Académique Documents
Professionnel Documents
Culture Documents
History Birads PDF
Transféré par
Tonny ChenTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
History Birads PDF
Transféré par
Tonny ChenDroits d'auteur :
Formats disponibles
NIH Public Access
Author Manuscript
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Published in final edited form as:
NIH-PA Author Manuscript
J Am Coll Radiol. 2009 December ; 6(12): 851–860. doi:10.1016/j.jacr.2009.07.023.
The ACR BI-RADS® Experience: Learning From History
Elizabeth S. Burnside, MD, MPH, MSa, Edward A. Sickles, MDb, Lawrence W. Bassett, MDc,
Daniel L. Rubin, MD, MSd, Carol H. Lee, MDe, Debra M. Ikeda, MDd, Ellen B. Mendelson,
MDf, Pamela A. Wilcoxg, Priscilla F. Butlerg, and Carl J. D’Orsi, MDh
a Department of Radiology, University of Wisconsin School of Medicine and Public Health,
Madison, Wisconsin
bDepartment of Radiology, University of California, San Francisco, Medical Center, San
Francisco, California
c David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California
d Department of Radiology, Stanford University School of Medicine, Stanford, California
e Department of Radiology, Memorial Sloan-Kettering Cancer Center, New York, New York
NIH-PA Author Manuscript
f Department of Radiology, Northwestern Memorial Hospital, Chicago, Illinois
g Department of Quality and Safety, American College of Radiology, Reston, Virginia
h Breast Imaging Center, Department of Radiology, Emory University Hospital, Atlanta, Georgia
Abstract
The Breast Imaging Reporting and Data System® (BI-RADS®) initiative, instituted by the ACR,
was begun in the late 1980s to address a lack of standardization and uniformity in mammography
practice reporting. An important component of the BI-RADS initiative is the lexicon, a dictionary
of descriptors of specific imaging features. The BI-RADS lexicon has always been data driven,
using descriptors that previously had been shown in the literature to be predictive of benign and
malignant disease. Once established, the BI-RADS lexicon provided new opportunities for quality
assurance, communication, research, and improved patient care. The history of this lexicon
illustrates a series of challenges and instructive successes that provide a valuable guide for other
groups that aspire to develop similar lexicons in the future.
NIH-PA Author Manuscript
Keywords
BI-RADS; breast cancer; breast imaging; diagnosis; informatics lexicon; mammography;
screening; structured reporting
The Breast Imaging Reporting and Data System® (BI-RADS®), the first practice
management system developed for imaging, is called a system because it contains several
important components, including 1) a lexicon of descriptors, 2) a recommended reporting
structure including final assessment categories with accompanying management
recommendations, and 3) a framework for data collection and auditing. In this paper, we
focus on the development of the lexicon within BI-RADS, but we also consider important
aspects of the system as a whole when indicated.
Copyright © 2009 American College of Radiology
Corresponding author and reprints: Elizabeth S. Burnside, MD, MPH, MS, University of Wisconsin Medical School, Department of
Radiology, E3/311 Clinical Science Center, 600 Highland Avenue, Madison, WI 53792-3252; eburnside@uwhealth.org.
Burnside et al. Page 2
A HISTORICAL PERSPECTIVE
As mammography utilization increased in the 1980s, wide variability of practices, including
NIH-PA Author Manuscript
disparate quality and inconsistent radiation doses, were cited as substantial problems [1–3].
Organizations such as the American Medical Association also asserted that mammography
reports too often contained unintelligible descriptions and ambiguous recommendations [4].
In response, the ACR convened a committee of radiologists, medical physicists, and a US
Food and Drug Administration (FDA) representative to develop a voluntary mammography
accreditation program in 1986 [5]. The ACR recognized that meaningful descriptors of
findings and the precise communication of recommendations in mammography reports were
important parts of a quality assurance program. Thus, a separate ACR committee also was
charged with drafting guidelines on mammography reporting and management under the
title of the Breast Imaging Reporting and Data System [6].
Many well-respected groups, including the American Medical Association, the National
Cancer Institute, the Centers for Disease Control and Prevention, the FDA, the American
College of Surgeons, and the College of American Pathologists, participated in this
development initiative to establish a broad base of support [6]. The inclusion of diverse
stakeholders in the development process helped promote consensus and facilitated
acceptance.
NIH-PA Author Manuscript
The first version of BI-RADS included recommendations for the conduct of mammographic
imaging, an overall structure for mammography reports, final assessment categories with
management recommendations, and a mammography lexicon. The introduction of the
document outlined the recommended method to provide efficient and cost-effective
mammography: screening mammography, which can be performed without an interpreting
physician in attendance (batch reading) to optimize efficiency; and diagnostic
mammography, which should be performed with “direct supervision” so that examinations
can be tailored to individual patients. Direct supervision is defined as a physician’s being
present and immediately available to furnish assistance and direction throughout the
performance of the procedure. Direct supervision may also be accomplished “via
telemammography as long as the interpreting physician is immediately available” [7]. These
guidelines clarified the optimal practice of mammography services for radiologists, referring
physicians, and patients.
The original BI-RADS document also described the overall structure of the breast imaging
report, which included a summary of breast density, a description of significant findings
(using appropriate descriptors as well as size and location), and a final assessment and
management section. The inclusion of a statement describing the general breast tissue type
NIH-PA Author Manuscript
arose from evidence in the literature establishing that increased breast density is
accompanied by decreased sensitivity [8–11]. Subsequently, evidence has mounted that
increased breast density also is associated with increased breast cancer risk [12–14].
Although these hypotheses are still active areas of research, the inclusion of 4 categories
describing breast density (ranging from the almost entirely fatty breast to the extremely
dense breast) in the standard mammography report is designed to improve the
communication of predicted mammographic performance and breast cancer risk.
The descriptors in the BI-RADS lexicon were selected on the basis of their ability to
discriminate between benign and malignant findings as determined by well-designed reader
studies. These studies suggested that feature lists containing predictive descriptors could
improve diagnostic accuracy. In the first study, investigators developed a decision aid
consisting of predictive terms and scales used to assess xero-radiography and reported
increased decision-making accuracy in a small reader study [15]. These early features were
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 3
then adapted to screen-film mammography and studied in a larger reader study of 150 cases
and 6 radiologists [16,17]. These initial studies helped refine the feature lists and led to the
development of computer programs to improve both sensitivity and specificity. Furthermore,
NIH-PA Author Manuscript
careful cataloging of descriptors significantly improved performance as case difficulty
increased and brought generalist performance to the level of the mammography specialist
[16,17]. The studies also guided the development of descriptors in the original BI-RADS
lexicon. Once the terms were chosen, they were precisely defined to eliminate ambiguity.
The final list of terms was intended to be evidence based and predictive and to foster the
clear and accurate communication of mammographic findings.
The BI-RADS Committee went beyond advocating the use of clear and standardized terms
and recommended that mammography be “decision oriented.” During the early days of
mammography, the American Medical Association specifically complained that
mammography interpretation was often indecisive and confusing [4]. In response, the BI-
RADS Committee recommended that final impressions be summarized by choosing only
one among several standardized final assessment categories at the end of a report, each of
which included a matched, also standardized management recommendation. These
categories currently are as follows: category 1: negative; category 2: benign finding(s);
category 3: probably benign finding—initial short-interval follow-up suggested; category 4:
suspicious abnormality— biopsy should be considered; category 5: highly suggestive of
malignancy—appropriate action should be taken; and category 6: known biopsy-proven
NIH-PA Author Manuscript
malignancy—appropriate action should be taken. An incomplete category was also
provided, category 0: need additional imaging evaluation and/or prior mammograms for
comparison. These categories also took an evidence-based approach. The “probably benign”
category was based on literature demonstrating that follow-up rather than biopsy is safe and
effective management for a clearly defined subset of findings that are very likely benign
[18]. The final assessment of “highly suggestive for malignancy” was included at the request
of representatives of the American College of Surgeons. Thus, the “highly suggestive”
category implied a “classic” finding for malignancy, which enabled women living in
underserved areas (without expertise in image-guided diagnosis) to be scheduled for
operative diagnosis, using frozen section, and immediate surgical management.
ENVIRONMENTAL INFLUENCES
Concurrent with the development of the BI-RADS lexicon, the mammography community
was recognizing the importance of automating clinical practice for a variety of mission
critical reasons. First, as a population-based initiative, screening mammography involves the
management of a substantial amount of data. Second, the conduct of medical audits to
ensure optimal performance and fulfill compliance requirements is onerous if performed
NIH-PA Author Manuscript
manually. Third, optimal tracking for the accurate communication of results requires reliable
and reproducible data storage and retrieval. Automated, computer-based management and
tracking of mammography results addressed these challenges more efficiently than
conventional manual methods [19,20].
The explosive increase in data was not unique to breast imaging practices during the late
1980s. The entire biomedical infrastructure was straining under the increases in data
inherent in clinical care and research. There were other, more general efforts to standardize
biomedical terminology that paralleled the construction of the BI-RADS mammography
lexicon. For example, the Unified Medical Language System (UMLS) was designed and
constructed by the National Library of Medicine in 1986 to enhance access to medical data
and the scientific literature. The UMLS provides the infrastructure to collect and link
controlled vocabularies to facilitate the development of computer systems that understand,
retrieve, and classify biomedical literature. The National Library of Medicine itself uses
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 4
components of UMLS for its PubMed system. The UMLS, a powerful tool to enable
computers to communicate about biomedical data generally, parallels BI-RADS, which
provides the same capabilities in the domain of breast imaging.
NIH-PA Author Manuscript
THE EVOLUTION OF BI-RADS
BI-RADS in general and the lexicon specifically were not intended to be static [6]. After the
initial creation of BI-RADS in 1993 [21], 3 more editions were created in 1995 [22], 1998
[23], and 2003 [24]. However, the path to a successfully adopted breast imaging lexicon was
not always smooth. Controversy involving the BI-RADS lexicon arose in 1994 with the
publication of an editorial in which the author contested that “expertise is the heart of the
problem, not terminology. BI-RADS, with its emphasis on words and definitions, is barking
up the wrong tree” [25]. The authors of the BI-RADS lexicon responded in a follow-up
editorial that addressed many concerns that were being voiced by the community. In
particular, the response clarified that the BI-RADS lexicon was intended to be a tool that
radiologists would use to communicate with clinicians to convey concise and orderly
descriptions of findings in understandable, standardized language, which in turn contributes
to an orderly thought process and logical assessments and recommendations. Furthermore,
BI-RADS was designed to encourage improvements in expertise because it provided
standardized recommendations that could be used for performance tracking: “Without
standardized terms to describe important features … there is no means of obtaining objective
NIH-PA Author Manuscript
data to improve. Indeed, this format is important for all reports we generate, not only
mammography” [26]. This thoughtful and productive debate strengthened support for the
lexicon.
Each BI-RADS revision added components that were important for clarification,
management, or quality assurance. The third edition of BI-RADS incorporated an atlas that
provided an artist’s renderings of examples of each descriptor. The fourth edition made
several changes in lexicon terminology [27]. To decrease confusion between terminology
for overall breast density and the descriptor density (referring to a noncalcified finding seen
on only one of the two standard mammographic views), the authors adopted the descriptor
term asymmetry in place of density. To parallel this change in terminology, asymmetric
breast tissue was renamed global asymmetry (a nonmass finding seen on at least two views
that occupies at least a quadrant), and focal asymmetric density was renamed focal
asymmetry (a nonmass finding seen on at least two views that occupies less than a quadrant).
Furthermore, a study demonstrating that amorphous microcalcifications carried a 20% risk
for malignancy [28] prompted the BI-RADS Committee to subcategorize suspicious
microcalcification descriptors into “intermediate risk” (including the amorphous descriptor)
and “higher probability of malignancy.” Additional published data related to
NIH-PA Author Manuscript
microcalcification descriptors demonstrated that the pleomorphic descriptor was not
stratifying risk beyond the overall risk for suspicious microcalcifications [29]. In response,
the BI-RADS Committee divided “pleomorphic” microcalcifications into two more specific
categories: “coarse heterogeneous” and “fine pleomorphic.” This distinction was
subsequently shown to effectively stratify the probability of malignancy among these types
of calcifications [30]. To further help with risk stratification, the fourth edition provided the
option to subdivide BI-RADS assessment category 4 into 4A (low suspicion for
malignancy), 4B (intermediate suspicion for malignancy), and 4C (moderate concern but not
classic for malignancy). These subdivisions provide assessment and reporting options
designed to help both physicians and patients understand likely biopsy findings and probable
follow-up recommendations [27,31].
The development of a breast ultrasound lexicon, BI-RADS–Ultrasound, first published as
part of the fourth edition of BI-RADS, demonstrated similar themes. In 1998, the ACR
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 5
received a grant from the Office on Women’s Health of the US Department of Health and
Human Services to support protocol development for research in breast ultrasound (contract
282-97-0076, Federal Technology Transfer Program to Advance Novel Breast Imaging
NIH-PA Author Manuscript
Technologies, US Public Health Service, Office on Women’s Health, Department of Health
and Human Services). An ACR expert working group of national and international breast
imagers with special interest in breast ultrasound met in Maine to design research projects
that might advance use of ultrasound in conjunction with mammography and other imaging
modalities. These potential studies included 1) the identification of criteria to differentiate
benign from malignant solid masses, 2) ultrasound for breast cancer screening, and 3) using
ultrasound to guide diagnostic interventions and as a therapeutic agent (high-frequency
ultrasound). Until that time, ultrasound was used primarily for cyst-solid differentiation,
despite FDA premarket approval of Advanced Technology Laboratories’ (Bothell,
Washington; now Philips Medical Systems, Andover, Massachusetts) “high-definition
imaging” in 1996. This approval was based on an international, multicenter study involving
ultrasound evaluation of nearly 1,000 breast lesions (published as a monograph by
Advanced Technology Laboratories rather than in the peer-reviewed literature), which
indicated that ultrasound improved specificity for masses found to be indeterminate on
mammography and physical examination. This study asserted the need for additional
research in defining diagnostic criteria for classifying solid breast masses on ultrasound.
The ACR expert working group proposed a standardized lexicon, similar to BI-RADS for
NIH-PA Author Manuscript
mammography, to provide a foundation for research characterizing solid masses for risk
stratification and evidence-based management. For example, this strictly defined lexicon
could be used to determine benign and probably benign masses. Soon after the Maine
meeting, a subcommittee of the ACR’s BI-RADS Committee was formed to officially
develop BI-RADS–Ultrasound. After several iterations, the consensus document was
presented and tested at select subspecialty meetings, including the Society of Breast
Imaging’s biennial meeting in San Diego in 2001. The descriptors and assessment categories
were validated by statistical analysis of interobserver consistency (κ), showing good
agreement for most terms among the experienced and novice breast imaging participants
[31].
BI-RADS–Ultrasound was predicated on high-quality images and real-time ultrasound
observations and encourages the assessment of combined features. Using descriptors from
several feature categories can balance the risk associated with all relevant features, but
usually, the most suspicious feature will dominate the final assessment and recommendation
[32]. Validation of the grouping of features is derived from univariate, bivariate, and
multivariate analyses of features characterizing the lesions submitted as evidence for FDA
premarket approval by Advanced Technology Laboratories for its high-definition imaging
NIH-PA Author Manuscript
system. Groups of features were also used in the development of the mass classification
algorithm proposed by Stavros et al [33]. For BI-RADS–Ultrasound, the three most
important feature categories, taken together are shape, margin, and orientation, the last a
feature unique to ultrasound.
BI-RADS–Ultrasound can also contribute to the investigation of emerging technologies.
Computer-assisted diagnosis programs use segmentation and feature extraction to classify
breast masses on the basis of features similar to BI-RADS–Ultrasound. Structured reporting,
rapidly evolving and currently available in breast imaging reporting software packages
initially designed for mammography, uses BI-RADS-Ultrasound to construct standardized
reports and encourage consistent communication.
The development of BI-RADS–Ultrasound that began more than a decade ago will continue.
New feature categories (eg, elasticity) will require standardization, evidence, and validation.
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 6
The continuing goals for BI-RADS of providing useful, comprehensive guides to breast
imagers for analyzing, assessing, reporting, and managing breast lesions is especially critical
for breast ultrasound, which has long been considered an operator-dependent modality.
NIH-PA Author Manuscript
The fourth edition of BI-RADS also incorporated breast MRI descriptors. Between the early
1970s and the late 1990s, contrast-enhanced breast MRI had shown great promise. Studies
demonstrated near 100% sensitivity for detecting early invasive breast cancer, though these
results were tempered by more modest specificity [34]. However, attempts to systematically
evaluate the literature were stymied by nonuniform approaches to image acquisition and
reporting. Variable magnet field strength, hardware, pulse sequences, and lesion
characterization (including both morphologic and kinetic data) led to freedom of innovation
but also impeded consensus development. A Web-based survey of the members of the
Society of Breast Imaging conducted from September 2006 to January 2007 showed that
poorly standardized breast MRI protocols were a serious problem [35]. Of 551 responding
facilities, 84% indicated they never or rarely would interpret contrast-enhanced breast MRI
examinations performed at other facilities because of protocol variability.
Recognizing a clear need to achieve consensus on MRI acquisition techniques and lesion
terminology, the Public Health Service’s Office on Women’s Health funded the
International Working Group for Breast MRI in 1997. This group’s goal was to disseminate
evidence-based consensus on the performance and interpretation of breast MRI. A subset of
NIH-PA Author Manuscript
the international working group, the Lesion Diagnosis Working Group, composed of
internationally recognized breast MRI investigators, was charged with developing a
standardized breast MRI lexicon and reporting system [36]. This group later became the
Subcommittee on MRI Lexicon Breast Cancer.
In 1998, the Lesion Diagnosis Working Group developed minimum reporting standards on
MRI scanning techniques, region-of-interest kinetic curve acquisition, lesion architecture,
and kinetic curve interpretation. These experts used the breast MRI literature to compile the
most important descriptors for lesion diagnosis that would prompt specific patient
management recommendations, such as biopsy. The morphologic descriptors were based on
terms used in the BI-RADS mammography lexicon, when appropriate, to facilitate use and
adoption in clinical practice. After the development of the preliminary breast MRI lexicon,
this group performed several reader studies (funded by the National Cancer Institute, the
Susan G. Komen Breast Cancer Foundation, and the ACR) to evaluate the reproducibility of
these descriptors for the characterization of biopsy-proven MRI abnormalities [37]. Using
the results of each study, portions of the lexicon were expanded and others eliminated in a
stepwise, progressive manner.
NIH-PA Author Manuscript
Optimization and testing of the lexicon continued for a period of 6 years, resulting in BI-
RADS–MRI, first published in the fourth edition of BI-RADS in 2003 [24]. The breast MRI
lexicon is now widely used for breast MRI reporting, teaching, research, and
communication. However, the work of the Subcommittee on MRI Lexicon Breast Cancer,
researchers, and practitioners is far from over; the accuracy and reproducibility of the
lexicon continue to be tested in the clinical and scientific arenas [38–41], and novel features
and techniques are emerging rapidly. For example, the importance of background
enhancement, the use of T2-weighted sequences, and bilateral scanning [42–44] prompted
the committee to recommend the routine use of these techniques. Major advances in
hardware, software, magnet field strength, and pulse sequence development, including
parallel imaging, diffusion-weighted imaging, and MR spectroscopy, among other advances,
promise to contribute to improved diagnostic capability as well as add complexity to the
lexicon [45]. For example, the increased use of computer-aided diagnosis has led to major
changes in breast MRI interpretation [35]. Furthermore, MRI-guided biopsy provides tissue
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 7
diagnosis for abnormalities seen only on MRI, enabling early breast cancer diagnosis as well
as the collection and calculation of accurate performance metrics. In fact, the ACR’s MRI
Lexicon Committee is planning a new edition for 2010 that will clarify existing descriptors,
NIH-PA Author Manuscript
eliminate descriptors that did not work in clinical practice, and add T2 weighting,
background enhancement, and breast implant descriptions. Clearly, the BI-RADS–MRI is an
evolving document that requires continued development to provide up-to-date, evidence-
based standard terminology for its continued contribution to improving the accuracy of
breast MRI. Technological progress, the multimodality evolution of breast imaging, and
maintenance of the data-driven lexicon will serve as the foundation of the fifth edition of BI-
RADS, which will include the second editions of BI-RADS–Ultrasound and BI-RADS–
MRI.
There also have been spinoffs from BI-RADS, including the National Mammography
Database (NMD) initiative [46]. The ACR originally launched the NMD in 1999 but
subsequently put the initiative on hold in 2002 because of limited resources. However, in
2007, the ACR began the development of the National Radiology Data Registry, a data
warehouse to collect quality improvement data across multiple modalities. The NMD is one
of the registries within the National Radiology Data Registry. BI-RADS licensed software
vendors are required to comply with NMD requirements, enabling the automatic upload of
facility audit data directly to the ACR. During the second half of 2009, the ACR pilot-tested
the NMD in early 2009 and opened NMD reporting to interested participants in the summer
NIH-PA Author Manuscript
of 2009. The ACR will provide outcomes reports to participants to enable comparison with
national benchmarks as well as practices similar in size, type, and region.
BI-RADS, MAMMOGRAPHY ACCREDITATION, AND THE MAMMOGRAPHY
QUALITY STANDARDS ACT
The fact that the ACR Mammography Accreditation Program and the BI-RADS Committee
were launched almost simultaneously (in 1987 and 1988, respectively) demonstrates that
these initiatives were closely tied and interdependent. The Committee on Mammography
Accreditation believed that once completed, compliance with BI-RADS reporting categories
and the medical audit would be a critical element in improving the quality of
mammography. BI-RADS was rapidly implemented by many experts in breast imaging, but
both the ACR and the FDA’s National Mammography Quality Assurance Advisory
Committee believed that the greatest potential for improvement in interpretation lay with
interpreting physicians who were not dedicated breast imagers. After the successful
implementation of the ACR’s Mammography Accreditation Program, the federal
government followed suit by passing the Mammography Quality Standards Act of 1992
(MQSA), mandating accreditation and certification for all mammography facilities. The
NIH-PA Author Manuscript
MQSA 1993 interim regulations [47] required that every mammography facility review
outcome data from all mammography performed, including follow-up on the disposition of
positive mammographic results and correlation of surgical biopsy results with
mammographic reports. The 1999 MQSA final rules [48] mandated that every
mammographic report include the language for a final assessment similar to those in BI-
RADS. The MQSA also encouraged all interpreting physicians to review their performance
compared with benchmarks established by the Agency for Health Care Policy and
Research’s [49] “Quality Determinants of Mammography,” a document intended to provide
guidance for the MQSA regulations.
The BI-RADS assessment categories have proved to be a unique resource for measuring and
improving the quality of mammographic interpretation. In its 2005 report “Improving Breast
Imaging Quality Standards,” the Institute of Medicine [50] recognized that BI-RADS
assessment provides an important tool for defining mammography positivity and negativity
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 8
to audit interpretive performance. In addition, the report stated that the audit requirements
under MQSA are inadequate for measuring or improving the quality of interpretation and
recommended that to achieve this improvement, an expanded BI-RADS-compatible audit
NIH-PA Author Manuscript
should be required.
BI-RADS AND EDUCATION
The structure of BI-RADS lends itself to consistent and rational evaluation of
mammographic findings and facilitates resident and breast imaging fellowship training. Both
the ACR and the Society of Breast Imaging recommend that breast imaging education
within residency and fellowship training should be designed to require the use of BI-RADS
terminology, assessment categories, and management recommendations [51]. In fact, a 2003
survey indicated that 98% of radiology residents were taught to use BI-RADS in their
mammographic reports [52]. Furthermore, the ACR’s mammography self-assessment
examinations, used by thousands of radiologists from 1993 to date, have exclusively used
BI-RADS terminology, assessment categories, and management recommendations [53].
Because the BI-RADS lexicon descriptors are precisely defined and tied (through the
literature) to breast cancer risk, proper term use leads logically to a final assessment and in
turn to an appropriate management recommendation. The literature shows that training in
BI-RADS can decrease variability and improve performance [54]. For example, scattered
NIH-PA Author Manuscript
distribution and punctate morphology of calcifications are benign features and should not
prompt a BI-RADS 4 or 5 final assessment or a recommendation for biopsy. However,
interobserver variability remains a challenge [54,55], and the appropriate use of BI-RADS
assessment categories and recommendations is not uniform [56–60].
BI-RADS AND COMMUNICATION
Although structured lexicons enable communication between humans and computers, they
also facilitate communication between various physicians involved with complex clinical
care. In particular, the BI-RADS final assessment categories and their accompanying
management recommendations have become the standard by which physicians determine
breast care on the basis of imaging. This standardization is unique among imaging reports
and greatly aids in communication and facilitates the comprehension of imaging results by
all members of the multidisciplinary breast care team: surgeons, pathologists, oncologists,
radiation oncologists, and other health care providers. For example, the BI-RADS final
assessment categories (eg, 4A, 4B, 4C, 5) are useful for communicating to pathologists the
level of suspicion of lesions undergoing imaging-guided biopsy. This helps pathologists
make accurate histologic diagnoses by encouraging imaging-pathology correlation. In
addition, clarity of communication between pathologists and radiologists can promote the
NIH-PA Author Manuscript
detection of possible sampling errors at percutaneous core biopsy and avoid a delay in
cancer detection by prompting excisional biopsy.
BI-RADS AND RESEARCH
BI-RADS has also served to generate substantial scientific investigation that otherwise may
not have been possible. A review of PubMed from 1985 to 2007 illustrates the possible
effect that BI-RADS may have had on the mammography literature (Figure 1). As a
baseline, papers catalogued in PubMed as addressing generic “mammography” or
“mammography standards” demonstrate a steady increase in number within these years
without apparent acceleration at any time point. However, publication rates for papers
presenting mammographic performance data or observer studies began to outpace the more
generic categories in the mid-1990s. Although it is difficult to assert a causal relationship
between the establishment of BI-RADS and these types of performance and observer
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 9
studies, one can surmise that a standardized reporting system would facilitate such research.
Reader studies that measure mammography performance, whether retrospective or
prospective, require standardized imaging outcome assessments such as those provided by
NIH-PA Author Manuscript
BI-RADS. Furthermore, the major North American peer-reviewed radiology journals
unofficially but effectively require the use of BI-RADS terminology for the acceptance of
papers related to breast imaging for publication, which in turn encourages the wide
international readership to use BI-RADS. Currently, the ACR’s BI-RADS Atlas has been
translated into (or is in the process of being translated into) 8 languages: French, Spanish,
Portuguese, Croatian, German, Russian, Mandarin Chinese, and Romanian.
BI-RADS provides a foundation on which to build a strong scientific tradition in breast
imaging. The large, prospective ACRIN® trials in breast imaging (eg, the Digital
Mammographic Imaging Screening Trial and the ultrasound screening trial) used
interpretation guidelines that were modeled after BI-RADS (though perhaps not identical).
In addition, decision support technologies using artificial intelligence algorithms such as
artificial neural networks [61] and Bayesian networks [62] have been developed on the basis
of standardized BI-RADS descriptors.
The National Cancer Institute–funded Breast Cancer Surveillance Consortium has used BI-
RADS and its audit data elements as the basis for its multistate registry (including academic
and private practices) since 1996. Participants in this registry include community-based
NIH-PA Author Manuscript
mammography facilities as well as academic practices. The consortium sites have published
290 articles across a broad range of research topics in mammography and breast cancer.
DEVELOPING TECHNOLOGY AND FUTURE DIRECTIONS
Many directions promise to further advance the goals of BI-RADS: standardization and
accuracy in breast imaging reporting. For example, technologies in medical informatics,
including structured reporting (using specific data elements or formats that allow automated
storage and indexing of report) and ontologies (the representation of a set of concepts and
the relationships between those concepts), have the potential to further improve the
application of the BI-RADS mammography, ultrasound, and MRI lexicons in clinical
practice. Structured reporting is a currently available but rapidly evolving technology that
provides reusable knowledge, such as templates or checklists, to the clinical reporting
process to aid radiologists’ consistency, accuracy, and completeness [63]. Structured
reporting usually uses a point-and-click interface, prescribed lexical conventions, and a
back-end database to record important variables and generate reports. For example, in breast
imaging, these modules incorporate a clearly defined set of data-driven features to generate
clinical reports and next management steps, mandating that a BI-RADS category be present
NIH-PA Author Manuscript
on every report, as required by the FDA. Such systems record this information in the back-
end database and allow the automated abstraction of important data for future use. However,
although advantageous for standardization, mandating the use of acceptable terms can limit
rich descriptive language that might be important in complex cases. Understandably,
radiologists do not want to be constrained in their descriptions and thereby risk losing
important subtleties of cases. So, structured reporting vendors invariably offer the ability to
report free text, which increases the chance that BI-RADS descriptors will be erroneously or
ambiguously applied.
Ontologies can be used to solve issues of ambiguity because they rigorously define
relationships between terms within and outside of the lexicon. Such ontologies provide a
mechanism for computers to understand concepts and the representation of these concepts in
human language. For example “is-a” relationships would link more general terms to their
more specific terms within a lexicon. “Associated” relationships would provide a conception
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 10
that there are specific terms sanctioned by BI-RADS that would be more appropriate than
seemingly similar terms not sanctioned by BI-RADS.
NIH-PA Author Manuscript
Merging structured reporting and ontologies could provide powerful, reusable tools that
could advance the accurate application of BI-RADS. For example, using relationships
defined in an ontology could enable reporting systems to guide radiologists away from
undesirable terms (eg, stellate) to more desirable BI-RADS terms (eg, spiculated) in real
time in the clinic. Such functionality is not currently possible with lexicons alone.
Furthermore, an ontology can clarify sources of confusion when a single descriptor may be
used in different contexts but the specific context is ambiguous in free text. For example, in
BI-RADS, the word linear may be used to describe 1) a microcalcification shape, 2) a
microcalcification distribution, or 3) an MRI enhancement pattern. Although linear is not an
official BI-RADS term on its own (fine linear and linear distribution are the precise terms
recommended), it is widely used. When relationships are defined in an ontology and
structured reporting is used on the basis of that ontology, it is possible to extract which
“linear” is being referenced. As a final example, a radiologist may enter “Linear and
heterogeneous microcalcifications are identified in the upper outer quadrant extending from
the nipple into the axillary tail” in free text in a structured reporting module. Although this
sentence is appropriately descriptive, the BI-RADS lexicon descriptors are not correct and
therefore lack precise meaning to enable decision-driven management. If an ontology of BI-
RADS terms were available, the structured reporting system would be able to suggest to the
NIH-PA Author Manuscript
radiologist in real time, “By linear, do you mean fine-linear morphology or linear
distribution?” as well as “By heterogeneous, are you referring to the morphology descriptor
‘coarse-heterogeneous’?” The radiologist would then know to improve the description and
adhere to BI-RADS, and the appropriate adjustment would be “coarse-heterogeneous
microcalcifications in a linear distribution are identified in the upper outer quadrant
extending from the nipple into the axillary tail.” Structured reporting systems combined with
ontologies have the potential to provide seamless and facile interfaces that “mandate” the
use of standardized terms such as BI-RADS, preferably using point-and-click entry, while
providing the freedom of expression that radiologists demand to generate precise and
descriptive reports.
However, these solutions will not work if each vendor creates a different system to convey
the concepts within BI-RADS; therefore, “harmonization” of BI-RADS with other lexicons
has the potential to further codify the breast imaging lexicon in the context of other lexicons.
Harmonization is a general term that can refer to defining the relationships of terms between
lexicons, which does not imply that terms are “integrated” or the meanings of terms
changed. Harmonization provides the opportunity to formalize an organizational structure
that encourages communication among groups responsible for developing and curating
NIH-PA Author Manuscript
lexicons. Harmonization, which encourages the use of uniform conventions but not
necessarily the same terms, would facilitate global radiology informatics endeavors. For
example, a harmonized collection of radiology lexicons provides the opportunity to develop
general algorithms (eg, natural language processing tools) that could extract information
from all types of radiology reports for data mining or quality assurance. The Radiological
Society of North America has created RadLex (http://www.rsna.org/radlex/), a controlled
terminology for radiology reporting, teaching, and research [64]. RadLex focuses on
compiling a comprehensive set of terms for radiology and making the relationships among
terms explicit in an ontology. Harmonization between BI-RADS and RadLex has the
potential to benefit both systems. For example, the ontology in RadLex would provide
reusable knowledge to standardize structured reporting interfaces and thereby encourage
radiologists to use these systems to uniformly create BI-RADS-compliant reports. BI-RADS
could help RadLex by providing a well-developed lexicon and a historical perspective
regarding techniques that contributed to the development and evolution of a successful
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 11
lexicon in radiology. This harmonization process will take careful thought and planning and
should be guided by experts in the field while informed by other stakeholders, as has been
the BI-RADS tradition. Furthermore, RadLex harmonization will succeed only if established
NIH-PA Author Manuscript
BI-RADS terminology and its principled, evidence-based evolution are rigorously
preserved.
LESSONS LEARNED
The BI-RADS system has followed a logical and evidence-based path from its inception.
The ACR committees have insisted on predictive features discovered through scientific
investigation whenever possible. BI-RADS was developed from scientific data, expert
guidance from leaders in breast imaging, and input from clinicians and other stakeholders. It
was always meant to be a “living” document that changes as new data are acquired and more
sophisticated patterns of breast care emerge. The BI-RADS lexicon can serve as an example
of a highly successful standard terminology upon which lexicons in other domains can be
modeled.
Acknowledgments
This study was supported by grant 1 K07 CA114181 from the National Cancer Institute of the National Institutes of
Health, Bethesda, Maryland.
NIH-PA Author Manuscript
References
1. McLelland R. Mammography 1984: challenge to radiology. AJR Am J Roentgenol. 1984; 143:1–4.
[PubMed: 6428201]
2. Galkin BM, Feig SA, Muir HD. The technical quality of mammography in centers participating in a
regional breast cancer awareness program. Radiographics. 1988; 8:133–45. [PubMed: 3353530]
3. Conway BJ, McCrohan JL, Rueter FG, Suleiman OH. Mammography in the eighties. Radiology.
1990; 177:335–9. [PubMed: 2217765]
4. Scott, W. Establishing mammographic criteria for recommending surgical biopsy. Chicago, Ill:
American Medical Association; 1989.
5. McLelland R, Hendrick RE, Zinninger MD, Wilcox PA. The American College of Radiology
Mammography Accreditation Program. AJR Am J Roentgenol. 1991; 157:473–9. [PubMed:
1872231]
6. D’Orsi CJ, Kopans DB. Mammography interpretation: the BI-RADS® method. Am Fam Physician.
1997; 55:1548–50. 52. [PubMed: 9105186]
7. American College of Radiology. Practice guidelines and technical standards. Reston, Va: American
College of Radiology; 2008. ACR practice guideline for the performance of screening and
diagnostic mammography; p. 525-34.
NIH-PA Author Manuscript
8. Fajardo LL, Hillman BJ, Frey C. Correlation between breast parenchymal patterns and
mammographers’ certainty of diagnosis. Invest Radiol. 1988; 23:505–8. [PubMed: 3170137]
9. van Gils CH, Otten JD, Verbeek AL, Hendriks JH, Holland R. Effect of mammographic breast
density on breast cancer screening performance: a study in Nijmegen, the Netherlands. J Epidemiol
Community Health. 1998; 52:267–71. [PubMed: 9616416]
10. Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic
detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000; 92:1081–
7. [PubMed: 10880551]
11. Mann BD, Giuliano AE, Bassett LW, Barber MS, Hallauer W, Morton DL. Delayed diagnosis of
breast cancer as a result of normal mammograms. Arch Surg. 1983; 118:23–4. [PubMed: 6848070]
12. Hainline S, Myers L, McLelland R, Newell J, Grufferman S, Shingleton W. Mammographic
patterns and risk of breast cancer. AJR Am J Roentgenol. 1978; 130:1157–8. [PubMed: 418657]
13. Carlile T, Kopecky KJ, Thompson DJ, et al. Breast cancer prediction and the Wolfe classification
of mammograms. JAMA. 1985; 254:1050–3. [PubMed: 4021043]
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 12
14. Whitehead J, Carlile T, Kopecky KJ, et al. Wolfe mammographic parenchymal patterns. A study of
the masking hypothesis of Egan and Mosteller. Cancer. 1985; 56:1280–6. [PubMed: 4027868]
15. Getty DJ, Pickett RM, D’Orsi CJ, Swets JA. Enhanced interpretation of diagnostic images. Invest
NIH-PA Author Manuscript
Radiol. 1988; 23:240–52. [PubMed: 3372189]
16. Swets JA, Getty DJ, Pickett RM, D’Orsi CJ, Seltzer SE, McNeil BJ. Enhancing and evaluating
diagnostic accuracy. Med Decis Making. 1991; 11:9–18. [PubMed: 2034078]
17. D’Orsi CJ, Getty DJ, Swets JA, Pickett RM, Seltzer SE, McNeil BJ. Reading and decision aids for
improved accuracy and standardization of mammographic diagnosis. Radiology. 1992; 184:619–
22. [PubMed: 1509042]
18. Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184
consecutive cases. Radiology. 1991; 179:463–8. [PubMed: 2014293]
19. Sickles EA. The usefulness of computers in managing the operation of a mammography screening
practice. AJR Am J Roentgenol. 1990; 155:755–61. [PubMed: 2119105]
20. Monticciolo DL, Sickles EA. Computerized follow-up of abnormalities detected at mammography
screening. AJR Am J Roentgenol. 1990; 155:751–3. [PubMed: 2119104]
21. American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®).
Reston, Va: American College of Radiology; 1992.
22. American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®). 2.
Reston, Va: American College of Radiology; 1995.
23. American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®). 3.
Reston, Va: American College of Radiology; 1998.
NIH-PA Author Manuscript
24. American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®). 4.
Reston, Va: American College of Radiology; 2003.
25. Heilbrunn KS. The American College of Radiology’s mammography lexicon: barking up the
wrong tree? AJR Am J Roentgenol. 1994; 162:593–4. [PubMed: 8109502]
26. D’Orsi CJ, Kopans DB. The American College of Radiology’s mammography lexicon: barking up
the only tree. AJR Am J Roentgenol. 1994; 162:595. [PubMed: 8109503]
27. D’Orsi CJ, Newell MS. BI-RADS® decoded: detailed guidance on potentially confusing issues.
Radiol Clin North Am. 2007; 45:751–63. [PubMed: 17888766]
28. Berg WA, Arnoldus CL, Teferra E, Bhargavan M. Biopsy of amorphous breast calcifications:
pathologic outcome and yield at stereotactic biopsy. Radiology. 2001; 221:495–503. [PubMed:
11687695]
29. Liberman L, Abramson AF, Squires FB, Glassman JR, Morris EA, Dershaw DD. The Breast
Imaging Reporting and Data System: positive predictive value of mammographic features and
final assessment categories. AJR Am J Roentgenol. 1998; 171:35–40. [PubMed: 9648759]
30. Burnside ES, Ochsner JE, Fowler KJ, et al. Use of microcalcification descriptors in BI-RADS® 4th
edition to stratify risk of malignancy. Radiology. 2007; 242:388–95. [PubMed: 17255409]
31. Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and
mammography: interobserver variability and positive predictive value. Radiology. 2006; 239:385–
NIH-PA Author Manuscript
91. [PubMed: 16569780]
32. Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-
RADS: ultrasound. Semin Roentgenol. 2001; 36:217–25. [PubMed: 11475068]
33. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use
of sonography to distinguish between benign and malignant lesions. Radiology. 1995; 196:123–34.
[PubMed: 7784555]
34. Stomper PC, Herman S, Klippenstein DL, et al. Suspect breast lesions: findings at dynamic
gadolinium-enhanced MR imaging correlated with mammographic and pathologic features.
Radiology. 1995; 197:387–95. [PubMed: 7480682]
35. Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J
Roentgenol. 2008; 191:332–9. [PubMed: 18647898]
36. Ikeda DM. Progress report from the American College of Radiology Breast MR Imaging Lexicon
Committee. Magn Reson Imaging Clin North Am. 2001; 9:295–302.
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 13
37. Ikeda DM, Hylton NM, Kinkel K, et al. Development, standardization, and testing of a lexicon for
reporting contrast-enhanced breast magnetic resonance imaging studies. J Magn Reson Imaging.
2001; 13:889–95. [PubMed: 11382949]
NIH-PA Author Manuscript
38. Macura KJ, Ouwerkerk R, Jacobs MA, Bluemke DA. Patterns of enhancement on breast MR
images: interpretation and imaging pitfalls. Radiographics. 2006; 26:1719–34. [PubMed:
17102046]
39. Nie K, Chen JH, Yu HJ, Chu Y, Nalcioglu O, Su MY. Quantitative analysis of lesion morphology
and texture features for diagnostic prediction in breast MRI. Acad Radiol. 2008; 15:1513–25.
[PubMed: 19000868]
40. Raza S, Vallejo M, Chikarmane SA, Birdwell RL. Pure ductal carcinoma in situ: a range of MRI
features. AJR Am J Roentgenol. 2008; 191:689–99. [PubMed: 18716095]
41. Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast
MR imaging: multicenter study. Radiology. 2006; 238:42–53. [PubMed: 16373758]
42. Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. Differentiation between
benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced MRI: a
sensitivity and specificity study. Magn Reson Med. 2008; 59:747–54. [PubMed: 18383287]
43. Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation,
diagnostic accuracy, and transfer to clinical practice. Radiology. 2007; 244:356–78. [PubMed:
17641361]
44. Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;
244:672–91. [PubMed: 17709824]
NIH-PA Author Manuscript
45. Yabuuchi H, Matsuo Y, Okafuji T, et al. Enhanced mass on contrast-enhanced breast MR imaging:
lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted
MR images. J Magn Reson Imaging. 2008; 28:1157–65. [PubMed: 18972357]
46. Osuch JR, Anthony M, Bassett LW, et al. A proposal for a national mammography database:
content, purpose, and value. AJR Am J Roentgenol. 1995; 164:1329–36. [PubMed: 7754870]
47. Mammography facilities: requirements for accrediting bodies and quality standards and
certification requirements—interim rules. Fed Reg. 1993; 58:67558–72.
48. State certification of mammography facilities. Fed Reg. 2002; 67:5446–69.
49. Bassett, LW.; Hendrick, RE.; Bassford, TL.; Butler, PF.; Carter, D.; DeBor, M. Clinical Practice
Guideline No. 13. AHCPR Publication No. 95-0632. Rockville, Md: Agency for Health Care
Policy and Research, Public Health Service, US Department of Health and Human Services; 1994.
Quality determinants of mammography.
50. Nass, SJ.; Ball, J. Improving breast imaging quality standards. Washington, DC: National
Academy of Science; 2005.
51. Sickles EA, Philpotts LE, Parkinson BT, et al. American College of Radiology/Society of Breast
Imaging curriculum for resident and fellow education in breast imaging. J Am Coll Radiol. 2006;
3:879–84. [PubMed: 17412188]
52. Bassett LW, Monsees BS, Smith RA, et al. Survey of radiology residents: breast imaging training
NIH-PA Author Manuscript
and attitudes. Radiology. 2003; 227:862–9. [PubMed: 12728182]
53. Sickles EA. The American College of Radiology’s Mammography Interpretive Skills Assessment
(MISA) examination. Semin Breast Dis. 2003; 6:133–40.
54. Berg WA, D’Orsi CJ, Jackson VP, et al. Does training in the Breast Imaging Reporting and Data
System (BI-RADS®) improve biopsy recommendations or feature analysis agreement with
experienced breast imagers at mammography? Radiology. 2002; 224:871–80. [PubMed:
12202727]
55. Baker JA, Kornguth PJ, Floyd CE Jr. Breast imaging reporting and data system standardized
mammography lexicon: observer variability in lesion description. AJR Am J Roentgenol. 1996;
166:773–8. [PubMed: 8610547]
56. Houssami N, Boyages J, Stuart K, Brennan M. Quality of breast imaging reports falls short of
recommended standards. Breast. 2007; 16:271–9. [PubMed: 17270445]
57. Geller BM, Barlow WE, Ballard-Barbash R, et al. Use of the American College of Radiology BI-
RADS® to report on the mammographic evaluation of women with signs and symptoms of breast
disease. Radiology. 2002; 222:536–42. [PubMed: 11818625]
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 14
58. Lehman C, Holt S, Peacock S, White E, Urban N. Use of the American College of Radiology BI-
RADS® guidelines by community radiologists: concordance of assessments and recommendations
assigned to screening mammograms. AJR Am J Roentgenol. 2002; 179:15–20. [PubMed:
NIH-PA Author Manuscript
12076896]
59. Taplin SH, Ichikawa LE, Kerlikowske K, et al. Concordance of breast imaging reporting and data
system assessments and management recommendations in screening mammography. Radiology.
2002; 222:529–35. [PubMed: 11818624]
60. D’Orsi CJ, Hall FM. BI-RADS® lexicon reemphasized. AJR Am J Roentgenol. 2006; 187:W557.
[PubMed: 17056895]
61. Baker JA, Kornguth PJ, Lo JY, Williford ME, Floyd CE Jr. Breast cancer: prediction with artificial
neural network based on BI-RADS® standardized lexicon. Radiology. 1995; 196:817–22.
[PubMed: 7644649]
62. Burnside ES, Rubin DL, Shachter RD. Using a Bayesian network to predict the probability and
type of breast cancer represented by microcalcifications on mammography. Stud Health Technol
Inform. 2004; 107:13–7. [PubMed: 15360765]
63. Kahn CE Jr, Langlotz CP, Burnside ES, et al. Toward best practices in radiology reporting.
Radiology. 2009; 252:852–6. [PubMed: 19717755]
64. Langlotz CP. RadLex: a new method for indexing online educational materials. Radiographics.
2006; 26:1595–7. [PubMed: 17102038]
NIH-PA Author Manuscript
NIH-PA Author Manuscript
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Burnside et al. Page 15
NIH-PA Author Manuscript
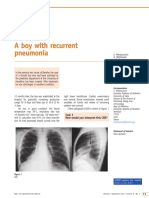
Fig 1.
Publication rate calculated on the basis of the percentage of papers published in a given 2-
year period as a percentage of the total in that category over the entire 22-year interval. The
slopes of the lines represent the publication rates within the respective categories. Papers
catalogued in PubMed demonstrate that observer and performance studies outpaced generic
mammographic or mammography standards studies after the mid-1990s, when BI-RADS
NIH-PA Author Manuscript
was increasingly adopted. The search was performed on May 14, 2009. Searches used
Medical Subject Heading (MeSH) terms as follows: mammography =
“Mammography[MeSH Terms]”; standards = “Mammography/standards[MAJR]”; observer
studies = “Mammography[MeSH Terms] AND Observer Variation[MeSH Terms]”; and
performance = “Mammography[MeSH Terms] AND Sensitivity and Specificity[MeSH
Term].”
NIH-PA Author Manuscript
J Am Coll Radiol. Author manuscript; available in PMC 2011 May 22.
Vous aimerez peut-être aussi
- Ovarian Adnexal Reporting Lexicon For MRI A WhiteDocument17 pagesOvarian Adnexal Reporting Lexicon For MRI A WhiteElena KaluçPas encore d'évaluation
- Bi Rads, C Rads, Cad Rads, Li Rads, Lung Rads, Ni RadsDocument5 pagesBi Rads, C Rads, Cad Rads, Li Rads, Lung Rads, Ni RadsMisael Mustafa SilvaPas encore d'évaluation
- Bethesda 2014Document11 pagesBethesda 2014David José FletesPas encore d'évaluation
- Pirads V2Document64 pagesPirads V2ekowahyudiPas encore d'évaluation
- Using Contextual Learning To Improve Diagnostic Accuracy Application in Breast Cancer ScreeningDocument13 pagesUsing Contextual Learning To Improve Diagnostic Accuracy Application in Breast Cancer ScreeningPriya HankarePas encore d'évaluation
- Best Practice Issue On "Abnormal Uterine Bleeding"Document21 pagesBest Practice Issue On "Abnormal Uterine Bleeding"Rosu GeorgePas encore d'évaluation
- Breast, Imaging, Reporting and Data System (BI RADS) - StatPearls - NCBI Bookshelf PDFDocument3 pagesBreast, Imaging, Reporting and Data System (BI RADS) - StatPearls - NCBI Bookshelf PDFnn hijo de alguienPas encore d'évaluation
- Bi-Rads Fifth Edition: A of ChangesDocument12 pagesBi-Rads Fifth Edition: A of ChangesDobritoiu FlorinPas encore d'évaluation
- Biospecimen Reporting For Improved Study Quality (BRISQ) : Original ArticleDocument10 pagesBiospecimen Reporting For Improved Study Quality (BRISQ) : Original ArticleSarandos KaptanisPas encore d'évaluation
- Asccp 2006 Consensus GuidelinesDocument10 pagesAsccp 2006 Consensus GuidelinesDiana Lopera CastrillonPas encore d'évaluation
- Biomarker Reproducibility ChalDocument25 pagesBiomarker Reproducibility ChalFenyl Isis GuigayomaPas encore d'évaluation
- 2015 - Swanson - How To Practice Evidence-Based MedicineDocument14 pages2015 - Swanson - How To Practice Evidence-Based MedicineAudrey VivierPas encore d'évaluation
- PIIS2468045123000603Document10 pagesPIIS2468045123000603daniaPas encore d'évaluation
- AUA-ASTRO Guideline Part I CA de Prostata LocalizadoDocument9 pagesAUA-ASTRO Guideline Part I CA de Prostata LocalizadoZuriPas encore d'évaluation
- Breast Radiology Entrustable Activity Supervision Tool ResidentsDocument46 pagesBreast Radiology Entrustable Activity Supervision Tool Residentsada.rodriguezPas encore d'évaluation
- Bethesda 2014Document12 pagesBethesda 2014VinhPas encore d'évaluation
- MIMIC-CXR, A De-Identified Publicly Available Database of Chest Radiographs With Free-Text ReportsDocument8 pagesMIMIC-CXR, A De-Identified Publicly Available Database of Chest Radiographs With Free-Text ReportsIsmii NhurHidayatiePas encore d'évaluation
- Equator Guidelines For Health ResearchDocument2 pagesEquator Guidelines For Health ResearchpecescdPas encore d'évaluation
- Forum DiskusiDocument6 pagesForum DiskusiIsmun SiregarPas encore d'évaluation
- Guideline 2013 Breast Recon Expanders ImplantsDocument23 pagesGuideline 2013 Breast Recon Expanders ImplantsBogdan TudorPas encore d'évaluation
- O RadsDocument15 pagesO RadsMurilo Campos100% (1)
- Integrating Pathology and Radiology Disciplines: An Emerging Opportunity?Document7 pagesIntegrating Pathology and Radiology Disciplines: An Emerging Opportunity?Peko PekoPas encore d'évaluation
- The Pap Test and Bethesda 2014: ReviewDocument12 pagesThe Pap Test and Bethesda 2014: ReviewIndhumathiPas encore d'évaluation
- Precision Medicine Resource GuideDocument480 pagesPrecision Medicine Resource GuideLaura Ximena Sánchez SagredoPas encore d'évaluation
- Bone Tumor Risk Stratification and Management System A Consensus Guideline from the ACR Bone Reporting and Data System CommitteeDocument15 pagesBone Tumor Risk Stratification and Management System A Consensus Guideline from the ACR Bone Reporting and Data System CommitteeJuana GalloPas encore d'évaluation
- Thesis On Breast Cancer DetectionDocument4 pagesThesis On Breast Cancer Detectionafkodkedr100% (2)
- Evidence-Practice Gaps in Lung Cancer: A Scoping Review: European Journal of Cancer Care October 2016Document13 pagesEvidence-Practice Gaps in Lung Cancer: A Scoping Review: European Journal of Cancer Care October 2016Dharpure Mr. Harshal Namdeorao --Pas encore d'évaluation
- Nhs Screening Breast Pinder 2023Document11 pagesNhs Screening Breast Pinder 2023Tania OrtegaPas encore d'évaluation
- Guidance To Best Tools and Practices ForDocument33 pagesGuidance To Best Tools and Practices Forcvdk8dc8sbPas encore d'évaluation
- AJCC TNM 8th Ed 2017Document7 pagesAJCC TNM 8th Ed 2017Danny. JayPas encore d'évaluation
- Radioembolization of Hepatic Malignancies - Background, Quality Improvement Guidelines, and Future DirectionsDocument15 pagesRadioembolization of Hepatic Malignancies - Background, Quality Improvement Guidelines, and Future DirectionsVeronica AlexanderPas encore d'évaluation
- Guideline 2017 Autologous Breast ReconstructionDocument14 pagesGuideline 2017 Autologous Breast ReconstructionPedroAngelToribioOrbegozoPas encore d'évaluation
- Quality and Safety Considerations in Stereotactic Radiosurgery and Stereotactic Body Radiation TherapyDocument16 pagesQuality and Safety Considerations in Stereotactic Radiosurgery and Stereotactic Body Radiation TherapyVerónica Bello HorizontePas encore d'évaluation
- PSGS - BSG 2013 CPG Early Breast CA Full TextDocument61 pagesPSGS - BSG 2013 CPG Early Breast CA Full Textaa628100% (1)
- The International Academy of Cytology Yokohama System For Reporting Breast Fine-Needle Aspiration Biopsy CytopathologyDocument17 pagesThe International Academy of Cytology Yokohama System For Reporting Breast Fine-Needle Aspiration Biopsy Cytopathologyunimo.prgPas encore d'évaluation
- Rosen's Breast Pathology IntroductionDocument18 pagesRosen's Breast Pathology IntroductionyoussPas encore d'évaluation
- Sensors 22 01160 PDFDocument15 pagesSensors 22 01160 PDFEnes mahmut kulakPas encore d'évaluation
- 723 - Standardization of EUS Imaging and Reporting in High-Risk Individuals of Pancreatic AdenocarcinomaDocument17 pages723 - Standardization of EUS Imaging and Reporting in High-Risk Individuals of Pancreatic AdenocarcinomaJhon Jaime CarvajalPas encore d'évaluation
- Exercise As An Intervention in Preventing and Reducing Chemotherapy-Induced Cardiotoxicity Among Breast Cancer Survivors - Protocol For A Scoping ReviewDocument4 pagesExercise As An Intervention in Preventing and Reducing Chemotherapy-Induced Cardiotoxicity Among Breast Cancer Survivors - Protocol For A Scoping ReviewdrpraveenkumarPas encore d'évaluation
- Art 3A10.1186 2Fs12879 016 1665 1Document9 pagesArt 3A10.1186 2Fs12879 016 1665 1tmrezatandiPas encore d'évaluation
- ACR Guidance Document On MR Safe Practices: 2013Document30 pagesACR Guidance Document On MR Safe Practices: 2013Clauzi Rodrigo GueriniPas encore d'évaluation
- The European Society of Gynaecological Oncology:European Society For Radiotherapy and Oncology:European Society of Pathology Guidelines For The Management of Patients With Cervical CancerDocument18 pagesThe European Society of Gynaecological Oncology:European Society For Radiotherapy and Oncology:European Society of Pathology Guidelines For The Management of Patients With Cervical CancerKaleb Rudy HartawanPas encore d'évaluation
- HHS Public Access: Cost Analysis of A Surgical Consensus Guideline in Breast-Conserving SurgeryDocument16 pagesHHS Public Access: Cost Analysis of A Surgical Consensus Guideline in Breast-Conserving SurgeryReza Rahman 15Pas encore d'évaluation
- SR and Guidelines For Periop MGMT of Peds Patients Undergoing Major Plastic SurgeryDocument10 pagesSR and Guidelines For Periop MGMT of Peds Patients Undergoing Major Plastic SurgeryPaul MarjiPas encore d'évaluation
- Curroncol 29 00552 v3Document30 pagesCurroncol 29 00552 v3Arun AdhikariPas encore d'évaluation
- EBMDocument9 pagesEBMHendra TPPas encore d'évaluation
- Radiation SafetyDocument4 pagesRadiation Safetybraith7811Pas encore d'évaluation
- Muscle Invasive Bladder Cancer 2017Document42 pagesMuscle Invasive Bladder Cancer 2017Cristian MortuPas encore d'évaluation
- 2013 Appropriate Utilization of Cardiovascular ImagingDocument8 pages2013 Appropriate Utilization of Cardiovascular ImagingAdi BestaraPas encore d'évaluation
- Machine Learning Methods For Quantitative Radiomic BiomarkersDocument11 pagesMachine Learning Methods For Quantitative Radiomic BiomarkerskeyojeliPas encore d'évaluation
- Breast Cancer Highlights From 2023Document26 pagesBreast Cancer Highlights From 2023Rogelio cuervoPas encore d'évaluation
- The Breast: SciencedirectDocument11 pagesThe Breast: SciencedirectYefry Onil Santana MartePas encore d'évaluation
- Downgrading of Breast Massess by Optoacoustic ImaginDocument11 pagesDowngrading of Breast Massess by Optoacoustic ImaginChadiIbrahimPas encore d'évaluation
- A Multimodal Deep Neural Network For Human Breast Cancer Prognosis Prediction by Integrating Multi-Dimensional DataDocument10 pagesA Multimodal Deep Neural Network For Human Breast Cancer Prognosis Prediction by Integrating Multi-Dimensional Datamanoj523Pas encore d'évaluation
- 2013 Surgical Prophylaxis ASHP, IDSA, SHEA, SISDocument8 pages2013 Surgical Prophylaxis ASHP, IDSA, SHEA, SISMara Medina - BorleoPas encore d'évaluation
- BI-RADS For UltrasonographyDocument11 pagesBI-RADS For UltrasonographyLucas GuadaPas encore d'évaluation
- Dellinger R Phillip Surviving Sepsis Campaign 2023Document14 pagesDellinger R Phillip Surviving Sepsis Campaign 2023Oscar SantosPas encore d'évaluation
- Guidelines Early Breast Cancer Diagnosis ManagementDocument53 pagesGuidelines Early Breast Cancer Diagnosis Managementabdulahad mohamedPas encore d'évaluation
- Optimizing Strategies for Clinical Decision Support: Summary of a Meeting SeriesD'EverandOptimizing Strategies for Clinical Decision Support: Summary of a Meeting SeriesPas encore d'évaluation
- Advanced Practice and Leadership in Radiology NursingD'EverandAdvanced Practice and Leadership in Radiology NursingKathleen A. GrossPas encore d'évaluation
- SalmonellaDocument1 pageSalmonellaTonny ChenPas encore d'évaluation
- 02 BIRADS Mammography ReportingDocument20 pages02 BIRADS Mammography ReportinglPiNGUSlPas encore d'évaluation
- Classification AO PediatricDocument36 pagesClassification AO PediatricdvcmartinsPas encore d'évaluation
- Drug Doses 2017Document127 pagesDrug Doses 2017Yuliawati HarunaPas encore d'évaluation
- Protokol2 Nefro PDFDocument8 pagesProtokol2 Nefro PDFTonny ChenPas encore d'évaluation
- Cme SG Guidelines BPHDocument8 pagesCme SG Guidelines BPHTonny ChenPas encore d'évaluation
- Hirschsprung's Disease - Diagnosis and Management (Kessmann, 2006) PDFDocument4 pagesHirschsprung's Disease - Diagnosis and Management (Kessmann, 2006) PDFMuhammad ReyhanPas encore d'évaluation
- Chole StasisDocument4 pagesChole StasisTonny ChenPas encore d'évaluation
- Rectal Prolapse CaseDocument20 pagesRectal Prolapse CaseTonny ChenPas encore d'évaluation
- Congenital Heart Disease Handout CHD PDFDocument82 pagesCongenital Heart Disease Handout CHD PDFTonny Chen100% (1)
- Dysmorphic InfantDocument5 pagesDysmorphic InfantTonny ChenPas encore d'évaluation
- CMV Induced Neonatal Cholestasis A Success Story PDFDocument2 pagesCMV Induced Neonatal Cholestasis A Success Story PDFTonny ChenPas encore d'évaluation
- Jco 2015 61 5906Document12 pagesJco 2015 61 5906Tonny ChenPas encore d'évaluation
- Recurrent Pneumonia in ChildrenDocument13 pagesRecurrent Pneumonia in ChildrenTonny ChenPas encore d'évaluation
- Guideline Kawasaki Disease AHA PDFDocument8 pagesGuideline Kawasaki Disease AHA PDFTonny ChenPas encore d'évaluation
- JNC 8Document14 pagesJNC 8amiwahyuniPas encore d'évaluation
- A Boy With Recurrent PneumoniaDocument6 pagesA Boy With Recurrent PneumoniaTonny ChenPas encore d'évaluation
- Recurrent Pneumonia - . - Not!: OmmentaryDocument2 pagesRecurrent Pneumonia - . - Not!: OmmentaryAnantaBenvenutoPas encore d'évaluation
- 1 s2.0 S1574626716300386 MainDocument7 pages1 s2.0 S1574626716300386 MainTonny ChenPas encore d'évaluation
- Fluid Electrolyte TherapyDocument15 pagesFluid Electrolyte Therapyaarm1990Pas encore d'évaluation
- 2016 Heartfailure - Eurheartj.ehw128.fullDocument85 pages2016 Heartfailure - Eurheartj.ehw128.fullTonny ChenPas encore d'évaluation
- Maintenance & Replacement Fluid Therapy: Moderated by DR - Madhuri EngadeDocument43 pagesMaintenance & Replacement Fluid Therapy: Moderated by DR - Madhuri EngadeTonny ChenPas encore d'évaluation
- CK NotesDocument25 pagesCK NotesFahad AliPas encore d'évaluation
- Easy Way To Learn ABGsDocument13 pagesEasy Way To Learn ABGsMunaim TahirPas encore d'évaluation
- Spirometry in PracticeDocument24 pagesSpirometry in Practiceuser_at_scribd100% (1)
- Clemency Is Not Granted by Jokowi To Dui Bali Nine CaseDocument1 pageClemency Is Not Granted by Jokowi To Dui Bali Nine CaseTonny ChenPas encore d'évaluation
- The Pathophysiology, Diagnosis and Current Management of Acute Compartment SyndromeDocument10 pagesThe Pathophysiology, Diagnosis and Current Management of Acute Compartment SyndromeTonny ChenPas encore d'évaluation
- JNC 8Document14 pagesJNC 8amiwahyuniPas encore d'évaluation
- Personal ReportDocument1 pagePersonal ReportTonny ChenPas encore d'évaluation
- Definition of operating modes and transmission fault codesDocument22 pagesDefinition of operating modes and transmission fault codesAhmetPas encore d'évaluation
- ASaP LNG Sampler Brochure 01-2018Document5 pagesASaP LNG Sampler Brochure 01-2018Arfan NPas encore d'évaluation
- Lotus Domino Designer 8.5.1 Classic Notes Applications (DDCNA851)Document5 pagesLotus Domino Designer 8.5.1 Classic Notes Applications (DDCNA851)marializaestellaPas encore d'évaluation
- 22 Alex - Balashov Kamailio TriviaDocument24 pages22 Alex - Balashov Kamailio TriviaerminPas encore d'évaluation
- Python BookDocument445 pagesPython BookShreyas Kolhe100% (3)
- Modern Statistics GuideDocument4 pagesModern Statistics GuideErille Julianne (Rielianne)100% (1)
- Distributed Systems WlsDocument456 pagesDistributed Systems WlsMadhav Madhausudhan Reddy KPas encore d'évaluation
- TLE - Davao Room Assignments For September 2013 LETDocument53 pagesTLE - Davao Room Assignments For September 2013 LETScoopBoyPas encore d'évaluation
- 2 2 T T T T M T 1 1 T 2 T 2 2 2 2 2 1 2 1 T 1 T 3 T 1 2 1! 2 2! 4 3!Document48 pages2 2 T T T T M T 1 1 T 2 T 2 2 2 2 2 1 2 1 T 1 T 3 T 1 2 1! 2 2! 4 3!AndersonHaydarPas encore d'évaluation
- Traffic MGMT QFXDocument1 196 pagesTraffic MGMT QFXmohamad septianto nugrohoPas encore d'évaluation
- Dentalia Setup GuideDocument3 pagesDentalia Setup GuideBryan AsenciosPas encore d'évaluation
- AOW Walkthrougth v043 Final PDFDocument193 pagesAOW Walkthrougth v043 Final PDFAlessandro PiranPas encore d'évaluation
- CMOS Processing Technology Chapter Explains FEOL and BEOL StepsDocument1 pageCMOS Processing Technology Chapter Explains FEOL and BEOL StepsCarlos SaavedraPas encore d'évaluation
- Assignment - 1 HCI: Task - 1Document3 pagesAssignment - 1 HCI: Task - 1Education materialPas encore d'évaluation
- AR Research PaperDocument18 pagesAR Research PaperShresh KumarPas encore d'évaluation
- 13 PDFDocument4 pages13 PDFAmritansh RanjanPas encore d'évaluation
- SPL Ree FracasDocument1 pageSPL Ree Fracasnicocla94maramPas encore d'évaluation
- 1770 KF2Document8 pages1770 KF2Chuy HernandezPas encore d'évaluation
- l1 Sep IntroductionDocument6 pagesl1 Sep IntroductionFact HubPas encore d'évaluation
- F6TesT EnglishDocument270 pagesF6TesT EnglishHamylto PamoPas encore d'évaluation
- DCRS 5960 28F DCDocument7 pagesDCRS 5960 28F DCPhạm Văn ThuânPas encore d'évaluation
- ERP Vendor ListDocument13 pagesERP Vendor Listpiyush3289Pas encore d'évaluation
- Aliza JaneDocument3 pagesAliza Janeواجد چوھدریPas encore d'évaluation
- CH-SIK-COAX-02 SpecificationDocument5 pagesCH-SIK-COAX-02 SpecificationDuong NathanPas encore d'évaluation
- Cyber Law Notes PDFDocument579 pagesCyber Law Notes PDFAdv NesamudheenPas encore d'évaluation
- Comptia Security Sy0 701 Exam Objectives (5 0)Document21 pagesComptia Security Sy0 701 Exam Objectives (5 0)Erick Camilo50% (4)
- E360 BrosurDocument6 pagesE360 BrosurPutra DalimaPas encore d'évaluation
- Waltonchain White Paper 2.0 - ENDocument72 pagesWaltonchain White Paper 2.0 - ENrlarapscribdPas encore d'évaluation
- Wi-Fi Certified Interoperatibility CertificateDocument1 pageWi-Fi Certified Interoperatibility CertificatelisandroPas encore d'évaluation
- Guia Modem Zyxel VMG862 3T50BDocument4 pagesGuia Modem Zyxel VMG862 3T50BJosé AntonioPas encore d'évaluation