Académique Documents
Professionnel Documents
Culture Documents
Vendor Audit Question Air Re
Transféré par
Mohit SaxenaDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Vendor Audit Question Air Re
Transféré par
Mohit SaxenaDroits d'auteur :
Formats disponibles
Standard Operating Procedure
Title: Vendor Selection and Evaluation
______________________________________________________________________________________
Department Quality Management Document no QMS-045
Prepared by: Date: Supersedes:
Checked by: Date: Date Issued:
Approved by: Date: Review Date:
Document Owner
Procurement / Quality Assurance Manager
Affected Parties
All colleagues working in a team for vendor selection and evaluation process from the Procurement, QA,
Technical Services and Laboratory Departments.
Purpose
To define the process by which Vendors are assessed and evaluated in accordance with the GMP
requirements and Corporate Policies to create conditions for adequate material availability and adherence to
specification.
Scope
Note: It is recommended to use this procedure in conjunction with SOP QMS-115.
This SOP describes the process for the selection of vendors for raw materials (Active Ingredients &
Excipients), Packaging Components, Laboratory Supplies, Engineering Supplies, Bulk Product & Imported
Finished Goods.
This SOP does not describe the process of selection of a Contract Manufacture, (see SOP QMS-110)
The process of vendor assessment and evaluation is divided into four phases.
Vendor Assessment
Phase 1. Technical discussions
Phase 2. General Vendor Audit
Phase 3. Item specific evaluation
Vendor Evaluation
Phase 4. Ongoing vendor evaluation
This phased approach is to be applied to the following purchasing activities. Specific details pertaining to
each group are outlined in the procedure.
The progress of each Vendor, through this phased process, may be changed by management by means of
documented discussion to be held on Vendor’s file.
A Vendor may be designated as approved without undergoing the following procedure under the following
conditions:
• Via the legislation of local regulatory authority.
• Following submission of data as part of a product registration process.
It is the responsibility of Quality Assurance to ensure that all relevant requirements are communicated and
adhered to.
This is not an approved copy unless stamped in red
File Location: Date Printed: Page 1 of 19
Standard Operating Procedure
Title: Vendor Selection and Evaluation
______________________________________________________________________________________
QMS-080 Audits
EHS Statement
EHS issues are to be part of the program when a Vendor is approved and reviewed. Their corporate
responsibility should be noted. All EHS information with respect to a purchased product is to be sourced.
Table of Contents
1. Procedure.............................................................................................................................. 3
2. Specific Requirements for each Phase - Actives.................................................................... 5
3. Specific Requirements for Each Phase - Excipients .............................................................. 5
4. Specific Requirements for Each Phase - Critical Packaging Components ............................. 5
5. Specific Requirements for Each Phase - Non Critical Packaging Components ...................... 5
6. Specific Requirements for Each Phase - Laboratory Supplies ............................................... 6
7. Specific Requirements for Each Phase - Engineering Supplies ............................................. 6
8. Specific Requirements for Each Phase - Bulk Product & Imported Finished Goods............... 6
9. Appendix 1 – Flowchart- Vendor Selection and Evaluation .................................................... 7
10. Summery of Changes ............................................................................................................ 8
1. Procedure
Responsibility PROCEDURE
See Flow diagram (Appendix - 1)
All employees Any unit within the organization may propose the need to source a new vendor of goods
or services.
Procurement The initiator of the request must contact Procurement who will then commence the
vendor assessment. It is the responsibility of Procurement to ensure the progression of
the vendor through the vendor assessment process
Phase 1
Procurement Source suitable vendor who is capable of manufacturing the material according to in-
house Requirements, as per SOP QMS-115.
Vendors are selected using the following parameters:
• Ability to consistently supply material to the specification (laboratory records)
• Ability to deliver the required material in the quantities ordered (purchasing records)
• Previous experience with the supplier (raw material records, laboratory records, reject
material/component forms)
• Cost of material
Ability to supply technical support where required.
This is not an approved copy unless stamped in red
File Location: Date Printed: Page 3 of 19
Standard Operating Procedure
Title: Vendor Selection and Evaluation
______________________________________________________________________________________
2. Specific Requirements for each Phase - Actives
Active materials can only be obtained from approved manufacturers and in accordance with the
registered details with the local regulatory authorities.
Phase 1, 2 & 3 are to be carried out in accordance with SOP QMS-115.
Phase 4
The evaluation is performed annually using information provided from laboratory trend cards
reporting test results, stability analyses from post production stability monitoring, production
records, special project minutes of meetings, and reject material/component forms.
If an adverse trend is detected by routine testing of the materials then the Laboratory
Manager, in conjunction with the Procurement staff, will review, discuss and take appropriate
action.
3. Specific Requirements for Each Phase - Excipients
There is no requirement to specify the manufacturer of an excipient material for the registration of a
product. It is mandatory that the material supplied must comply with the grade and material
specification registered as part of the registration process. The specification to which the material
must comply may have been registered under the following conditions:
The manufacturer may be local or overseas based.
Phase 1, See SOP QMS-115.
Phase 2, 3 & 4, as per the procedure identified in this SOP.
4. Specific Requirements for Each Phase - Critical Packaging Components
In most instances the manufacturers of critical packaging components are identified as part of the
registration process, yet this is dependent upon the class of material. This may be via the local
regulatory guidelines or submission of actual data for the more recent registrations.
The manufacturer may be local or overseas based.
Phase 1, 2, 3 & 4, See SOP QMS-115.
5. Specific Requirements for Each Phase - Non Critical Packaging Components
The manufacturer may be local or overseas based.
Phase 1, 2
As per the procedure identified in this SOP.
Phase 3
Material Performance is conducted according to the material specification and performance
in production. Results to be recorded on Form-390 and filed in Vendor File kept in
procurement.
Phase 4
The evaluation is performed by QA and Procurement Departments annually using
information provided from compilation of number of deliveries, degree of rejects, number and
extent of problems encountered in production, stability data showing untoward trends
attributed to material deficiencies or changes.
This is not an approved copy unless stamped in red
File Location: Date Printed: Page 5 of 19
Standard Operating Procedure
Title: Vendor Selection and Evaluation
______________________________________________________________________________________
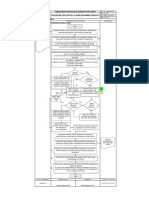
9. Appendix 1 – Flowchart- Vendor Selection and Evaluation
Need to
source new
vendor is
identified
QA, Technical,
Procurement liaise
for vendor
Identify possible
vendors
Send Supplier
Questionnaire
Purchase,
Sample & Testing
No Discussion
to proceed
Yes
Phase 2
required
Yes No
General Vendor
Audit
No Accepted
Chart Continued -
This is not an approved copy unless stamped in red
File Location: Date Printed: Page 7 of 19
Form-385
Issue date
Vendor Audit Questionnaire
(Ref. SOP QMS-045; QMS-080)
Vendor Company Name:
Supplier Site Address: Supplier Business Address (if different):
Phone No: Phone No:
Fax No: Fax No:
E Mail: E Mail:
Material supplied to Sydco, covered by this questionnaire:
Is the Company a division/subsidiary of another corporation? Yes No N/A
If Yes, Please Specify
This questionnaire was completed by:
Name:
Job Title:
Date:
Signature:
All information contained within this document will be treated as confidential between the Supplier and Buyer.
File Location: Date Printed: Page 9 of 19
Form-385
Issue date
Vendor Audit Questionnaire
(Ref. SOP QMS-045; QMS-080)
Can you please provide full Supply chain(s) for the referenced material(s)
(i.e. Manufacturer, Testers, Providers of C of A / C of C, Yes N/A
Packers / Repackers and Storage & Distribution)
If “Yes”, please list & explain:
Quality Management System
What is the basis of your quality system, i.e. ISO?
Please state your Certificate/Registration reference and appropriate dates:
Have any regulatory agencies inspected your facility in the last five years? Yes No N/A
If ‘Yes’, by whom, when and what were the results?
Are all procedures documented and approved? Yes No N/A
Are there change control procedures in place? Yes No N/A
Is there a procedure to notify customers of change? Yes No N/A
Are QA/QC responsibilities well defined and independent? Yes No N/A
Does QA/QC approve all analytical specifications and methods? Yes No N/A
How is a batch (standard quantity) defined?
What is the batch numbering system? (Please explain in detail)
All information contained within this document will be treated as confidential between the Supplier and Buyer.
File Location: Date Printed: Page 11 of 19
Form-385
Issue date
Vendor Audit Questionnaire
(Ref. SOP QMS-045; QMS-080)
Warehouse
Are storage facilities/equipment/ rented or personnel contracted? Yes No N/A
If “Yes”, please provide details.
Are receipt and release procedures documented? Yes No N/A
Is the supply chain documented? Yes No N/A
How is material status controlled? (i.e. Physical, system or labelling)
How is rejected material controlled? (i.e. Physical, system or labelling)
Is there an identified sampling area? Yes No N/A
Are all containers identified? Yes No N/A
Is a First-In-First-Out or First-Expiry-First-Out system in use? (Identify) Yes No N/A
Are shelf life/expiration dates used? Yes No N/A
Is Temperature (T°°), controlled and documented? Yes No N/A
Comments:
Is Relative humidity (RH%), controlled and documented? Yes No N/A
Comments:
Production
Is there more than one site or plant used for the manufacture
Yes No N/A
of the specified material(s)?
If “Yes”, please provide details.
Is plant equipment labelled as to its status and contents? Yes No N/A
Is Pipe work labelled? Yes No N/A
Are critical processes validated? Yes No N/A
Does process documentation include:
Yes No N/A
Process instructions
Cleaning instructions Yes No N/A
Cleaning records
Yes N/A
Area clearance Yes No N/A
Are cleaning processes validated? Yes No N/A
All information contained within this document will be treated as confidential between the Supplier and Buyer.
File Location: Date Printed: Page 13 of 19
Form-385
Issue date
Vendor Audit Questionnaire
(Ref. SOP QMS-045; QMS-080)
Are there label disposal procedures? Yes No N/A
How are containers security sealed?
Is material clearly labelled, including waste and reject material?
No N/A
Computerized Systems
Do you have a list of the Computerized systems used by this facility? Yes No N/A
If “Yes”, do you identify the Computerized systems that are considered to
Yes No N/A
have an impact on Quality of Product, or Service offered?
If “Yes”, how is this documented?
Does your Quality system cover the quality of Computerized systems? Yes No N/A
Do you have procedures in place for disaster recovery and restoring
Yes No N/A
of data archives?
Do you have access security levels for the Computerized systems?
Yes N/A
Do your procedures for validation cover the Computerized systems? Yes No N/A
Do you have anti-virus protection? Yes No N/A
Does the Change Control procedure include Computerized systems? Yes No N/A
Laboratories, QA & QC
Is an equipment use log in place? Yes No N/A
Are all instruments qualified (IQ, OQ, PQ)? Yes No N/A
Are all instruments calibrated? Yes No N/A
Is there a preventative maintenance program? Yes No N/A
Are there documented procedures for:
Yes No N/A
Sampling
Sample handling Yes No N/A
Sample labelling Yes No N/A
Re-testing / Re-sampling Yes No N/A
Specification generation Yes No N/A
Analytical method generation Yes No N/A
Control and review of analytical methods
Yes N/A
All information contained within this document will be treated as confidential between the Supplier and Buyer.
File Location: Date Printed: Page 15 of 19
Form-385
Issue date
Vendor Audit Questionnaire
(Ref. SOP QMS-045; QMS-080)
If Agent/Distributor involved, is the pipe work used on delivery to the Yes No N/A
agent/distributor dedicated?
Are the Agent/Distributor storage facilities dedicated?
No N/A
If “No”, what other substances are stored in the facilities?
Does the Agent/Distributor use dedicated filling lines?
No N/A
What instructions are given to the haulier for delivery to a Sydco site e.g. dedicated hoses,
dedicated tanks, dedicated pumps, temperature control, and paperwork to accompany delivery?
Facilities & Housekeeping
Are there procedures for health and hygiene? Yes No N/A
Are rest/change/wash facilities separated from production areas? Yes No N/A
Are access restrictions implemented as needed? Yes No N/A
Do any production areas have special containment needs? Yes No N/A
Are waste disposal systems in place? Yes No N/A
Are there procedures documenting a pest control program? Yes No N/A
Are material Safety Data Sheets maintained?
Yes N/A
Training
Is there a written training program? Yes No N/A
Are job-training needs evaluated?
No N/A
Is completed training evaluated and approved?
Yes N/A
Are there completed written training records for all employees? Yes No N/A
Questionnaire reviewed for Buyer lead audit site by:
All information contained within this document will be treated as confidential between the Supplier and Buyer.
File Location: Date Printed: Page 17 of 19
Form-390
Issue date
New Supplier Assessment
(Ref. SOP QMS-045)
Supplier Name:
Results of the testing of first three deliveries:
Instructions on how to use this form:
1. Include all the material code numbers delivered on the one delivery and results of the testing in
the corresponding row. If for example, more than one material code was delivered in the first
delivery, list them all under Delivery 1 and so on for the subsequent deliveries. If more space
is needed use a new form.
2. The materials are to be fully tested according to the respective Laboratory Control Test
Methods.
3. Return the completed form to Procurement when completed.
Tested by:
Material Supplier C of A (Signature)
Material code Batch conforms to if available is in
number number current agreement with
specification? test results
Date:
Delivery 1
Date:
Delivery 2
Date:
Delivery 3
Date:
Comments:
Laboratory Manager: Date:
File Location: Date Printed: Page 19 of 19
Vous aimerez peut-être aussi
- SopDocument87 pagesSopskynyrd75100% (3)
- Vender Appoval SOP (PHARMA IND)Document8 pagesVender Appoval SOP (PHARMA IND)Prem Goel91% (23)
- SOP For Vendor ManagementDocument8 pagesSOP For Vendor ManagementShankar Mano83% (6)
- Supplier Performance Measure Rating SystemDocument12 pagesSupplier Performance Measure Rating SystemPaulo Lindgren0% (1)
- Supplier Qualification...Document5 pagesSupplier Qualification...elmiraPas encore d'évaluation
- QP Nonconforming Product Sample 2014Document2 pagesQP Nonconforming Product Sample 2014Anna Maree100% (1)
- SupplierAudit FormateDocument5 pagesSupplierAudit FormateimamudeenPas encore d'évaluation
- Supplier Assessment ChecklistDocument2 pagesSupplier Assessment ChecklistCOLONEL ZIKRIA100% (1)
- New Supplier Survey FormDocument14 pagesNew Supplier Survey Formsutharitessh100% (1)
- QAD Procedure - Control of Non-Conforming Products P2Document2 pagesQAD Procedure - Control of Non-Conforming Products P2sumanPas encore d'évaluation
- Internal Audit Checklist New Product Dev.Document10 pagesInternal Audit Checklist New Product Dev.dhir.ankur100% (3)
- Management Review Meeting ChecklistDocument1 pageManagement Review Meeting ChecklistGaruda84Pas encore d'évaluation
- Vendor Qualification ProcessDocument11 pagesVendor Qualification ProcessGautam Kumar100% (4)
- Supplier Audit Checklist - ZftvsDocument3 pagesSupplier Audit Checklist - Zftvsaboo2uPas encore d'évaluation
- Supplier QMS Survey and Audit ExampleDocument7 pagesSupplier QMS Survey and Audit ExampleHaleem Ur Rashid BangashPas encore d'évaluation
- Supplier Approval, Qualification and CertificationDocument1 pageSupplier Approval, Qualification and Certificationqmdocs60% (5)
- Supplier Pre-Assessment Questionnairevendor Evaluation Form: Document No. Revision 0Document12 pagesSupplier Pre-Assessment Questionnairevendor Evaluation Form: Document No. Revision 0Priyank Srivastava100% (1)
- Supplier Audit Check Sheet.Document1 pageSupplier Audit Check Sheet.ALI ASGHARPas encore d'évaluation
- D-1 Incoming Material ReceivingDocument3 pagesD-1 Incoming Material ReceivingAlonso Diaz0% (1)
- Supplier Assessment Form NewDocument49 pagesSupplier Assessment Form NewChinh Lê ĐìnhPas encore d'évaluation
- Food Law in Vietnam PDFDocument33 pagesFood Law in Vietnam PDFThanh Tâm TrầnPas encore d'évaluation
- Purchase Policy and Procedure by Puruhutjit SurjitDocument2 pagesPurchase Policy and Procedure by Puruhutjit SurjitSurjit PuruhutjitPas encore d'évaluation
- Revised SOP Policy Vendor ManagementDocument19 pagesRevised SOP Policy Vendor Managementnikhil patel100% (1)
- SOP - 0111 - 10 - Vendor Audit SOPDocument9 pagesSOP - 0111 - 10 - Vendor Audit SOPrana_ehsan1163100% (2)
- SOP 2 (Supplier Approval)Document18 pagesSOP 2 (Supplier Approval)Prince MoniPas encore d'évaluation
- SOPSP05 VendorSelection BSDocument3 pagesSOPSP05 VendorSelection BStroubledsoul100% (1)
- Keller SBM5e Accessible CH04Document29 pagesKeller SBM5e Accessible CH04Vương Trần Cao PhướcPas encore d'évaluation
- Productr Process Quality ChecklistDocument8 pagesProductr Process Quality ChecklistSenthil_KPas encore d'évaluation
- Supplier Vendor Qualification QuestionnaireDocument12 pagesSupplier Vendor Qualification QuestionnaireManu arava100% (2)
- Supplier Quality QuestionnaireDocument4 pagesSupplier Quality QuestionnairesutharitesshPas encore d'évaluation
- Supplier Audit Checklist: Nisan Scientific Process Equipments PVT - LTDDocument6 pagesSupplier Audit Checklist: Nisan Scientific Process Equipments PVT - LTDPratik KarekarPas encore d'évaluation
- 025 SOP Self InspectionDocument6 pages025 SOP Self InspectionMhammad Naveed50% (2)
- Supplier Quality/Purchasing Controls Successful PracticesDocument23 pagesSupplier Quality/Purchasing Controls Successful PracticesJessica Christy Sitio100% (1)
- SOP-06 (Supplier Evalutation)Document11 pagesSOP-06 (Supplier Evalutation)Farhan75% (4)
- Vendor Selection and Evaluation PDFDocument3 pagesVendor Selection and Evaluation PDFJayant Kumar JhaPas encore d'évaluation
- Supplier Audit ChecklistDocument20 pagesSupplier Audit ChecklistSteven Singleton100% (1)
- SOP No. 20.0 Sample Shipping ProceduresDocument6 pagesSOP No. 20.0 Sample Shipping ProceduresMahayudin SaadPas encore d'évaluation
- Supplier Selection & Evaluation TemplateDocument5 pagesSupplier Selection & Evaluation Templatek9pup100% (1)
- SOP For Vendor ManagementDocument8 pagesSOP For Vendor ManagementAndrei YabutPas encore d'évaluation
- Vendor Scorecard TemplateDocument8 pagesVendor Scorecard TemplatechristinathomasPas encore d'évaluation
- Sop PurchasingDocument11 pagesSop Purchasinghr multicraft100% (6)
- RecallDocument8 pagesRecallgmpaudittraining100% (1)
- Supplier Process Audit Checklist TemplateDocument8 pagesSupplier Process Audit Checklist TemplateYang LishengPas encore d'évaluation
- SOP Corrective Action: SOP As Approved by The Program Signing AuthorityDocument1 pageSOP Corrective Action: SOP As Approved by The Program Signing Authoritysudar1477Pas encore d'évaluation
- Quality Assurance ProceduresDocument35 pagesQuality Assurance ProcedurestrikjohPas encore d'évaluation
- Vendor Audit QuestionnaireDocument9 pagesVendor Audit QuestionnairebaluchakpPas encore d'évaluation
- Vendor Qualification ProcedureDocument2 pagesVendor Qualification ProcedurePrem Goel80% (10)
- Packaging Design Process TipsDocument23 pagesPackaging Design Process TipsAmadeo de los RíosPas encore d'évaluation
- Sop 7 4 1 B Qualifying New and Existing VendorsDocument7 pagesSop 7 4 1 B Qualifying New and Existing Vendorsיוליה רחמילובPas encore d'évaluation
- Pt. Patco Elektronik Teknologi Standard Operating Procedure PurchasingDocument8 pagesPt. Patco Elektronik Teknologi Standard Operating Procedure Purchasingmochammad iqbal100% (1)
- QMSSOP049 - 01 Supplier QualificationDocument13 pagesQMSSOP049 - 01 Supplier QualificationMohamed Kamal100% (1)
- Document Change RequestDocument1 pageDocument Change Requestsuresh kumarPas encore d'évaluation
- Quality Assurance Incoming Raw Materials Inspection PlanDocument2 pagesQuality Assurance Incoming Raw Materials Inspection PlanAlvin NodaPas encore d'évaluation
- Green Products Cosmetics and ToiletriesDocument12 pagesGreen Products Cosmetics and ToiletriesAKPas encore d'évaluation
- Vendor Audit Checklist PDFDocument3 pagesVendor Audit Checklist PDFMaria Magdalena Palacios HernandezPas encore d'évaluation
- SOP For Qualification of VendorsDocument2 pagesSOP For Qualification of VendorsDeepak Bhanji100% (1)
- F4E-QA-102 Supplier Audit Implementation 296E7T v2 3Document18 pagesF4E-QA-102 Supplier Audit Implementation 296E7T v2 3Jai BhandariPas encore d'évaluation
- Procedure For Recall - AOCDocument6 pagesProcedure For Recall - AOCMohamed EzzatPas encore d'évaluation
- Service Guide 2012Document180 pagesService Guide 2012Chang YuPas encore d'évaluation
- Supplier AuditDocument14 pagesSupplier AuditJayant Kumar JhaPas encore d'évaluation
- Technical Regulation For Machinery Safety - Part 1: Portable And/or Hand-Oriented MachinesDocument53 pagesTechnical Regulation For Machinery Safety - Part 1: Portable And/or Hand-Oriented MachinesEyad OsPas encore d'évaluation
- Supplier Guiding Principles ChecklistDocument8 pagesSupplier Guiding Principles ChecklistCuong Phan100% (1)
- Change Control Alaxan FR Capsule 2020Document6 pagesChange Control Alaxan FR Capsule 2020Rio FebriansyahPas encore d'évaluation
- T-ENG CardDocument14 pagesT-ENG CardViswaChaitanya NandigamPas encore d'évaluation
- Good Distribution Practices A Complete Guide - 2021 EditionD'EverandGood Distribution Practices A Complete Guide - 2021 EditionPas encore d'évaluation
- Is 14534.1998 Plastic Recycle StandardDocument9 pagesIs 14534.1998 Plastic Recycle StandardSukumar Linga ReddyPas encore d'évaluation
- Resistance To Airflow, Cooling Characteristics and Quality of Pomegranate Fruit Inside Ventilated PackagingDocument127 pagesResistance To Airflow, Cooling Characteristics and Quality of Pomegranate Fruit Inside Ventilated PackagingJohan YepesPas encore d'évaluation
- Manuale捲板機Document96 pagesManuale捲板機Andy WuPas encore d'évaluation
- Aerospace Material Specification: Nickel Alloy, Corrosion and Heat-Resistant, Wire 74ni - 15.5Cr - 8.0fe AnnealedDocument6 pagesAerospace Material Specification: Nickel Alloy, Corrosion and Heat-Resistant, Wire 74ni - 15.5Cr - 8.0fe AnnealedAnonymous T6GllLl0Pas encore d'évaluation
- High-Tech and Reliable: Automatic Stick-Type Packaging MachineDocument5 pagesHigh-Tech and Reliable: Automatic Stick-Type Packaging Machineem sajikuPas encore d'évaluation
- Starwest 2020 Wholesale CatalogDocument115 pagesStarwest 2020 Wholesale CatalogKay TiPas encore d'évaluation
- Garment Finishing Process and Equipments: Presented By: Akansha Gupta Devika Rastogi Isha Milap Sanskriti VarmaDocument33 pagesGarment Finishing Process and Equipments: Presented By: Akansha Gupta Devika Rastogi Isha Milap Sanskriti Varmaagga1111Pas encore d'évaluation
- Mix Up PresentationDocument21 pagesMix Up Presentationjai soniPas encore d'évaluation
- Evaluation of Costs and BenefitsDocument96 pagesEvaluation of Costs and BenefitsAdrian GasmanPas encore d'évaluation
- Data Sheet BZJ-5038Document3 pagesData Sheet BZJ-5038realesjPas encore d'évaluation
- Chapter 5 Global Product and Brand StrategyDocument55 pagesChapter 5 Global Product and Brand StrategyKidus AbebePas encore d'évaluation
- 3.0 Production Operation ManagementDocument5 pages3.0 Production Operation Managementhaidil abd hamidPas encore d'évaluation
- 2019 IPI Winter Web CompressedDocument92 pages2019 IPI Winter Web CompressedKamran AlamPas encore d'évaluation
- Business PlanDocument15 pagesBusiness PlanKa LaiPas encore d'évaluation
- A Model For Quantifying Construction Waste in Projects According To The European Waste ListDocument16 pagesA Model For Quantifying Construction Waste in Projects According To The European Waste ListMuhammad NaeemPas encore d'évaluation
- IATA Li-On ShippingDocument22 pagesIATA Li-On ShippingMatthew WilsonPas encore d'évaluation
- China Filling Machine Manufacturer, Beverage, Packing Machine SupplierDocument1 pageChina Filling Machine Manufacturer, Beverage, Packing Machine SupplierSulemanPas encore d'évaluation
- D 5138 - 99 Rduxmzg - PDFDocument7 pagesD 5138 - 99 Rduxmzg - PDFMarko's Brazon'Pas encore d'évaluation
- Grade 6 DLL Mapeh q4 Week 4Document5 pagesGrade 6 DLL Mapeh q4 Week 4Ivy PacatePas encore d'évaluation
- Dove PresentationDocument16 pagesDove Presentationfaraz_3524100% (1)
- Operational PlanDocument3 pagesOperational PlanLewellyn MirandaPas encore d'évaluation
- European and National Legislation On Packaging and The EnvironmentDocument112 pagesEuropean and National Legislation On Packaging and The EnvironmentmariaPas encore d'évaluation
- Diksha ProjectDocument49 pagesDiksha ProjectdevashishPas encore d'évaluation
- Company Overview: - CENIZA, Kate Angelou - DOCTORA, Dale - GULBEN, Krisha Kate - ORIG, Lyssa Mei PauleenDocument2 pagesCompany Overview: - CENIZA, Kate Angelou - DOCTORA, Dale - GULBEN, Krisha Kate - ORIG, Lyssa Mei PauleenDale DoctoraPas encore d'évaluation