
- Foundation PROJECT T2-1: Technical University "Gh. Asachi", Iasi Faculty of Civil Engineering Job NumberDocument1 pageFoundation PROJECT T2-1: Technical University "Gh. Asachi", Iasi Faculty of Civil Engineering Job NumberGafita FlavianPas encore d'évaluation
- Diagrama de Molière R134a: RefrigerantesDocument1 pageDiagrama de Molière R134a: RefrigerantesHumnerPas encore d'évaluation
- Denah FixDocument1 pageDenah FixJaka PranaPas encore d'évaluation
- Ficha Tecnica U 150 KSBDocument1 pageFicha Tecnica U 150 KSBElizardo Isaias Campos CamposPas encore d'évaluation
- Jindal Steel and Power LTD: 1.1 Fundamentalanalysis (Company Analysis by Financial Ratio'S)Document3 pagesJindal Steel and Power LTD: 1.1 Fundamentalanalysis (Company Analysis by Financial Ratio'S)yash chauhanPas encore d'évaluation
- Denah Konsbang2dwg-ModelDocument1 pageDenah Konsbang2dwg-ModelrifkicimakPas encore d'évaluation
- MELTING - I Kearney. Type 0 OJ. ............ KP:'.) .RNEY. ANS I. C3 7 - 4 2 .. 1981 ............................ J - 1100Document2 pagesMELTING - I Kearney. Type 0 OJ. ............ KP:'.) .RNEY. ANS I. C3 7 - 4 2 .. 1981 ............................ J - 1100JUAN DAVID MINA CASARANPas encore d'évaluation
- CO2 Mollier Chart PDFDocument1 pageCO2 Mollier Chart PDFmarko quirozPas encore d'évaluation
- Foundation Details For GatpDocument1 pageFoundation Details For GatpMaheshPas encore d'évaluation
- Indola V6S6360Document1 pageIndola V6S6360Rydel CuachonPas encore d'évaluation
- Gandhi Chouk To Manbharan tihar-CH-0-600Document1 pageGandhi Chouk To Manbharan tihar-CH-0-600shivshankar kushwahaPas encore d'évaluation
- Example Airflow Chart: 6500 7000 8000 VEL Ocit Y, FP MDocument1 pageExample Airflow Chart: 6500 7000 8000 VEL Ocit Y, FP MShiva ShakthiPas encore d'évaluation
- Peta Sequence Elev 35-40Document1 pagePeta Sequence Elev 35-40haltimberdikariPas encore d'évaluation
- 7 - Pile Layout-Rev00Document1 page7 - Pile Layout-Rev00layaljamal2Pas encore d'évaluation
- Hallite CatalogueDocument374 pagesHallite Cataloguegeovane cardosoPas encore d'évaluation
- J230023P 05Document1 pageJ230023P 05Ka Wai ChengPas encore d'évaluation
- Peta UTS 2020Document1 pagePeta UTS 2020Maharani RengganisPas encore d'évaluation
- KL2205 Week09Document75 pagesKL2205 Week09mutiara indahPas encore d'évaluation
- ROAD-3: 450mm THKDocument1 pageROAD-3: 450mm THKAhmed MandorPas encore d'évaluation
- HALLITEDocument318 pagesHALLITERubensPas encore d'évaluation
- Intro 2 - 8 - IntroDocument8 pagesIntro 2 - 8 - IntroJosé HurtadoPas encore d'évaluation
- Lead Screw Nut Mounting Details 1Document1 pageLead Screw Nut Mounting Details 1shubham tadePas encore d'évaluation
- Ramp3: Panvel UranDocument1 pageRamp3: Panvel UranDeepak_pethkarPas encore d'évaluation
- Revit Domoty 1Document1 pageRevit Domoty 1SAN RAKSAPas encore d'évaluation
- 290322-DTP 3 Bangunan TesisDocument21 pages290322-DTP 3 Bangunan TesisNUNING SRI RAHAYUPas encore d'évaluation
- Interland: A-TypeDocument1 pageInterland: A-TypeDuy Giáp NguyễnPas encore d'évaluation
- ANN 400 Cooper Datasheet 13045334Document1 pageANN 400 Cooper Datasheet 13045334assdaPas encore d'évaluation
- Stage Zouari Med PDFDocument1 pageStage Zouari Med PDFMohamed ZouariPas encore d'évaluation
- Section (Ceiling) 3: Scale A4 NTSDocument1 pageSection (Ceiling) 3: Scale A4 NTSDenver Esyaben Sin-otPas encore d'évaluation
- Chainage: Center LevelDocument1 pageChainage: Center LevelShivam singh baghelPas encore d'évaluation
- R 404 AmoliereDocument1 pageR 404 AmoliereCris Carvajal BonillaPas encore d'évaluation
- Unnamed Owner A101: No. Description DateDocument1 pageUnnamed Owner A101: No. Description DateJason CaddyPas encore d'évaluation
- Lapter DrainaseDocument1 pageLapter DrainaseDwi mandiri JayaPas encore d'évaluation
- How PDFDocument2 pagesHow PDFFelipe Cáceres S.Pas encore d'évaluation
- Non Forniti Con Questi Modelli Not Supplied With These ModelsDocument1 pageNon Forniti Con Questi Modelli Not Supplied With These ModelsFelipe Cáceres S.Pas encore d'évaluation
- Bombas de Calor CLIMAVENETA AW - HT - 0122 - 0302 - Ficha TécnicaDocument28 pagesBombas de Calor CLIMAVENETA AW - HT - 0122 - 0302 - Ficha TécnicaFelipe Cáceres S.Pas encore d'évaluation
- Calderas de Condensación COSMOGAS Mydens T - Ficha TécnicaDocument24 pagesCalderas de Condensación COSMOGAS Mydens T - Ficha TécnicaFelipe Cáceres S.Pas encore d'évaluation
- VGF Flanged Globe Valves: FeaturesDocument4 pagesVGF Flanged Globe Valves: FeaturesFelipe Cáceres S.Pas encore d'évaluation
- Digitron 2000 Series User ManualDocument8 pagesDigitron 2000 Series User ManualFelipe Cáceres S.Pas encore d'évaluation
- (Ug, PG & PHD) Fellowship: Tih-Iot Chanakya GroupDocument3 pages(Ug, PG & PHD) Fellowship: Tih-Iot Chanakya GroupVijay M.MPas encore d'évaluation
- Void Engineers (Convention: Mage The Ascension)Document6 pagesVoid Engineers (Convention: Mage The Ascension)Beth0% (1)
- Comparative Study On Analysis of Plain and RC Beam Using AbaqusDocument9 pagesComparative Study On Analysis of Plain and RC Beam Using Abaqussaifal hameedPas encore d'évaluation
- OB Case Study Care by Volvo UK 2020Document1 pageOB Case Study Care by Volvo UK 2020Anima AgarwalPas encore d'évaluation
- Moc3040 MotorolaDocument3 pagesMoc3040 MotorolaBryanTipánPas encore d'évaluation
- Empowerment Series Social Work With Groups Comprehensive Practice and Self Care 10Th Edition Charles Zastrow Full ChapterDocument67 pagesEmpowerment Series Social Work With Groups Comprehensive Practice and Self Care 10Th Edition Charles Zastrow Full Chapterruby.levi441100% (5)
- Cambridge IGCSE ™: Combined ScienceDocument11 pagesCambridge IGCSE ™: Combined ScienceAhmed Jomaa Salem0% (1)
- 19 71 Hydrologic Engineering Methods For Water Resources DevelopmentDocument654 pages19 71 Hydrologic Engineering Methods For Water Resources DevelopmentMartha LetchingerPas encore d'évaluation
- Biosynthesis and Characterization of Silica Nanoparticles From RiceDocument10 pagesBiosynthesis and Characterization of Silica Nanoparticles From Riceanon_432216275Pas encore d'évaluation
- Aicte Internship Approval Pending 1Document7 pagesAicte Internship Approval Pending 1Anisha KumariPas encore d'évaluation
- HCH - 15 04 004Document5 pagesHCH - 15 04 004NarvaxisPas encore d'évaluation
- Evidence MODULE 1 Evidence DefinitionDocument8 pagesEvidence MODULE 1 Evidence Definitiondave BarretoPas encore d'évaluation
- SoundsDocument61 pagesSoundsJemabel RosarioPas encore d'évaluation
- 5 Grade: Daily MathDocument130 pages5 Grade: Daily MathOLIVEEN WILKS-SCOTT100% (3)
- Emerson Mentor MP ManualDocument182 pagesEmerson Mentor MP ManualiampedrooPas encore d'évaluation
- Ab 2023Document5 pagesAb 2023Cristelle Estrada-Romuar JurolanPas encore d'évaluation
- William Ury Power of A Positive No Bantam - 2007Document227 pagesWilliam Ury Power of A Positive No Bantam - 2007Tam Jeopardy100% (1)
- Teachers Guide Lower Secondary Science PDFDocument141 pagesTeachers Guide Lower Secondary Science PDFNuzhat IbrahimPas encore d'évaluation
- I.A.-1 Question Bank EM-3 (Answers)Document11 pagesI.A.-1 Question Bank EM-3 (Answers)UmmPas encore d'évaluation
- 61annual Report 2010-11 EngDocument237 pages61annual Report 2010-11 Engsoap_bendPas encore d'évaluation
- Lennox IcomfortTouch ManualDocument39 pagesLennox IcomfortTouch ManualMuhammid Zahid AttariPas encore d'évaluation
- Thesis - A Surlyn® Ionomer As A Self-Healing and Self-Sensing Composite - 2011 - UKDocument194 pagesThesis - A Surlyn® Ionomer As A Self-Healing and Self-Sensing Composite - 2011 - UKAhmedPas encore d'évaluation
- Stress: Problem SetDocument2 pagesStress: Problem SetDanielle FloridaPas encore d'évaluation
- S25580 MSDS Corn Starch FisherchiDocument6 pagesS25580 MSDS Corn Starch FisherchiProcurement ProlinePas encore d'évaluation
- Use The Analysis ToolPak To Perform Complex Data Analysis - Excel - OfficeDocument5 pagesUse The Analysis ToolPak To Perform Complex Data Analysis - Excel - OfficedakingPas encore d'évaluation
- College of Engineering Cagayan State UniversityDocument16 pagesCollege of Engineering Cagayan State UniversityErika Antonio GutierrezPas encore d'évaluation
- EXPERIMENT 1 - Bendo Marjorie P.Document5 pagesEXPERIMENT 1 - Bendo Marjorie P.Bendo Marjorie P.100% (1)
- Bombas KMPDocument42 pagesBombas KMPReagrinca Ventas80% (5)
- Mathematics4 q4 Week4 v4Document11 pagesMathematics4 q4 Week4 v4Morales JinxPas encore d'évaluation
- "Large Quote Goes Here.": Title or Heading HereDocument2 pages"Large Quote Goes Here.": Title or Heading HereHesti RianaPas encore d'évaluation