Académique Documents
Professionnel Documents
Culture Documents
THE Dynamical THE: Abcdefg
Transféré par
Mara ScisciTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
THE Dynamical THE: Abcdefg
Transféré par
Mara ScisciDroits d'auteur :
Formats disponibles
424 THE DYNAMICAL EVIDENCE OF THE
The relation between pressure and density arising from such an action
between the particles is of this kind.
As the density increases from zero, the pressure at first depends almost
entirely on the motion of the particles, and therefore varies almost exactly as
the pressure, according to Boyle's Law. As the density continues to increase,
the effect mutual attraction of the particles becomes sensible, and this
of the
causes the rise of pressure to be less than that given by Boyle's Law. If
the temperature is low, the effect of attraction may become so large in pro-
portion to the effect of motion that the pressure, instead of always rising as
the density increases, may reach a maximum, and then begin to diminish.
At length, however, as the average distance of the particles is still further
diminished, the effect of repulsion will prevail over that of attraction, and the
pressure will increase so as not only to be greater than that given by Boyle's
Law, but so that an exceedingly small increase of density will produce an
enormous increase of pressure.
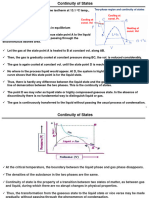
Hence the relation between pressure and volume may be represented by
the curve ABCDEFG, where the horizontal ordinate represents the volume,
and the vertical ordinate represents the pressure.
As the volume diminishes, the pressure increases up to the point C, then
diminishes to the point E, and finally increases without limit as the volume
diminishes.
We have hitherto supposed the experiment to be conducted in such a
way that the density is the same in every part of the medium. This, how-
ever, is impossible in practice, as the only condition we can impose on the
medium from without is that the whole of the medium shall be contained
MOLECULAR CONSTITUTION OF BODIES. 425
within a certain vessel. Hence, if it is possible for the medium to arrange
itself so that part has one density and part another, we cannot prevent it from
doing so.
Now the points B and F represent two states of the medium in which
the pressure is the same but the density very different. The whole of the
medium may pass from the state B to the state F, not through the inter-
mediate states CDF, but by small successive portions passing directly from the
state B to the state F. In this way the successive states of the medium as
a whole by points on the straight line BF, the point B
will be represented
representing it when entirely in the rarefied state, and F representing it when
entirely condensed. This is what takes place when a gas or vapour is liquefied.
Under ordinaiy circumstances, therefore, the relation between pressure and
vokune at constant temperature is represented by the broken line ABFG. If,
however, the medium when liquefied is carefully kept from contact with vapour,
it be preserved in the liquid condition and brought into states represented
may
by the portion of the curve betw^een F
and F. It is also possible that methods
mav be devised whereby the vapour may be prevented from condensing, and
brought into states represented by points in BC.
The portion of the hypothetical curve from C to E represents states which
are essentially unstable, and which cannot therefore be realised.
Now let us suppose the medium to pass from B to F along the hypo-
thetical curve BCDEF in a state always homogeneous, and to return along
the straight line FB in the form of a mixture of liquid and vapour. Since
the temperature has been constant throughout, no heat can have been trans-
formed into work. Now the heat transformed into work is represented by the
excess of the area FDE over BCD. Hence the condition which determines
the maximum pressure of the vapour at given temperature is that the line
BF cuts ofi" equal areas from the curve above and below.
The higher the temperature, the greater the part of the pressure which
depends on motion, as compared with that which depends on forces between
the particles. Hence, as the temperature rises, the dip in the curve becomes
less and at a certain temperature the curve, instead of dipping, merely
marked,
becomes horizontal at a certain point, and then slopes upward as before. This
point is called the critical point. It has been determined for carbonic acid
by the masterly researches of Andrews. It corresponds to a definite temperature,
pressure and density.
VOL. II. ^^
426 THE DYNAMICAL EVIDENCE OF THE
At higher temperatures the curve slopes upwards throughout, and there is
nothing corresponding to Uquefaction in passing from the rarest to the densest
state.
The molecular theory of the continuity of the liquid and gaseous states
forms the subject of an exceedingly ingenious thesis by Mr Johannes Diderick
van der Waals'"', a graduate of Leyden. There are certain points in which I
think he has fallen into mathematical errors, and his final result is certainly
not a complete expression for the interaction of real molecules, but his attack
on this difficult question is so able and so brave, that it cannot fail to give
a notable impulse to molecular science. It has certainly directed the attention
of more than one inquker to the study of the Low-Dutch language in which
it is written.
The purely themiodynamical relations of the different states of matter do
not belong to our subject, as they are independent of particular theories about
molecules. I must not, however, omit to mention a most important American
contribution to this part of thermodynamics by Prof. Willard Gibbsf, of Yale
College, U.S., who has given us a remarkably simple and thoroughly satisfactory
method of representing the relations of the different states of matter by means
of a model. By means of this model, problems which had long resisted the
efforts of myself and others may be solved at once.
Let us now return to the case of a highly rarefied gas in which the
pressure is due entirely to the motion of its particles. It is easy to calculate
the mean square of the velocity of the particles from the equation of Clausius,
since the pressure, and the mass are all measurable quantities.
the volume,
Supposing the velocity of every particle the same, the velocity of a molecule
of oxygen would be 461 metres per second, of nitrogen 492, and of hydrogen
1844, at the temperature of O'C.
The explanation of the pressure of a gas on the vessel which contains it
by the impact of its particles on the surface of the vessel has been suggested
at various times by various writers. The fact, however, that gases are not ob-
served to disseminate themselves through the atmosphere wdth velocities at all
approaching those just mentioned, remained unexplained, till Clausius, by a
* Over de continuiteit van den gas en vloeistoftoestand. (Leiden : A. W. Sijtlioff, 1873.)
t " method of geometrical representation of the thermodynamic properties of substances by means
A
of surfaces." Transactions of the Connecticut Academy of Arts and Sciences, Vol. ii. Part 2.
Vous aimerez peut-être aussi
- Turbomachinery Centrifugal Compressors: Class 13Document70 pagesTurbomachinery Centrifugal Compressors: Class 13Manoj BaishyaPas encore d'évaluation
- Natural Gas Engineering Hand Book (2005) Ch.2Document21 pagesNatural Gas Engineering Hand Book (2005) Ch.2Abdelrahman Saeed89% (9)
- Surface TensionDocument59 pagesSurface TensionPANKAJ SHARMAPas encore d'évaluation
- Charles Law Strategic Intervention Material in ChemistryDocument11 pagesCharles Law Strategic Intervention Material in ChemistryDwell Joy Armada78% (9)
- Charle's LawDocument37 pagesCharle's LawGarren Jude AquinoPas encore d'évaluation
- Hidrodinámica BernoulliDocument25 pagesHidrodinámica BernoulliFélix IbarraPas encore d'évaluation
- Mass Transfer Coeffiicent and Interface Mass Transfer IIDocument42 pagesMass Transfer Coeffiicent and Interface Mass Transfer IImohitPas encore d'évaluation
- Lecture - 5 - 16-01-2024Document12 pagesLecture - 5 - 16-01-2024Rakshak AwasthiPas encore d'évaluation
- Fluid MechanicsDocument45 pagesFluid MechanicsCholwe Essau MbilimaPas encore d'évaluation
- MbouyancechanicsDocument45 pagesMbouyancechanicsCholwe Essau MbilimaPas encore d'évaluation
- Physica: Viscosity of Carbon Dioxide in The Liquid PhaseDocument32 pagesPhysica: Viscosity of Carbon Dioxide in The Liquid PhasejtorerocPas encore d'évaluation
- Compare and ContrastDocument1 pageCompare and ContrastLezyl UntalanPas encore d'évaluation
- Compare and ContrastDocument1 pageCompare and ContrastLezyl UntalanPas encore d'évaluation
- Principles of ExperimentDocument3 pagesPrinciples of ExperimentAnnie AlbertoPas encore d'évaluation
- Lec 6Document43 pagesLec 6Hadi SiddiqiPas encore d'évaluation
- Asc0304 Chemistry I: Phase EquilibriumDocument27 pagesAsc0304 Chemistry I: Phase EquilibriumhadassahhadidPas encore d'évaluation
- CH 8 - Mass TransferDocument15 pagesCH 8 - Mass Transferahmed mohamedPas encore d'évaluation
- Mass Transfer SlideDocument12 pagesMass Transfer Slidea187213Pas encore d'évaluation
- 1902 - Rayleigh - On The Distillation of Binary MixturesDocument17 pages1902 - Rayleigh - On The Distillation of Binary MixturesmontoyazumaetaPas encore d'évaluation
- Chapter 1 Fluid PropertiesDocument55 pagesChapter 1 Fluid PropertiesKhaled Md Saiful Islam100% (1)
- Exploiting Wetting Phenomena To Tailor 1D Nano-And MicrostructuresDocument15 pagesExploiting Wetting Phenomena To Tailor 1D Nano-And Microstructuress1hahriarPas encore d'évaluation
- L Is Garten 1971Document20 pagesL Is Garten 1971t8e7w2koPas encore d'évaluation
- The Equation of State For Gases and Liquids: Nobel Lecture, December 12, 1910Document12 pagesThe Equation of State For Gases and Liquids: Nobel Lecture, December 12, 1910Nataly CGPas encore d'évaluation
- Fluid Mechanics Chapter on Viscosity, Buoyancy and StabilityDocument38 pagesFluid Mechanics Chapter on Viscosity, Buoyancy and StabilityMansi TayalPas encore d'évaluation
- Using Surface Integrals For Checking The Archimedes' Law of BuoyancyDocument16 pagesUsing Surface Integrals For Checking The Archimedes' Law of BuoyancysusioktaviaPas encore d'évaluation
- 04.properties of Liquids and Solids and Zeroth Thermodinamic LawDocument18 pages04.properties of Liquids and Solids and Zeroth Thermodinamic LawMirawati EfendiPas encore d'évaluation
- The Advection-Diffusion Equation States That The Rate of Change of A Property Depends On The Divergence of The Advective and Diffusive FluxesDocument2 pagesThe Advection-Diffusion Equation States That The Rate of Change of A Property Depends On The Divergence of The Advective and Diffusive FluxesGonçalo Machete BotelhoPas encore d'évaluation
- Fluid Statics - Ii: CSE20202: Fluid Mechanics For Civil EngineeringDocument27 pagesFluid Statics - Ii: CSE20202: Fluid Mechanics For Civil EngineeringWY SPas encore d'évaluation
- Materi 04 - Kimia Teknik - by Waluyo Nuswantoro 2021Document11 pagesMateri 04 - Kimia Teknik - by Waluyo Nuswantoro 2021Ryan BageurPas encore d'évaluation
- Van Der Waals LectureDocument12 pagesVan Der Waals LectureEdgar SanchezPas encore d'évaluation
- LN 2Document1 pageLN 2R S PappuPas encore d'évaluation
- The Equation of State for Gases and LiquidsDocument12 pagesThe Equation of State for Gases and LiquidsAMIT PRAJAPATIPas encore d'évaluation
- Chapter 4: Unsteady State (Transient) Mass Transfer: Prof. Do Hyun Kim, CBE332 Heat and Molecular Transfer, Fall, 2021Document20 pagesChapter 4: Unsteady State (Transient) Mass Transfer: Prof. Do Hyun Kim, CBE332 Heat and Molecular Transfer, Fall, 2021JW MPas encore d'évaluation
- C 2, According T O: BY TVDocument15 pagesC 2, According T O: BY TVMax J PowerPas encore d'évaluation
- Age 204Document69 pagesAge 204Usman Samuel BabalolaPas encore d'évaluation
- Communicating vessels fluid equilibrium levelsDocument8 pagesCommunicating vessels fluid equilibrium levelsJuan Carlos BordaPas encore d'évaluation
- Lec - 6Document12 pagesLec - 6Arif AhmedPas encore d'évaluation
- Hgpe Formulas 2022Document21 pagesHgpe Formulas 2022Malikah AliPas encore d'évaluation
- Archimedes Surface IntegralsDocument13 pagesArchimedes Surface IntegralsMuhammad Nur ArifinPas encore d'évaluation
- PURE SUBSTANCES AND PHASE CHANGESDocument29 pagesPURE SUBSTANCES AND PHASE CHANGESRuel Japhet BitoyPas encore d'évaluation
- Fluid Mechanics WK 3Document26 pagesFluid Mechanics WK 3JAMES CLEARPas encore d'évaluation
- Turner 1969Document16 pagesTurner 1969Alain BastidePas encore d'évaluation
- Floatation and Stability: What Is It?Document3 pagesFloatation and Stability: What Is It?BETSYPas encore d'évaluation
- Heat 4e Chap06 Lecture 1Document146 pagesHeat 4e Chap06 Lecture 1Abir RashidPas encore d'évaluation
- Viscosity PDFDocument15 pagesViscosity PDFRajat AroraPas encore d'évaluation
- Fiziks: Chapter - 2Document13 pagesFiziks: Chapter - 2SURAJ PRATAP SINGHPas encore d'évaluation
- Why Is SurfaceDocument10 pagesWhy Is SurfaceGiovanniPas encore d'évaluation
- Why Is SurfaceDocument10 pagesWhy Is SurfaceGiovanniPas encore d'évaluation
- Why Is Surface Tension Parallel To The Surface - by Antonin MarchandDocument10 pagesWhy Is Surface Tension Parallel To The Surface - by Antonin MarchandAaryan DewanPas encore d'évaluation
- Phase Equilibrium: Phases, Components, and Degrees of FreedomDocument69 pagesPhase Equilibrium: Phases, Components, and Degrees of FreedomSyahirah FazialPas encore d'évaluation
- Deney 3Document22 pagesDeney 3baratniloy1Pas encore d'évaluation
- Double LayerDocument4 pagesDouble LayerJose Eduardo Osuna AraizaPas encore d'évaluation
- Liquid State FosDocument32 pagesLiquid State FosSrirama LakshmiPas encore d'évaluation
- 74. Natural ConvectionDocument39 pages74. Natural Convectionraghu.entrepreneurPas encore d'évaluation
- P V N B: Excluded Volume and The Van Der Waals EquationDocument2 pagesP V N B: Excluded Volume and The Van Der Waals EquationTamara KrsticPas encore d'évaluation
- CALCULATING SURFACE FREE ENERGY USING CONTACT ANGLE DATADocument3 pagesCALCULATING SURFACE FREE ENERGY USING CONTACT ANGLE DATAAlper Baran SözmenPas encore d'évaluation
- Intermolecular Forces ChartDocument5 pagesIntermolecular Forces ChartCarlos Mella-RijoPas encore d'évaluation
- 04 - Interfacial PhenomenaDocument79 pages04 - Interfacial PhenomenaPuspa DasPas encore d'évaluation
- 徐牧恒科学C2Document27 pages徐牧恒科学C2中华雅思王Pas encore d'évaluation
- Using Surface Integrals For Checking The Archimedes' Law of BuoyancyDocument17 pagesUsing Surface Integrals For Checking The Archimedes' Law of BuoyancyLuanna Silva de Pires Campos AlvesPas encore d'évaluation
- Models of Electrical Double LayerDocument5 pagesModels of Electrical Double LayeriamakingPas encore d'évaluation
- DensityDocument3 pagesDensityWheeuu RawrPas encore d'évaluation
- 9259478Document64 pages9259478Brajesh Kumar100% (1)
- Multiphase Systems - Part IDocument20 pagesMultiphase Systems - Part I랄뚜기Pas encore d'évaluation
- Control ValveDocument1 pageControl ValveAlvin SmithPas encore d'évaluation
- Eng Therm and Fluid Mech E1 Level 4 Exam MAY 2021Document10 pagesEng Therm and Fluid Mech E1 Level 4 Exam MAY 2021Brody CrossPas encore d'évaluation
- UNIT 5 Changes of StateDocument6 pagesUNIT 5 Changes of StatePham Van Tin B1909842Pas encore d'évaluation
- Steam Tables (Rajput)Document21 pagesSteam Tables (Rajput)saifPas encore d'évaluation
- Conversion of NM3/Min to M3/Min volume and other unitsDocument36 572 pagesConversion of NM3/Min to M3/Min volume and other unitsOmkar JoshiPas encore d'évaluation
- Layers of Earth's Atmosphere: TroposphereDocument4 pagesLayers of Earth's Atmosphere: TroposphereCarlos EduardoPas encore d'évaluation
- CHE 406 Transport Phenomena Lecture 5 Shell Momentum BalanceDocument26 pagesCHE 406 Transport Phenomena Lecture 5 Shell Momentum BalanceAhmad IjazPas encore d'évaluation
- Chapter 3 Permeability Part 2Document18 pagesChapter 3 Permeability Part 2Pugal100% (1)
- 3Document10 pages3Ariel Carlos De LeonPas encore d'évaluation
- Dew Point Calculation ExampleDocument4 pagesDew Point Calculation Examplesandeep lal100% (1)
- Boyles LawDocument18 pagesBoyles Lawcale suarezPas encore d'évaluation
- Tutorial 1 - Statistical Thermodynamics 1Document15 pagesTutorial 1 - Statistical Thermodynamics 1JoserinePas encore d'évaluation
- States of MatterDocument30 pagesStates of MatterSwapnil MandalPas encore d'évaluation
- 4.3.4 Assignments - 4.3 Separation - Liquid - Liquid - Material Del Curso CHEM01x - EdxDocument5 pages4.3.4 Assignments - 4.3 Separation - Liquid - Liquid - Material Del Curso CHEM01x - EdxRicardo NuñezPas encore d'évaluation
- Me F214 1084 C 2013 2Document3 pagesMe F214 1084 C 2013 2siddharth deshmukhPas encore d'évaluation
- 8.8 Calculation of Fugacity (Liquids) : Example S8.1 Vapor and Liquid Fugacities Using The Virial EquationDocument1 page8.8 Calculation of Fugacity (Liquids) : Example S8.1 Vapor and Liquid Fugacities Using The Virial EquationtruffelovePas encore d'évaluation
- HYSYSDocument26 pagesHYSYSquimicocad9891Pas encore d'évaluation
- Fluid DyanamicsDocument19 pagesFluid DyanamicsSanthoshMBSanthuPas encore d'évaluation
- MCQ Astm Distillation: Abdul Majeed Ahmed Hossam IsmailDocument4 pagesMCQ Astm Distillation: Abdul Majeed Ahmed Hossam IsmailAbdul Majeed AhmedPas encore d'évaluation
- Cryogenic Refrigeration Systems and Ultra-Low Temperature ApplicationsDocument35 pagesCryogenic Refrigeration Systems and Ultra-Low Temperature ApplicationsHosnii QamarPas encore d'évaluation
- Partition FunctionDocument2 pagesPartition FunctionmanishcmetPas encore d'évaluation
- Vapor Liquid EquilibriumDocument28 pagesVapor Liquid EquilibriumKhloud MadihPas encore d'évaluation
- Assignment 3 PDFDocument3 pagesAssignment 3 PDFk l mandalPas encore d'évaluation
- Ref Review ProblemsDocument3 pagesRef Review ProblemsGiancarlo SantosPas encore d'évaluation
- Question Bank BSPH351Document10 pagesQuestion Bank BSPH351buntysharma8218Pas encore d'évaluation