Académique Documents
Professionnel Documents
Culture Documents
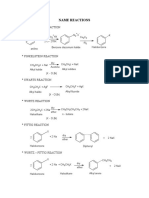
6carboxylic Acids
Transféré par
sharmimiameerasanadyDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
6carboxylic Acids
Transféré par
sharmimiameerasanadyDroits d'auteur :
Formats disponibles
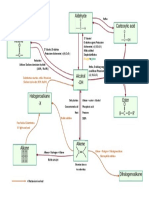
Flow Chart of organic reactions in CHM096
o
Cl2 or Br2/ hv or high heat (300-400 C) Free radical substitution
Alkyl halides/NaOH
3o Amines Quaternary ammonium salt
+
Alkyl halides/NaOH o Con NH3/ NaOH
Mg/dry ether Grignard Dilute H
o 1 Amines
2 Amines e.g CH3CHNH2CH3
Reagent
Alkyl halides/NaOH Nucleophilic
substitution HBr or HCl in ether or H2O
Alkynes 2 mol H2/Pt/Ni/Pd
NaCN/H
+
Nucleophilic hydrohalogenation
Alkyl Halides
Alkane Nitrile OR HCN substitution
e.g CH3CHBrCH3 (major) NaOH/EtOH Elimination 1 mol H2/Pt/Ni/Pd
e.g CH3CHCNCH3 (major) CH3CH2CHBr (minor)
CH3CH2CHCN (minor)
Nucleophilic Alkanes
NaOH/H2O substitution Free redical substitution
Alkoxide e.g CH3CH2CH3
e.g CH3ONa
Metal e.g Na Nucleophilic Alkenes
Carboxylic acid Cold, dil alkaline
+ H2SO4
substitution eg. CH3CH=CH2 hydrogenation
Ester Alcohols PCl3 or PCl5 or PBr3 or SOCl2 SN2 KMnO4 Electrophilic addition
Oxidation 1 mol H2/Pt/Ni/Pd
CH3OH Diol Hot,
1 o 1o eg. CH3CHOHCHOH acidified Br2 or Cl2/CCl4
Nucleophilic HBr or HI / heat
2o substitution
KMnO4
2o HCl/ZnCl2/heat (Lucas reagent)
C=C bond cleavage
Halogenation
3o SN1
3o =C ketoneI
Alcohols Elimination =CH carboxylic acid
e.g Alcohols
CH3CHOHCHBr (major) Conc. H2SO4. (Dehydration)
CH3CHBrCHOH (minor) =CH2 CO2 + H2O
(Elimina Dihalide alkanes
e.g CH3CHBrCHBr
+
H /H2O Hydration
+ + Br2 or Cl2/H2O
Hot KMnO4/H or Hot K2Cr2O7/H
Oxidation
Oxidation Hot KMnO4/H+ or Halohydrin
H2/Pt or LiAlH4 Hot K2Cr2O7/H+ Alcohols + con H2SO4 e.g CH3CHOHCHBr (major)
Aldehydes Carboxylic Acid Ester CH3CHBrCHOH (minor)
H2/Pt or LiAlH4 Tollens reagent +
e.g CH3COOH Dil H /Reflux
Formaldehyde -
Dil OH /Reflux
Reduction
+ Metal e.g Na or alkali e.g NaOH Eg. NaOH
HCN or NaCN/H
H2/Pt or LiAlH4 +
HCN or NaCN/H Alkanoate (salt of
Ketones Cyanohydrin carboxylic acid)
Hot KMnO4/H+ or Oxidation
e.g CH3COONa
Hot K2Cr2O7/H+
Oxidation Iodoform Test : 1) Alcohol with CH3CHOH-
No Reaction 2) Etanal CH3CH=O
3) Ketone with CH3C=O
T. Syed Illah and Dr LHSim
Vous aimerez peut-être aussi
- Haloalkanes and Haloarenes1Document15 pagesHaloalkanes and Haloarenes1Poorni RenuPas encore d'évaluation
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMPas encore d'évaluation
- Reagent ListDocument9 pagesReagent ListArka MukhopadhyayPas encore d'évaluation
- 11 Alcohols Phenols and EthersDocument2 pages11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- 7 Coordination CompoundsDocument329 pages7 Coordination CompoundsArka100% (1)
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocument9 pagesOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Organic-Chemistry (As Level)Document8 pagesOrganic-Chemistry (As Level)Pirate HunterPas encore d'évaluation
- Name Reactions of Organic ChemistryDocument7 pagesName Reactions of Organic ChemistryNaynam SharmaPas encore d'évaluation
- 18.0 Carbonyl CompoundsDocument9 pages18.0 Carbonyl CompoundsKudzayi Tusaumwe100% (1)
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- WWW - Crackjee.xyz: Organic ChemistryDocument9 pagesWWW - Crackjee.xyz: Organic ChemistryRau100% (1)
- AIEEE Chemistry Quick ReviewDocument1 pageAIEEE Chemistry Quick ReviewYashwanth KalyanPas encore d'évaluation
- NamereactionorganicDocument13 pagesNamereactionorganicdeykrishna654100% (1)
- Reaction List v002Document5 pagesReaction List v002cecil3414Pas encore d'évaluation
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocument8 pages12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456Pas encore d'évaluation
- Reaction Reactants Products Conditions Mechanism Other: AlkanesDocument3 pagesReaction Reactants Products Conditions Mechanism Other: AlkanesInzamam A HaquePas encore d'évaluation
- X UV Light or Heat: Reactions in Topic XIDocument3 pagesX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- Haloalkanes MADDocument31 pagesHaloalkanes MADggdfjkkvvPas encore d'évaluation
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Document47 pages12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Swaroop SurendraPas encore d'évaluation
- SGDGDDDocument33 pagesSGDGDDyopoboy100% (1)
- Organic ReagentsDocument3 pagesOrganic ReagentsKushagra Rai100% (1)
- Revision Notes On AlcoholsDocument13 pagesRevision Notes On AlcoholsMuredzwa MuzendaPas encore d'évaluation
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Document1 pageA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- DPP Percentage - 2 (1) 5321633Document2 pagesDPP Percentage - 2 (1) 5321633netra7222Pas encore d'évaluation
- Hydrocarbons NotesDocument13 pagesHydrocarbons NotesShivansh Pundir100% (1)
- Isomerism - Handwritten NotesDocument7 pagesIsomerism - Handwritten Notesgovind_galamPas encore d'évaluation
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982Pas encore d'évaluation
- 12 Chemistry Notes Ch12 Aldehydes Ketones and CarboxylicacidDocument11 pages12 Chemistry Notes Ch12 Aldehydes Ketones and Carboxylicacidankajkumar100% (1)
- Alcohol, Phenols and Ethers Ch-10Document19 pagesAlcohol, Phenols and Ethers Ch-10Literal ShTPas encore d'évaluation
- Aldehydes Ketones and Carboxylic AcidsDocument37 pagesAldehydes Ketones and Carboxylic Acidsssheeladevi84100% (1)
- Roadmap Problem - 9Document1 pageRoadmap Problem - 9abhyudaipathwayPas encore d'évaluation
- IsomerismDocument62 pagesIsomerismsubesinghPas encore d'évaluation
- 12 Chemistry Keypoints Revision Questions Chapter 12Document20 pages12 Chemistry Keypoints Revision Questions Chapter 12sangam patraPas encore d'évaluation
- Organic Chem ReactionsDocument7 pagesOrganic Chem ReactionsTeo Jia Ming NickolasPas encore d'évaluation
- Solved Example: Chemistry For Neet & AiimsDocument24 pagesSolved Example: Chemistry For Neet & AiimsAnup KPas encore d'évaluation
- Excel JEE Booster (3A, 3B) Chemistrty Alcohol Phenol and EtherDocument21 pagesExcel JEE Booster (3A, 3B) Chemistrty Alcohol Phenol and Ethersourav gargPas encore d'évaluation
- Alkyl HalideDocument8 pagesAlkyl HalideMegh Raj BhattPas encore d'évaluation
- Alkyl Aryl Halides PDFDocument21 pagesAlkyl Aryl Halides PDFLakshit SanghrajkaPas encore d'évaluation
- 01 D and F Block Elements Theory Final EDocument17 pages01 D and F Block Elements Theory Final Etech 2 life100% (1)
- Organic Chemistry ReagentsDocument7 pagesOrganic Chemistry ReagentsRishabhPas encore d'évaluation
- 3 - Aldehydes and Ketones (Assignment) Booklet-2Document15 pages3 - Aldehydes and Ketones (Assignment) Booklet-2kraken monsterPas encore d'évaluation
- CBSE Class-12 Chemistry Quick Revision Notes Chapter-12: Aldehydes, Ketones and Carboxylic Acid AldehydesDocument11 pagesCBSE Class-12 Chemistry Quick Revision Notes Chapter-12: Aldehydes, Ketones and Carboxylic Acid AldehydesManoj PrakashPas encore d'évaluation
- Reactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionDocument51 pagesReactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionmacybnzPas encore d'évaluation
- Chemistry SME Notes (Organic Chemmistry)Document14 pagesChemistry SME Notes (Organic Chemmistry)Sayeef MahdiPas encore d'évaluation
- 1 Roh Carboxylic Acids: H CroDocument15 pages1 Roh Carboxylic Acids: H CroandrewwroblePas encore d'évaluation
- Reactions and Preparations of AlkenesDocument9 pagesReactions and Preparations of AlkenesGolda Meyer VidalPas encore d'évaluation
- Alcohol Phenol & EtherDocument13 pagesAlcohol Phenol & EtherAbir DuttaPas encore d'évaluation
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnPas encore d'évaluation
- CBSE Class 12 Alcohol Phenol and Ether Study NotesDocument378 pagesCBSE Class 12 Alcohol Phenol and Ether Study NotesV T PRIYANKAPas encore d'évaluation
- Organic 6 CDocument26 pagesOrganic 6 CDr.Rajarshi PatelPas encore d'évaluation
- 1.aldehydes, Ketones and Carboxylic AcidsDocument117 pages1.aldehydes, Ketones and Carboxylic AcidsKRISHNARJUNA NPas encore d'évaluation
- NCERT Important Name Reactions For RevisionDocument34 pagesNCERT Important Name Reactions For Revisionyimisa2927Pas encore d'évaluation
- Art Integrated Learning: Kendriya Vidyalaya C.I.S.F. Bhilai Subject: Chemistry Class: XIIDocument32 pagesArt Integrated Learning: Kendriya Vidyalaya C.I.S.F. Bhilai Subject: Chemistry Class: XIIRANJEETA UIKEY 12APas encore d'évaluation
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- 100 Organic Reagentspptx - 230327 - 085539 PDFDocument15 pages100 Organic Reagentspptx - 230327 - 085539 PDFHeera MeenaPas encore d'évaluation
- Organometallic Transition Metal Catalysis: A Holistic Approach to Understanding and Predicting their MechanismsD'EverandOrganometallic Transition Metal Catalysis: A Holistic Approach to Understanding and Predicting their MechanismsPas encore d'évaluation
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoPas encore d'évaluation
- Imogen SlidesCarnivalDocument29 pagesImogen SlidesCarnivalZarith Emily Burgoa AguilarPas encore d'évaluation
- Year 3 EnglishDocument39 pagesYear 3 EnglishsharmimiameerasanadyPas encore d'évaluation
- ProposalDocument33 pagesProposalsharmimiameerasanadyPas encore d'évaluation
- 4alkyl Halides and AlcoholsDocument85 pages4alkyl Halides and AlcoholssharmimiameerasanadyPas encore d'évaluation
- 6carboxylic Acids PDFDocument29 pages6carboxylic Acids PDFsharmimiameerasanadyPas encore d'évaluation
- Learning About Gravity I. Free Fall: A Guide For Teachers and Curriculum DevelopersDocument33 pagesLearning About Gravity I. Free Fall: A Guide For Teachers and Curriculum DeveloperssharmimiameerasanadyPas encore d'évaluation
- 5carbonyl CompoundsDocument25 pages5carbonyl CompoundssharmimiameerasanadyPas encore d'évaluation
- Chemical Kinetics PDFDocument162 pagesChemical Kinetics PDFsharmimiameerasanadyPas encore d'évaluation
- 4alkyl Halides and AlcoholsDocument85 pages4alkyl Halides and AlcoholssharmimiameerasanadyPas encore d'évaluation
- Intro To Organic Chemistry PDFDocument78 pagesIntro To Organic Chemistry PDFsharmimiameerasanadyPas encore d'évaluation
- Storyboard Gerhana Bulan PDFDocument2 pagesStoryboard Gerhana Bulan PDFsharmimiameerasanadyPas encore d'évaluation
- Labelling Bilik Sains - For MergeDocument6 pagesLabelling Bilik Sains - For MergesharmimiameerasanadyPas encore d'évaluation
- Fieldtrip SafetyDocument5 pagesFieldtrip SafetysharmimiameerasanadyPas encore d'évaluation
- Functional Group Priority GroupingDocument2 pagesFunctional Group Priority GroupingrosePas encore d'évaluation
- Test No 3 AROMATIC HYDROCARBONSDocument11 pagesTest No 3 AROMATIC HYDROCARBONSMuhammad. Hafeez.MughalPas encore d'évaluation
- AlcoholsDocument33 pagesAlcoholsSakib AhmedPas encore d'évaluation
- 5 6289838506726392936Document16 pages5 6289838506726392936GtyPas encore d'évaluation
- Class 10 Syllabus Breakup ChemistryDocument4 pagesClass 10 Syllabus Breakup ChemistryUmme AbdullahPas encore d'évaluation
- 3 NomenclatureDocument45 pages3 Nomenclaturerusnah chungPas encore d'évaluation
- Lecture Planner - Organic Chemistry - Lakshya JEE 2025Document3 pagesLecture Planner - Organic Chemistry - Lakshya JEE 2025Dharmanshu GuptaPas encore d'évaluation
- Reaction of AlkynesDocument25 pagesReaction of AlkynesGabrielaAmandaPas encore d'évaluation
- Organic Chemistry Nomenclature Workbook 3.12Document101 pagesOrganic Chemistry Nomenclature Workbook 3.12Muhammad IzuanPas encore d'évaluation
- Infrared Spectroscopy:: Fundamentals and Interpretation of Organic CompoundsDocument75 pagesInfrared Spectroscopy:: Fundamentals and Interpretation of Organic CompoundsRajkishor YadavPas encore d'évaluation
- 7 Benzene and AromaticsDocument72 pages7 Benzene and Aromaticshamdy solimanPas encore d'évaluation
- Aldehydes and KetonesDocument20 pagesAldehydes and KetonesA BeheraPas encore d'évaluation
- Expt 7 Classification Tests For HydrocarbonsDocument10 pagesExpt 7 Classification Tests For Hydrocarbonssean goPas encore d'évaluation
- Metano y Propano K-FactorsDocument4 pagesMetano y Propano K-FactorsDany GarciaPas encore d'évaluation
- Bab 5 Chemistry Organometallic Compounds - IntroductionDocument14 pagesBab 5 Chemistry Organometallic Compounds - IntroductionPutrik AgustinaPas encore d'évaluation
- Evans PKa TableDocument5 pagesEvans PKa Tablecris1104Pas encore d'évaluation
- SHORTCUT DISTILLATION: Fenske Underwood Gilliland (FUG) : Chemical Engineer's GuideDocument87 pagesSHORTCUT DISTILLATION: Fenske Underwood Gilliland (FUG) : Chemical Engineer's GuideAmey BodkePas encore d'évaluation
- CARBOHYDRATES Lecture Notes (Autosaved)Document27 pagesCARBOHYDRATES Lecture Notes (Autosaved)Faith OluwalePas encore d'évaluation
- 11th-2022-23 Assignment - 05 Common Name Dt. 17-05-22 - 922612Document7 pages11th-2022-23 Assignment - 05 Common Name Dt. 17-05-22 - 922612Ravindra PatilPas encore d'évaluation
- Subject: Biochemistry Assignment On: LipidsDocument8 pagesSubject: Biochemistry Assignment On: LipidsMehran AhmadPas encore d'évaluation
- Organic Chemistry Lesson 2 ALKANESDocument13 pagesOrganic Chemistry Lesson 2 ALKANESSaddam GuiaminPas encore d'évaluation
- Aldehid Dan KetonDocument65 pagesAldehid Dan KetonAdi Kurniawan Effendi100% (1)
- Lecture 1Document50 pagesLecture 1Maria Cleo Conde PasionPas encore d'évaluation
- 5PY023 - Org Chem - L8 - OnlineDocument10 pages5PY023 - Org Chem - L8 - OnlineKelvin Opoku AddoPas encore d'évaluation
- CHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Document5 pagesCHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Lucy ZuluPas encore d'évaluation
- Presented in A: Cherry AccordDocument2 pagesPresented in A: Cherry AccordWu WeiPas encore d'évaluation
- Science9 - q2 - Mod4 - Carbon Compound - v3Document32 pagesScience9 - q2 - Mod4 - Carbon Compound - v3Kristine Ibarreta-Jazul100% (2)
- IUPAC Nomenclature of Organic ChemistryDocument10 pagesIUPAC Nomenclature of Organic ChemistryYashwanth SrinivasaPas encore d'évaluation
- Bayer Villager OxidationDocument11 pagesBayer Villager OxidationSabitry YadavPas encore d'évaluation