Académique Documents
Professionnel Documents
Culture Documents
Atomic Properties of The Elements: Group
Transféré par
barlosTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Atomic Properties of The Elements: Group
Transféré par
barlosDroits d'auteur :
Formats disponibles
P E R I O D I C T A B L E
Group
1
IA
Atomic Properties of the Elements 18
VIIIA
1 2
S1/2 FREQUENTLY USED FUNDAMENTAL PHYSICAL CONSTANTS § Physical Measurement Laboratory www.pml.nist.gov 2 1
S0
1
H 1 second = 9 192 631 770 periods of radiation corresponding to the
transition between the two hyperfine levels of the ground state of 133Cs
§ For the most accurate
Standard Reference Data www.nist.gov/srd He
Hydrogen speed of light in vacuum c 299 792 458 m s
−1
(exact) Helium
1.008* values of these and 4.002602
1s
2 Planck constant h 6.626 070 x 10
−34
Js (ħ /2 ) other constants, visit 13 14 15 16 17 1s
2
−19
13.5984 IIA elementary charge e 1.602 177 x 10 C pml.nist.gov/constants IIIA IVA VA VIA VIIA 24.5874
−31
electron mass me 9.109 384 x 10 kg
3 2
S1/2 4 1
S0
mec
2
0.510 999 MeV
5 2
P°
1/2 6 3
P0 7 4
°
S3/2 8 3
P2 9 2
°
P3/2 10 1
S0
Solids
2
Li Be proton mass
fine-structure constant
mp 1.672 622 x 10
1/137.035 999
−27
kg
Liquids
B C N O F Ne
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
6.94*
2
9.0121831
2 2
Rydberg constant R 10 973 731.569 m
−1
Gases 10.81*
2 2
12.011*
2 2 2
14.007*
2 2 3
15.999*
2 2 4
18.99840316*
2 2 5
20.1797
2 2 6
1s 2s 1s 2s R c 3.289 841 960 x 10 Hz
15 1s 2s 2p 1s 2s 2p 1s 2s 2p 1s 2s 2p 1s 2s 2p 1s 2s 2p
5.3917 9.3227 Artificially 8.2980 11.2603 14.5341 13.6181 17.4228 21.5645
R hc 13.605 693 eV
11 2
S1/2 12 1
S0 electron volt eV 1.602 177 x 10
-19
J
Prepared 13 2
°
P1/2 14 3
P0 15 4
°
S3/2 16 3
P2 17 2
°
P3/2 18 1
S0
3
Na Mg Boltzmann constant
molar gas constant
k
R
1.380 65 x 10
−23
−1
8.314 5 J mol K
JK
−1
−1
Al Si P S Cl Ar
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
22.98976928 24.305*
2
3 4 5 6 7 8 9 10 11 12 26.9815385
2
28.085*
2 2
30.97376199*
2 3
32.06*
2 4
35.45*
2 5
39.948
2 6
[Ne]3s [Ne]3s [Ne]3s 3p [Ne]3s 3p [Ne]3s 3p [Ne]3s 3p [Ne]3s 3p [Ne]3s 3p
5.1391 7.6462 IIIB IVB VB VIB VIIB VIII IB IIB 5.9858 8.1517 10.4867 10.3600 12.9676 15.7596
19 2
S1/2 20 1
S0 21 2D3/2 22 3
F2 23
4
F3/2 24
7
S3 25
6
S5/2 26
5
D4 27 4
F9/2 28
3
F4 29 2
S1/2 30
1
S0 31 2
°
P1/2 32 3
P0 33 4
°
S3/2 34 3
P2 35
2
°
P3/2 36 1
S0
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Br Kr
Period
4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton
39.0983 40.078 44.955908 47.867 50.9415 51.9961 54.938044 55.845 58.933194 58.6934 63.546 65.38 69.723 72.630 74.921595 78.971 79.904* 83.798
2 2 2 2 3 2 5 5 2 6 2 7 2 8 2 10 10 2 10 2 10 2 2 10 2 3 10 2 4 10 2 5 10 2 6
[Ar]4s [Ar]4s [Ar]3d4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p
4.3407 6.1132 6.5615 6.8281 6.7462 6.7665 7.4340 7.9025 7.8810 7.6399 7.7264 9.3942 5.9993 7.8994 9.7886 9.7524 11.8138 13.9996
37 2
S1/2 38 1
S0 39 2
D3/2 40 3
F2 41 6
D1/2 42 7
S3 43 6
S5/2 44 5
F5 45 4
F9/2 46 1
S0 47 2
S1/2 48 1
S0 49 2
°
P1/2 50 3
P0 51 4
°
S3/2 52 3
P2 53 2
°
P3/2 54 1
S0
5
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon
85.4678 87.62 88.90584 91.224 92.90637 95.95 (98) 101.07 102.90550 106.42 107.8682 112.414 114.818 118.710 121.760 127.60 126.90447 131.293
2 2 2 2 4 5 5 2 7 8 10 10 10 2 10 2 10 2 2 10 2 3 10 2 4 10 2 5 10 2 6
[Kr]5s [Kr]5s [Kr]4d5s [Kr]4d 5s [Kr]4d 5s [Kr]4d 5s [Kr]4d 5s [Kr]4d 5s [Kr]4d 5s [Kr]4d [Kr]4d 5s [Kr]4d 5s [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p
4.1771 5.6949 6.2173 6.6339 6.7589 7.0924 7.1194 7.3605 7.4589 8.3369 7.5762 8.9938 5.7864 7.3439 8.6084 9.0097 10.4513 12.1298
55 2
S1/2 56 1
S0 72 3
F2 73 4
F3/2 74 5
D0 75 6
S5/2 76 5
D4 77 4
F9/2 78 3
D3 79 2
S1/2 80 1
S0 81 2
°
P1/2 82 3
P0 83 4
°
S3/2 84 3
P2 85 2
°
P3/2 86 1
S0
Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
6 Cesium Barium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon
132.9054520* 137.327 178.49 180.94788 183.84 186.207 190.23 192.217 195.084 196.966569 200.592 204.38* 207.2 208.98040 (209) (210) (222)
2 14 2 2 14 3 2 14 4 2 14 5 2 14 6 2 14 7 2 14 9 14 10 14 10 2 2 3 4 5 6
[Xe]6s [Xe]6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Hg]6p [Hg]6p [Hg]6p [Hg]6p [Hg]6p [Hg]6p
3.8939 5.2117 6.8251 7.5496 7.8640 7.8335 8.4382 8.9670 8.9588 9.2256 10.4375 6.1083 7.4167 7.2855 8.414 9.3175 10.7485
87 2
S1/2 88 1
S0 104 3
F2 105 4
F3/2 106 0 107 5/2 108 4 109 110 111 112 113 114 115 116 117 118
Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og
7 Francium Radium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessine Oganesson
(223) (226) (267) (268) (271) (270) (269) (278) (281) (282) (285) (286) (289) (289) (293) (294) (294)
2 14 2 2 14 3 2 14 4 2 14 5 2 14 6 2
[Rn]7s [Rn]7s [Rn]5f 6d 7s [Rn]5f 6d 7s [Rn]5f 6d 7s [Rn]5f 6d 7s [Rn]5f 6d 7s
4.0727 5.2784 6.01 6.8 7.8 7.7 7.6
Atomic Ground-state 57 2
D3/2 58 1
G° 59 4
°
I9/2 60 5
I4 61 6
H° 62 7
F0 63 8
S° 64 9
D° 65 6
H° 66 5
I8 67 4
°
I15/2 68 3
H6 69 2
F° 70 1
S0 71 2
D3/2
Lanthanides
4 5/2 7/2 2 15/2 7/2
Number Level
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Symbol
58 1
G°
4 Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium
Ce
138.90547 140.116 140.90766 144.242 (145) 150.36 151.964 157.25 158.92535 162.500 164.93033 167.259 168.93422 173.045 174.9668
2 2 3 2 4 2 5 2 6 2 7 2 7 2 9 2 10 2 11 2 12 2 13 2 14 2 14 2
[Xe]5d6s [Xe]4f5d6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 5d6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 6s [Xe]4f 5d6s
Name 5.5769 5.5386 5.473 5.5250 5.582 5.6437 5.6704 6.1498 5.8638 5.9391 6.0215 6.1077 6.1843 6.2542 5.4259
Cerium
Standard 140.116 89 2
D3/2 90 3
F2 91 4
K11/2 92 5
L°
6 93 6
L11/2 94 7
F0 95 8
°
S7/2 96 9
D°
2 97 6
H°
15/2 98 5
I8 99 4
°
I15/2 100 3
H6 101 2
F°
7/2 102 1
S0 103 2
°
P1/2
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Actinides
Atomic 2
† [Xe]4f5d6s
Weight (Da)
5.5386 Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium
(227) 232.0377 231.03588 238.02891 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (266)
Ground-state Ionization [Rn]6d7s
2 2
[Rn]6d 7s
2 2
[Rn]5f 6d7s
2 3
[Rn]5f 6d7s
2 4
[Rn]5f 6d7s
2 6
[Rn]5f 7s
2 7
[Rn]5f 7s
2 7
[Rn]5f 6d7s
2 9
[Rn]5f 7s
2 10
[Rn]5f 7s
2 11
[Rn]5f 7s
2 12
[Rn]5f 7s
2 13
[Rn]5f 7s
2 14
[Rn]5f 7s
2 14 2
[Rn]5f 7s 7p
Configuration Energy (eV) 5.3802 6.3067 5.89 6.1941 6.2655 6.0258 5.9738 5.9914 6.1978 6.2817 6.3676 6.50 6.58 6.65 4.96
† 12
Based upon C. () indicates the mass number of the longest-lived isotope. *For the most precise value, visit ciaaw.org. For a description of the data, visit pml.nist.gov/data
NIST SP 966 (February 2017)
Vous aimerez peut-être aussi
- Atomic Properties of The Elements: 1 IA 18 ViiiaDocument1 pageAtomic Properties of The Elements: 1 IA 18 ViiiabarlosPas encore d'évaluation
- 2016 Periodic Table Page 1 of 2Document2 pages2016 Periodic Table Page 1 of 2yakupyldz251Pas encore d'évaluation
- NIST Periodic Table July 2018Document2 pagesNIST Periodic Table July 2018Jsn JsnPas encore d'évaluation
- Atomic Properties of The Elements: GroupDocument2 pagesAtomic Properties of The Elements: GroupYunet SozaPas encore d'évaluation
- Atomic Properties of The Elements: GroupDocument2 pagesAtomic Properties of The Elements: GroupsahraPas encore d'évaluation
- Handbook of XPS PDFDocument193 pagesHandbook of XPS PDFBernay CifuentesPas encore d'évaluation
- Nist Periodictable July2019Document4 pagesNist Periodictable July2019SE Rural WQMISPas encore d'évaluation
- Is 9972 Sprinkler-HeadsDocument30 pagesIs 9972 Sprinkler-HeadsNanu Patel100% (1)
- Schematic Esp8266Document1 pageSchematic Esp8266mihais100% (2)
- Periodic Table of The Elements: Li Be B CDocument13 pagesPeriodic Table of The Elements: Li Be B Cvroom victorPas encore d'évaluation
- Periodic Table of The Elements: Li Be B CDocument13 pagesPeriodic Table of The Elements: Li Be B CVăn Thanh BùiPas encore d'évaluation
- VK3HN OLED SchematicDocument1 pageVK3HN OLED Schematicluistorcatt72Pas encore d'évaluation
- Input Voltage: 5-16V: Usart1 - RX Usart1 - TXDocument1 pageInput Voltage: 5-16V: Usart1 - RX Usart1 - TXLeevan Bautista100% (1)
- Toyota ALPHARD VELLFIRE (EM2441E) - Overall Electrical Wiring Diagram PDFDocument133 pagesToyota ALPHARD VELLFIRE (EM2441E) - Overall Electrical Wiring Diagram PDFpengelana satu88% (17)
- Electrical Diagram A860 - v1 1 - 10092012 PDFDocument9 pagesElectrical Diagram A860 - v1 1 - 10092012 PDFFriends TVSHOWPas encore d'évaluation
- Darlington: Silicon PNP Epitaxial Planar TransistorDocument1 pageDarlington: Silicon PNP Epitaxial Planar TransistorFábio PellegattiPas encore d'évaluation
- Internal: Confidentiality Class Acc. To 1102 KDocument1 pageInternal: Confidentiality Class Acc. To 1102 KHudson LuppiPas encore d'évaluation
- Periodic TableDocument2 pagesPeriodic TableE KaraPas encore d'évaluation
- Schottky, !. Barrier (,1, .. T .Rir., ., ,,, Hot Carrier Power Rectifiers RECTI FI ER&F's:j$r . ,. .,, .Document4 pagesSchottky, !. Barrier (,1, .. T .Rir., ., ,,, Hot Carrier Power Rectifiers RECTI FI ER&F's:j$r . ,. .,, .gdiliogPas encore d'évaluation
- DD Expander - MB - Diy - Schematics - Rev1Document3 pagesDD Expander - MB - Diy - Schematics - Rev1JosephPas encore d'évaluation
- Dell Inspiron 14 3420 CODEC SchematicDocument1 pageDell Inspiron 14 3420 CODEC SchematicLHMFPas encore d'évaluation
- ACRD6XXP SchematicDocument2 pagesACRD6XXP SchematicMasab AnisPas encore d'évaluation
- Half Rectifier Snubber: Connecter Pins A La MasseDocument1 pageHalf Rectifier Snubber: Connecter Pins A La MasseMiftah HudaPas encore d'évaluation
- 35 Sequoia (Cont. Next Page) : Air ConditionerDocument4 pages35 Sequoia (Cont. Next Page) : Air Conditionerوليدمطهر الشجاعPas encore d'évaluation
- (Cont. Next Page) 1 Toyota Rav4: Power Source (Except Korea)Document3 pages(Cont. Next Page) 1 Toyota Rav4: Power Source (Except Korea)sasha211230% (1)
- Toyota Fortuner (EM3275E) - Overall Electrical Wiring DiagramDocument159 pagesToyota Fortuner (EM3275E) - Overall Electrical Wiring Diagramnur ali88% (17)
- Periodic TableDocument1 pagePeriodic TableEunice JorgePas encore d'évaluation
- fsp488-4f01 Power Supply SCHDocument1 pagefsp488-4f01 Power Supply SCHCarlos Junior PereiraPas encore d'évaluation
- HW5 SolDocument15 pagesHW5 Sol蒲念文Pas encore d'évaluation
- Ms DD 4810 Ics DWG 0213 - Rev1Document1 pageMs DD 4810 Ics DWG 0213 - Rev1lhecemponPas encore d'évaluation
- Gain and Frequency Circuit DesignDocument2 pagesGain and Frequency Circuit DesignTV BoxPas encore d'évaluation
- Formula SHeet and Periodic Table For CHMA10 Fall 2023Document2 pagesFormula SHeet and Periodic Table For CHMA10 Fall 2023jiachouhan7Pas encore d'évaluation
- Breakbox: Designator1 Cpu - Schdoc Designator2 Io - Schdoc Designator3 Power - SchdocDocument4 pagesBreakbox: Designator1 Cpu - Schdoc Designator2 Io - Schdoc Designator3 Power - SchdocimmanemmPas encore d'évaluation
- Hydraulic circuit and componentsDocument36 pagesHydraulic circuit and componentsIBrahym Souiri100% (1)
- Keeping your Toyota Fortuner well maintainedDocument159 pagesKeeping your Toyota Fortuner well maintainedrabitosanPas encore d'évaluation
- Periodic TableDocument1 pagePeriodic TablevxlrPas encore d'évaluation
- 83 Vios / Yaris: Remote Controlmirror (S/D From Aug. 2018 Production)Document1 page83 Vios / Yaris: Remote Controlmirror (S/D From Aug. 2018 Production)VôĐốiPas encore d'évaluation
- 1 Hilux (Cont. Next Page) : Power SourceDocument3 pages1 Hilux (Cont. Next Page) : Power SourceautocomtrucksPas encore d'évaluation
- Horn PDFDocument1 pageHorn PDFAgus SetiyawanPas encore d'évaluation
- Horn PDFDocument1 pageHorn PDFAgus SetiyawanPas encore d'évaluation
- Abs 1Document1 pageAbs 1budi yantoPas encore d'évaluation
- Fiesta en Corraleja 6Document1 pageFiesta en Corraleja 6Multipapel SabanalargaPas encore d'évaluation
- Fiesta en Corraleja 6Document1 pageFiesta en Corraleja 6Multipapel SabanalargaPas encore d'évaluation
- LAYOUT of OT Shelter PDFDocument1 pageLAYOUT of OT Shelter PDFBalaji YPas encore d'évaluation
- Stock pre final machine titleDocument1 pageStock pre final machine titleEduardo RodriguezPas encore d'évaluation
- 2SD 2017 PDFDocument1 page2SD 2017 PDFtabassam7801Pas encore d'évaluation
- Rustboro City: Pokémon Ruby/Sapphire/EmeraldDocument2 pagesRustboro City: Pokémon Ruby/Sapphire/EmeraldNyx OcPas encore d'évaluation
- Schematics-478020 RouterDocument5 pagesSchematics-478020 RouterALFONZO DANIELPas encore d'évaluation
- Periodic TableDocument1 pagePeriodic TableBetoPas encore d'évaluation
- KSP90 Electric Wiring DiagramDocument204 pagesKSP90 Electric Wiring DiagramMuhammad Zubair Sherwani93% (14)
- Logic Probe SchematicDocument1 pageLogic Probe SchematicAnonymous HPlNDhM6ejPas encore d'évaluation
- Hisense FA y LED DriveDocument2 pagesHisense FA y LED Driveatomo33Pas encore d'évaluation
- CN391 - Single ESSP Interface Between Smart Payout and Hopper AssemblyDocument1 pageCN391 - Single ESSP Interface Between Smart Payout and Hopper AssemblyAymen CheffiPas encore d'évaluation
- Post Concrete ChimneyDocument1 pagePost Concrete ChimneySubhakanta SwainPas encore d'évaluation
- Periodic Table (Quantum Mechanics)Document1 pagePeriodic Table (Quantum Mechanics)Harry PutrPas encore d'évaluation
- Darlington: Silicon PNP Epitaxial Planar Transistor (Complement To Type 2SD1785)Document1 pageDarlington: Silicon PNP Epitaxial Planar Transistor (Complement To Type 2SD1785)isaiasvaPas encore d'évaluation
- 20kV CommonDocument1 page20kV CommonReza PahlepiPas encore d'évaluation
- GRP Tanks - Approvals & ReferencesDocument24 pagesGRP Tanks - Approvals & ReferencesUttam KumarPas encore d'évaluation
- The Rough Guide to Beijing (Travel Guide eBook)D'EverandThe Rough Guide to Beijing (Travel Guide eBook)Évaluation : 2 sur 5 étoiles2/5 (1)
- Uiuach: Listado de VehículosDocument6 pagesUiuach: Listado de VehículosbarlosPas encore d'évaluation
- FormulaDocument3 pagesFormulabarlosPas encore d'évaluation
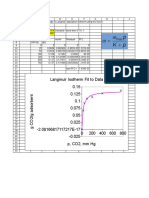
- Langmuir Isotherm DevelopmentDocument16 pagesLangmuir Isotherm DevelopmentbarlosPas encore d'évaluation
- A Flock Is A GDocument4 pagesA Flock Is A GbarlosPas encore d'évaluation
- Font ReadMe - Shorthalt Font FamilyDocument1 pageFont ReadMe - Shorthalt Font FamilybarlosPas encore d'évaluation
- IQ Scores: Sub-Test MeasuresDocument1 pageIQ Scores: Sub-Test MeasuresbarlosPas encore d'évaluation
- Ex20 1Document3 pagesEx20 1barlosPas encore d'évaluation
- Geography of Prosperity Scotland Press Release October 2015 PDFDocument3 pagesGeography of Prosperity Scotland Press Release October 2015 PDFbarlosPas encore d'évaluation
- History 8Document2 pagesHistory 8barlosPas encore d'évaluation
- Ex20 1Document3 pagesEx20 1barlosPas encore d'évaluation
- Espec VisionLite OpmanDocument38 pagesEspec VisionLite OpmanbarlosPas encore d'évaluation
- Lindenwood University St. Louis Community College: HISTORY (BA) Transfer GuideDocument2 pagesLindenwood University St. Louis Community College: HISTORY (BA) Transfer GuidebarlosPas encore d'évaluation
- Learn about people, events of the past with Junior Cert HistoryDocument2 pagesLearn about people, events of the past with Junior Cert HistorybarlosPas encore d'évaluation
- WaterSA 2004 04 12 PDFDocument8 pagesWaterSA 2004 04 12 PDFbarlosPas encore d'évaluation
- Geography Major at Utah State UniversityDocument4 pagesGeography Major at Utah State UniversitybarlosPas encore d'évaluation
- Paper9 PDFDocument7 pagesPaper9 PDFbarlosPas encore d'évaluation
- School GEOGRAPHY Curriculum Planning Outline WebsiteDocument4 pagesSchool GEOGRAPHY Curriculum Planning Outline WebsitebarlosPas encore d'évaluation
- Final TG Active Ingredient Product Chemistry May 2016Document20 pagesFinal TG Active Ingredient Product Chemistry May 2016barlosPas encore d'évaluation
- Department of Geography Postgraduate Supervision Agreement 2016Document9 pagesDepartment of Geography Postgraduate Supervision Agreement 2016barlosPas encore d'évaluation
- Geography General Y11 Sample Assessment Tasks WACE 201516 PDFDocument23 pagesGeography General Y11 Sample Assessment Tasks WACE 201516 PDFbarlosPas encore d'évaluation
- Chemistry Stage 6 Syllabus 2017Document76 pagesChemistry Stage 6 Syllabus 2017barlosPas encore d'évaluation
- MCcab Thiele by Van LaarDocument11 pagesMCcab Thiele by Van LaarbarlosPas encore d'évaluation
- Year 3 Geography Areas of LearningDocument1 pageYear 3 Geography Areas of LearningbarlosPas encore d'évaluation
- Introduction To GeographyDocument4 pagesIntroduction To GeographybarlosPas encore d'évaluation
- Healthcare in NicaDocument9 pagesHealthcare in NicabarlosPas encore d'évaluation
- Holtville High School graduation and A-G requirementsDocument2 pagesHoltville High School graduation and A-G requirementsbarlosPas encore d'évaluation
- Final TG Active Ingredient Product Chemistry May 2016Document20 pagesFinal TG Active Ingredient Product Chemistry May 2016barlosPas encore d'évaluation
- Researcher CategoriesDocument22 pagesResearcher CategoriesbarlosPas encore d'évaluation
- Basic Info About Glaze FormulationsDocument3 pagesBasic Info About Glaze FormulationsThongMaVanPas encore d'évaluation
- AITECH Accredited October 2015Document17 pagesAITECH Accredited October 2015Jiane NavalPas encore d'évaluation
- Astm E1426.11123 PDFDocument6 pagesAstm E1426.11123 PDFRicardo Martins SilvaPas encore d'évaluation
- Umc Technical SpecsDocument3 pagesUmc Technical SpecsMunir AhmadPas encore d'évaluation
- 02 WholeDocument121 pages02 WholeBarohPas encore d'évaluation
- 3D Printed Tooling For Thermoforming of Medical DevicesDocument7 pages3D Printed Tooling For Thermoforming of Medical DevicesAlex BurdePas encore d'évaluation
- 3 - SWCC Specification M02 Polyethylene Coating Rev0Document30 pages3 - SWCC Specification M02 Polyethylene Coating Rev0EngineerSajidAlam100% (1)
- Important To Write The Correct Structure FromDocument16 pagesImportant To Write The Correct Structure FromR A V IPas encore d'évaluation
- NSCP Definition of TermsDocument25 pagesNSCP Definition of TermsMary AnnePas encore d'évaluation
- Boedeker Plastics - Anti-Static and Conductive PlasticsDocument7 pagesBoedeker Plastics - Anti-Static and Conductive PlasticsVladimir MilasinovicPas encore d'évaluation
- ASTM A694 F60 - Heat Treatment and Mechanical Properties - ICRF 2012Document3 pagesASTM A694 F60 - Heat Treatment and Mechanical Properties - ICRF 2012Noushad Bin JamalPas encore d'évaluation
- Introduction To Composite Materials (Laminated Composite Materials)Document60 pagesIntroduction To Composite Materials (Laminated Composite Materials)soma_durga6606Pas encore d'évaluation
- Chapter2 Di Pa TaposDocument13 pagesChapter2 Di Pa TaposDianne VillanuevaPas encore d'évaluation
- Fracture Toughness Testing - TwiDocument5 pagesFracture Toughness Testing - TwiSergio MunhosPas encore d'évaluation
- Haselrieder 2015Document8 pagesHaselrieder 2015Daiana Medone AcostaPas encore d'évaluation
- Astm A-297 HPDocument2 pagesAstm A-297 HPeduardo_exsys100% (1)
- Palruf PVC BrochureDocument19 pagesPalruf PVC BrochureSerguei DobrinPas encore d'évaluation
- Ormex Butterfly ValvesDocument38 pagesOrmex Butterfly ValvesVishalPas encore d'évaluation
- Brookes Bell-Carriage of SulphurDocument4 pagesBrookes Bell-Carriage of SulphurAntonio Perez100% (1)
- Thermodynamically Stabilized B-Cspbi - Based Perovskite Solar Cells With Efficiencies 18%Document6 pagesThermodynamically Stabilized B-Cspbi - Based Perovskite Solar Cells With Efficiencies 18%HalfAton BaiPas encore d'évaluation
- Synthetic ester oils for air compressorsDocument1 pageSynthetic ester oils for air compressorsironitePas encore d'évaluation
- Construction Tech II Door TypesDocument105 pagesConstruction Tech II Door Typessydney augustPas encore d'évaluation
- Chapter 5 ConsolidationDocument11 pagesChapter 5 ConsolidationAwokePas encore d'évaluation
- Brundle Stock Part 1Document54 pagesBrundle Stock Part 1Colin SimpsonPas encore d'évaluation
- HT Unit - VDocument3 pagesHT Unit - VoctoviancletusPas encore d'évaluation
- 2019 - 1.2. Zavarivanje Gasnim Plamenom I Srodni PostupciDocument25 pages2019 - 1.2. Zavarivanje Gasnim Plamenom I Srodni PostupciticmaPas encore d'évaluation
- Et 0000 0 000 04 750Document83 pagesEt 0000 0 000 04 750RenatoPas encore d'évaluation
- REPORTING INCIDENTSDocument6 pagesREPORTING INCIDENTSDivyansh Singh ChauhanPas encore d'évaluation
- Nitofill EPLV : Constructive SolutionsDocument4 pagesNitofill EPLV : Constructive SolutionsmilanbrasinaPas encore d'évaluation