Académique Documents
Professionnel Documents
Culture Documents
Are There Parallel Channels in The Vestibular Nerve?: Ellengene H. Peterson
Transféré par
ramopavelTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Are There Parallel Channels in The Vestibular Nerve?: Ellengene H. Peterson
Transféré par
ramopavelDroits d'auteur :
Formats disponibles
rons in the hypothalamus project to various areas 6. Houston, J. P., H. L. Haas, F. Boix, M. Pfister, U.
Decking,
in the brain. Indeed, central administration of PRL J. Schrader, and R. K. Schwarting. Extracellular adenosine
also enhances REMS in rats and VIP induces levels in neostriatum and hippocampus during rest and
activity periods of rats. Neuroscience 73: 99–107, 1996.
increases in hypothalamic PRL mRNA levels. 7. Jimenez-Anguiano, A., A. Baez-Saldana, and R. Drucker-
In conclusion, within the past decade, great Colin. Cerebrospinal fluid (CSF) extracted immediately
strides have been made in our understanding of after REM sleep deprivation prevents REM rebound and
the humoral regulation of sleep. This knowledge contains vasoactive intestinal peptide (VIP). Brain Res.
may promote insights into sleep function. 631: 345–348, 1993.
8. Jouvet, M., C. Buda, R. Cespuglio, N. Castrette, M.
Denoyer, M. Sallanon, and J. P. Sastre. Hypogenic effects
We thank Laureen Poesy for additional help in preparing
this manuscript. of some hypothalamo-pituitary peptides. Clin. Neu-
This work was supported in part by National Institute of ropharmacol. 9: 465–467, 1986.
Neurological Disorders and Stroke Grants NS-25378, NS- 9. Krueger, J. M. Cytokine involvement in sleep responses to
27250, and NS-31453 and by the Hungarian National Sci- infection and physiological sleep. In: Cytokines in the
ence Foundation (OTKA-16080). Nervous System, edited by N. J. Rothwell. Austin, TX:
Landes, 1996, p. 41–71.
10. Krueger, J. M., and J. A. Majde. Microbial products and
References cytokines in sleep and fever regulation. Crit. Rev.
Immunol. 14: 355–379, 1994.
1. Blatteis, C. M., and E. Sehic. Fever: how may circulating 11. Krueger, J. M., and F. Obál, Jr. Growth hormone-releasing
pyrogens signal the brain? News Physiol. Sci. 12: 1–9, hormone and interleukin-1 in sleep regulation. FASEB J.
1997. 7: 645–652, 1993.
2. Borbély, A. A., and I. Tobler. Endogenous sleep-promot- 12. Obál., F., Jr., R. F. Floyd, C. Kapás, B. Bodosi, and J. M.
ing substances and sleep regulation. Physiol. Rev. 69: Krueger. Effects of GHRH on sleep in intact and
605–670, 1989. hypophysectomized rats. Am J. Physiol. 270 (Endocrinol.
3. Bourgin, P., C. Lebrand, P. Escourrou, C. Gaultier, B. Metab. 33): E230–E237, 1996.
Franc, M. Hamon, and J. Adrien. Vasoactive intestinal 13. Obál, F., Jr., B. Kacsoh, S. Bredow, N. Guha-Thakurta,
polypeptide microinjections into the oral pontine and J. M. Krueger. Sleep in rats rendered chronically
tegmentum enhance rapid eye movement sleep in the hyperprolactinemic with anterior pituitary grafts. Brain
rat. Neuroscience 77: 351–360, 1997. Res. 755: 130–136, 1997.
4. Fang, J., Y. Wang, and J. M. Krueger. Mice lacking the TNF 14. Roky, R., F. Obál, Jr., J. L. Valatx, S. Bredow, J. Fang, L. P.
55kD receptor fail to sleep more after TNF treatment. J. Pangano, and J. M. Krueger. Prolactin and rapid eye
Neurosci. 17: 5949–5955, 1997. movement sleep. Sleep 18: 536–542, 1995.
5. Hayaishi, O. Sleep-wake regulation by prostaglandin D2 15. Steriade, M., and R. W. McCarley. Brainstem Control of
and E2. J. Biol. Chem. 263: 14593–14596, 1988. Wakefulness and Sleep. New York: Plenum, 1990.
Are There Parallel Channels in the
Vestibular Nerve?
Ellengene H. Peterson
A popular concept in neurobiology is that sensory information is transmitted
to the central nervous system over parallel channels of neurons that play
different functional roles. But alternative organizing schemes are
possible, and it is useful to ask whether some other framework might
better account for the diversity of vestibular primary afferents.
I n sensory systems, incoming information is
sometimes carried over parallel populations of
neurons that differ from each other and appear to
distinctive, parallel chains of neurons from spinal
cord to brain stem and cortex. In vision, similarly,
information about pattern and movement is ana-
“In vision . . . pattern encode separate stimulus attributes. For example, lyzed by parallel systems of neurons that are said
and movement are nociceptive and tactile information is conveyed to be separable from retina to visual cortex.
analyzed by parallel from body surface to spinal cord over different Characterization of such “parallel channels” is
systems. . . .” populations of somatosensory afferents; signals a popular and intuitively attractive framework for
from these afferent populations are routed over sensory system analysis, and several investigators
have wondered whether a similar paradigm might
usefully be applied to the vestibular system. This
E. H. Peterson is in the Dept. of Biological Sciences, Ohio review summarizes evidence for parallel informa-
University, Athens, OH 45701-2979, USA. tion channels in the first and most thoroughly
194 News Physiol. Sci. • Volume 13 • August 1998 0886-1714/98 5.00 @ 1998 Int. Union Physiol. Sci./Am.Physiol. Soc.
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
characterized neurons of the vestibular system: inner ear and transmits signals from vestibular
primary afferents. It emphasizes afferents from the hair cells toward the brain stem, 2) a cell body in
semicircular canals because they are the best the vestibular (Scarpa’s) ganglion, and 3) a cen-
understood. There are significant lacunae in this tral process that enters the brain stem and
evidence, in particular, an exceedingly sketchy synapses on vestibular nucleus neurons and
understanding of how signals from afferents are other central nervous system (CNS) targets.
routed in the central nervous system. Neverthe- Differences in peripheral terminal morphology
less, a fairly mature body of information exists can be dramatic, and these are the basis of most
about the peripheral morphology and physiologi- morphological classifications (2, 3, 6, 9, 11). In
cal properties of canal afferents in several species, amniotes (reptiles, birds, mammals), the terminal
so we can begin to ask if there are parallel chan- arbor may bear a cluster of boutons (bouton affer-
nels in the vestibular nerve. ents; Fig. 1, A and B), one or more calyceal end-
ings (calyceal afferents; Fig. 1C ), or both (dimor-
phic afferents; Fig. 1D); these calyceal and bou-
What are parallel channels?
ton endings contact different receptor (hair cell)
Where parallel channels have been identified types (for review, see Ref. 14). Anamniotes (fish
in the periphery of other sensory systems they and amphibians) appear not to have calyceal “Differences in
usually share the following characteristics. endings; however, some anamniotes (toadfish, peripheral terminal
1) The peripheral neurons are heterogeneous. bullfrog) and turtles may have structurally distinct morphology can be
Anatomic and physiological parameters describ- subpopulations of bouton afferents (Fig. 1, A and dramatic. . . .”
ing these neurons, e.g., axon diameter or resting B). The axon diameters of afferents can differ by
discharge rate, assume a wide range of values. an order of magnitude or more, and soma diam-
2) These parameter values covary in orderly eters are almost as variable (2 , 3, 6, 9, 11).
ways. Among somatosensory afferents, for Finally, terminal location has aroused substantial
example, receptor type and axon diameter are interest during the last decade because several
clearly related. physiological parameters vary systematically with
3) Parameter values tend to cluster into groups. the position of the terminal on the neuroepithe-
Individual variable distributions may or may not lium (2, 3, 5, 9, 11; see Do structural and physio-
be bi- or multimodal, but where peripheral neu- logical parameters covary?)
rons are described by n parameters, their parame- PHYSIOLOGICAL PARAMETERS. Canal afferents are
ter values form clusters in n-dimensional space. most commonly characterized by their resting
For example, different ganglion cell types at a sin- discharge as regularly or irregularly firing (Fig. 2)
gle retinal locus clearly vary in axon and soma (1–5, 7, 9, 11, 13, 15); sometimes an “intermedi-
diameter, dendritic structure, spatial filtering prop- ate” category is recognized, and some authors
erties, temporal dynamics, and central targets. emphasize that discharge regularity is a contin-
4) The neurons described by different variable uum. If the head is sinusoidally rotated at or near
clusters appear to play qualitatively different the plane of the canal, afferents can also be
functional roles in the sensory system. For exam- described by their “response dynamics” (Fig. 2):
ple, afferents arising from free nerve endings and gain [incremental sensitivity to changes in head
Pacinian corpuscles in the skin encode quite dif- velocity (in spikes 3 s–1/degrees 3 s–1)], and the
ferent somatosensory attributes. Attributes here, phase of the afferent’s response (in degrees) rela-
and elsewhere in this review, mean subcate- tive to the head’s sinusoidally oscillating velocity.
gories or submodalities of a stimulus that are per- An afferent’s gain or phase may change with the
ceived to have qualitatively different information frequency of oscillation, and some afferents
content, e.g., pattern vs. movement or tempera- exhibit a pronounced gain increase and phase
ture vs. touch. advance at higher frequencies (2, 3, 5, 11). Affer-
To what extent do vestibular afferents share ents also differ in their sensitivity to electrical cur-
these characteristics? rents (4, 13), the time constant of their responses
to velocity ramps or rapid head turns (i.e., they
are relatively tonic or phasic; e.g., Ref. 7), and in
Properties of vestibular afferents
their operating range and sensitivity to head
Are vestibular afferents heterogeneous? Vestibu- speed [rate-intensity function (5)].
lar afferents are unquestionably diverse; they differ Do structural and physiological parameters
structurally and physiologically. covary? Clearly, they do. In mammals (2, 7, 11),
ANATOMIC PARAMETERS. Like other primary affer- irregularly discharging afferents have relatively
ents, vestibular afferents have a three-part struc- phasic responses to rapid head turns and
ture: 1) a peripheral process that arborizes in one response phases shifted toward head accelera-
of the semicircular canals or otolith organs of the tion; regularly discharging afferents have more
News Physiol. Sci. • Volume 13 • August 1998 195
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
FIGURE 1. Peripheral terminals of vestibular afferents in posterior semicircular canal of a turtle, Pseudemys scripta. Bouton affer-
ents, which receive their input from type II hair cells (A, B), vary considerably in axon diameter and terminal morphology; mul-
tivariate statistical analyses suggest that they may be divisible into 2 groups based on their size and location on the neuroep-
ithelium (6). Calyceal afferents (C ) bear 1 or more cuplike endings that enclose type I hair cells. Calyx-bearing afferents that also
bear bouton sprays are called dimorphs (D). Such differences in terminal structure and receptor (hair cell) type have led inves-
tigators to ask whether these afferents might play distinctive roles in vestibular signaling. Drawings by Alan Brichta.
tonic responses to rapid head turns and firing response phase also holds for afferents in toad-
rates in phase with head velocity (Fig. 2). The fish (3) and bullfrog (9) and for bouton afferents
relation between discharge regularity and in turtle (5).
196 News Physiol. Sci. • Volume 13 • August 1998
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
characterized, both calyceal and dimorphic affer-
ents occupy the epithelial center, where they can
be equally irregular (2, 11). Indeed, of the com-
mon physiological descriptors, only afferent gain
appears linked to terminal type in mammals:
calyceal afferents clearly have lower gains than
dimorphic afferents even though they may have
similar discharge regularities, response phases,
and epithelial locations (2, 11).
Thus structural and physiological properties of
vestibular afferents covary in orderly ways, but
the pattern of variation is complex. If we think of
n afferent parameters plotted in n-dimensional
space, the resulting data cloud is structurally
more intricate than the familiar X-Y plots of intro-
ductory statistics texts.
Do afferents cluster into groups? Orderly
covariation of afferent properties is not a sufficient “. . .analyses suggest
reason to posit parallel channels. It may simply that there may be a
reflect a gradient, e.g., a spatial gradient, that continuous gradation
encodes a continuously variable stimulus of terminals. . . .”
attribute such as frequency in hearing or spatial
acuity in vision or touch. Faced with a heteroge-
neous population of afferents, how do we decide
whether the observed variation reflects distinct
afferent classes, which may play different func-
tional roles in vestibular signaling? One approach
FIGURE 2. Relationship between resting discharge regular-
ity (CV*) and gain (A) or phase (B; re. head velocity) of the has been to focus on apparent discontinuities in
response to 2-Hz sinusoidal head rotations. CV*, normalized peripheral terminal morphology and suggest that
coefficient of variation for resting interspike interval, i.e., a bouton, dimorphic, and calyceal afferents repre-
measure of discharge regularity. Note that the response phase sent different classes of afferents. But detailed
of more irregular afferents (high CV*) is advanced by 45° or
anatomic analyses suggest that there may be a
more toward head acceleration; in some nonmammals, affer-
ents are phase advanced by almost 90°, firing maximally with continuous gradation of terminals, with pure
peak head acceleration (3, 5, 9). Vestibular afferents (n = 125) calyceal and bouton endings at the extremes, and
have been sorted into 3 morphological classes (B, bouton; D, between them a series of dimorphic terminals
dimorph; C, calyx). Lines are the best-fitting power law for with different proportions of the two ending types
bouton and dimorphic units (A) and best-fitting semilogarith-
[turtle (6); mammals (2, 11)]. Several other affer-
mic relation for all units (B ). [From Lysakowski et al. (11).]
ent parameters, e.g., axon diameter, sensitivity to
electrical currents, and response dynamics,
Discharge regularity and response phase also appear to have continuous distributions; only dis-
covary with the location of the afferent terminal charge regularity tends to be bimodal (2, 5, 7, 11).
on the canal neuroepithelium. In mammals, for So with few, sometimes arguable, exceptions,
example, average discharge regularity increases individual parameters provide little evidence for
and response phase advances as one moves from multiple classes of vestibular afferents.
peripheral to central zones of the sensory surface A second approach has been to control for the
(2, 11). Similarly, toadfish (3) and turtle (5) exhibit role of spatial gradients in afferent heterogeneity
finely graded changes in discharge regularity and (see Do structural and physiological parameters
response phase, as well as several morphological covary? above) and ask whether afferent proper-
parameters (6), as one moves from the periphery ties differ if one holds terminal location constant
to the center of the canal. (2, 3, 5, 6, 11). Such studies suggest two possible
In contrast, some afferent parameters follow instances of heterogeneity that may be indepen-
this pattern of covariation less clearly if at all. Ter- dent of epithelial location. First, in mammals,
minal morphology (and by implication, receptor both calcyeal and irregular dimorphic afferents
type) is less tightly correlated with discharge reg- occupy the epithelial center and there exhibit dif-
ularity, response phase, and epithelial location. ferent axon diameters and different incremental
For example in mammals, in which the distinctive sensitivities to head rotation (2,11). Second, in
physiological properties of bouton, calyceal, and toadfish (3), bullfrog (9), and turtle (5, 6), two
dimorphic canal afferents have been most fully classes of bouton afferents with different mor-
News Physiol. Sci. • Volume 13 • August 1998 197
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
phologies and/or response dynamics have been modeling studies require experimental verifica-
described; some evidence suggests they occur tion, but both suggest that one function of
side by side near the center of the canal [i.e., that calyceal terminals and their associated type I hair
they differ, even when location is held constant cells may be to extend the dynamic range of the
(3, 6)], but this is not yet firmly established (5). canals. Thus differences in terminal type and
Thus calyceal and irregular dimorphic afferents their associated hair cells could simply reflect the
provide the only clear evidence for afferent het- need to encode a continuous range of stimulus
erogeneity that is independent of epithelial locus. intensities (e.g., head velocities), rather than dis-
A third approach has been to capitalize on the tinctive stimulus attributes.
large number of available afferent parameters
and use statistical methods, for example, princi-
How are afferent signals routed in the
pal components or discriminant analysis, to ask
central nervous system?
whether afferents fall into distinct groups when
multiple parameters are considered simultane- The argument for distinctive functional roles of
ously (5, 6). Such studies have revealed tenden- vestibular afferents would be materially strength-
cies for afferents to cluster into groups based on ened if we knew that different afferents are
their parameter values, but separation between routed into distinctive central circuits. In vision,
groups tends to be modest. for example, the concept that different ganglion
Thus there is presently no clear answer to the cell types play different functional roles was
question of whether afferents can be subdivided greatly reinforced when it was discovered that
into groups based on their anatomic and physio- the axons of ganglion cells with different recep-
logical parameter values. There is a limited tive field properties project to different central
amount of positive evidence for distinct groups targets. In vestibular neuroscience, work address-
(i.e., groups that are separable in n-dimensional ing this issue has taken two forms.
parameter space). On the other hand, there is Several recent studies have used physiological
ample evidence that afferent heterogeneity takes methods to assess the contribution of regular and
the form of spatial gradients (see Do structural irregular afferents to vestibuloocular and vestibu-
and physiological parameters covary?). lospinal reflexes (see Refs. 4, 7, 13 for reviews).
Do different afferents play distinctive functional Most of these have taken advantage of the fact that
roles in vestibular signaling? It is well established irregularly discharging afferents, which tend to
that canal afferents signal temporal characteristics have the largest axon diameters, are more suscep-
of head movement. Some afferents modulate their tible than regular afferents to externally applied
firing frequency in phase with head velocity and currents. Thus irregular afferents can be preferen-
so are said to be “velocity signaling.” Other affer- tially stimulated by low depolarizing currents or
ents have their response phase advanced toward “ablated” by hyperpolarizing currents (13). These
“. . .canal afferents head acceleration (Fig. 2). In all vertebrates for studies suggest that there may be only limited seg-
signal temporal which data exist, these two “groups” represent the regation of regular and irregular afferents within
characteristics of extremes of a continuous phase distribution that, the CNS. For example, it has been argued that the
head movement.” as noted above, is tightly correlated with epithelial evoked discharge characteristics of regular (tonic)
locus but not with terminal type (2, 3, 5, 9, 11). and irregular (phasic) afferents are best matched to
The functional significance of different termi- the mechanical demands of the vestibuloocular
nal types (Fig. 1) remains unclear, but recent and vestibulocollic reflexes, respectively and that
experimental and modeling results suggest some as a result, vestibuloocular reflex (VOR) neurons
new possibilities (8, 14). The unusual geometry might receive their peripheral inputs from regular
of calyceal endings and their enclosed type I hair afferents, whereas vestibulocollic reflex (VCR) neu-
cells may increase the ability of calyx-bearing rons might receive irregular inputs.1 Several physi-
afferents to reach high firing rates and so extend ological experiments have been designed to test
the range over which spike frequency is a linear this hypothesis, but their results have provided lim-
function of head velocity (8). Other work indi- ited support for this hypothesis. Intracellular
cates that type I hair cells in turtles may have cil- recordings in the vestibular nuclei (see Ref. 4 for
iary bundle morphologies that are significantly review) suggest that, whereas VOR neurons are
different from those on type II hair cells, and more likely to receive regular than irregular mono-
computational analyses suggest that one conse-
quence may be increased stiffness of type I cil- 1 The VOR assists in stabilizing gaze during head movement
iary bundles (14). This could make calyceal affer- by producing eye rotation at approximately the same speed but
ents relatively insensitive to low head velocities opposite in direction to the head rotation. Vestibulospinal
reflexes include “postural” reflexes of the limbs triggered by head
but enable calyx-bearing afferents to respond to movement (e.g., antigravity reflexes), and the vestibulocollic
higher head velocities without saturating. These (neck) reflex, which acts with the VOR to help stabilize gaze.
198 News Physiol. Sci. • Volume 13 • August 1998
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
statistically significant differences in morphology
(12). Similarly, the central axons of regular and
irregular afferents in cats appear quite similar at
the light microscopic level (see Ref. 15 for review),
even though only the extremes of the available
intracellular sample were compared (i.e., afferents
with intermediate discharge regularities were not
analyzed). The only statistically significant differ-
ences between regular and irregular afferent struc-
ture in cats are in axonal and varicosity size, num-
ber of varicosities per afferent, and mitochondrial
shape. Furthermore, regular and irregular afferents
have comparable proportions of terminals in the
various vestibular nuclei, except for the lateral
nucleus where regular terminals are rare.
Even when afferent axons appear to be com-
pletely filled and the full range of afferent sizes is “. . .our
included in the analysis (10), the central axons of understanding of
afferents appear similar (Fig. 4). There are some afferent central
clear qualitative differences; for example, some circuitry is relatively
afferents project to the cerebellum and some do immature.”
not (Fig. 4, single arrowheads). But, at least in tur-
tles and at the present level of analysis, most dif-
FIGURE 3. Distributions of %I (index), the percentage of total
ferences between central axons are quantitative.
monosynaptic ipsilateral vestibular nerve input contributed by
irregular afferents for various classes of secondary vestibular For example, the tendency in cats (15) for larger
neurons. A: vestibulocollic cells to cervical segment C1, pro- diameter afferents to have more terminals in the
jecting in the medial (MVST, solid bars) or lateral (LVST, open lateral vestibular nucleus is also seen in turtles
bars) vestibulospinal tracts or else unassigned to a tract (10), but this appears to reflect a gradient in which
(hatched bars). B: vestibulospinal cells projecting to C6 and
afferent terminals are emitted more caudally as
assigned to a projection pathway as in A. C: vestibulo-oculo-
collic (VOC) cells projecting as far caudal as cervical segments parent axon size decreases, not an identifying fea-
C1 (solid bars) or C6 (open bars). D: LVST cells projecting to the ture of a distinct afferent group (Fig. 4).
lumbar segment (L1). E: vestibuloocular (VOR) cells projecting Thus the available anatomic and physiological
to the occulomotor nucleus, recorded in and around the ven- data provide only limited support for the hypoth-
tral lateral vestibular nucleus (LV, solid bars) or the superior
esis that different afferents are routed into idio-
vestibular nucleus (SV, open bars). [From Boyle et al. (4).]
syncratic central circuits, and this weakens any
argument that there are parallel channels in the
synaptic inputs, most monosynaptic inputs from vestibular nerve. Still, our understanding of affer-
the vestibular nerve are mixed (Fig. 3); conversely, ent central circuitry is relatively immature. There
some spinal-projecting vestibular neurons are are intriguing suggestions of central differences at
dominated by irregular inputs, but inputs to most of the ultrastructural level; for example, irregular
these secondary neurons come from a range of afferents in cats are significantly more likely than
afferent types (Fig. 3). Behavioral assays of afferent regular afferents to contact large vestibular
input to the monkey VOR have further complicated nucleus cells and to have a higher fraction of their
the issue by suggesting that irregular inputs to the synapses on somata and proximal dendrites (15).
VOR are negligible (13), significant (7), or depen- It is also possible that one of several afferent
dent on conditions of stimulation (1). It does inputs to a vestibular secondary neuron may
appear that pure calyceal afferents in monkeys do somehow be selected according to behavioral
not contribute to the VOR, i.e., that irregular inputs, state (4).
if indeed they are present, probably arise from
dimorphs (7, 13). But with this potential exception,
Conclusion
the available physiological data suggest that segre-
gation of regular and irregular canal afferents in the Are there parallel channels in the vestibular
central nervous system is limited at best. nerve? Of the four characteristics outlined above
Anatomic analyses present a similar picture of (see What are parallel channels?) only the first
overlapping central trajectories, at least at the light two are clearly shared by vestibular afferents. 1)
microscopic level. In toadfish, the three physio- Vestibular afferents are heterogeneous and 2)
logical classes of afferents have largely (but not their anatomic and physiological parameters
exclusively) overlapping central axons, with few covary in orderly, if sometimes complex, pat-
News Physiol. Sci. • Volume 13 • August 1998 199
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
FIGURE 4. Computer reconstructions of the central axons of 2 vestibular afferents from a turtle posterior canal. Afferents enter
brain stem from vestibular nerve (CN8) and bifurcate into ascending and descending limbs that emit transversely oriented collater-
als. Top: lateral view of turtle brain stem showing the 2 afferents in situ. Bottom: horizontal view of the same 2 afferents; cerebel-
lar outline has been removed for clarity. Both views show considerable overlap between the 2 afferents as well as some differences,
including 1) a long descending limb that almost reaches the spinal cord (double arrowheads), 2) a long ascending limb that tra-
verses cerebellar cortex (single arrowheads), and 3) long ventromedially oriented collaterals that extend toward somatic motor col-
umn. These features are present in 1 afferent (gray) but not the other (black). The 2 descending limb morphologies are part of a struc-
tural continuum that is correlated with the diameter of afferent’s parent axon (10). Axonal reconstructions by Janice Huwe.
terns. It is much less clear 3) whether this het- ried by canal nerves. Separable groups raise the
erogeneity takes the form of gradients or distinct possibility that these groups play different func-
afferent groups and 4) the extent to which differ- tional roles (characteristic 4); a gradient, for
ent afferents play idiosyncratic roles in vestibu- example, in response phase or terminal mor-
lar signaling. phology, suggests that a continuous variable may
The issues surrounding characteristics 3 and 4 be encoded. Thus the pattern of variability influ-
are closely linked, and they are important as we ences the functional questions we ask. Many
develop hypotheses about the information car- authors have made it clear that dividing afferents
200 News Physiol. Sci. • Volume 13 • August 1998
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
into morphological or physiological “types” is responses from morphological fiber classes in the turtle
more a convenience for communication and posterior crista. Ann. NY Acad. Sci. 781: 183–195, 1996.
6. Brichta, A. M., and E. H. Peterson. Functional architec-
analysis than a reflection of reality, but one unin-
ture of vestibular primary afferents from the posterior
tended consequence may be to obscure a pattern semicircular canal of a turtle, Pseudemys (Trachemys)
of continuous variation that has important func- scripta elegans. J. Comp. Neurol. 344: 481–507, 1994.
tional significance. This may be part of the reason 7. Brontë-Stewart, H. M., and S. G. Lisberger. Physiological
we have not yet achieved a satisfying picture of properties of vestibular primary afferents that mediate
motor learning and normal performance of the vestibulo-
the information content in canal signals and how
ocular reflex in monkeys. J. Neurosci. 14: 1290–1308,
this information content is related to observed 1994.
heterogeneity in canal afferents. 8. Goldberg, J. M. Theoretical analysis of intercellular com-
munication between the vestibular type I hair cell and its
Drs. R. A. Eatock, J. Huwe, A. Lysakowski, M. Rowe, and R. calyx ending. J. Neurophysiol. 76: 1942–1957, 1996.
Waters provided very helpful suggestions on the manuscript. 9. Honrubia, V., L. F. Hoffman, S. Sitko, and I. R. Schwartz.
The author’s work is supported by National Institute on Anatomic and physiological correlates in bullfrog
Deafness and Other Communication Disorders Grant DC- vestibular nerve. J. Neurophysiol. 61: 688–701, 1989.
00618 and National Science Foundation Grant IBN- 10. Huwe, J. A., and E. H. Peterson. Differences in the brain
9319630. stem terminations of large- and small-diameter vestibular
primary afferents. J. Neurophysiol. 74: 1362–1366,
1995.
References 11. Lysakowski, A., L. B. Minor, C. Fernandez, and J. M.
Goldberg. Physiological identification of morphologi-
1. Angelaki, D. E., and A. A. Perachio. Contribution of irreg- cally distinct afferent classes innervating the cristae
ular semicircular canal afferents to the horizontal ampullares of the squirrel monkey. J. Neurophysiol. 73:
vestibuloocular response during constant velocity rota- 1270–1281, 1995.
tion. J. Neurophysiol. 69: 996–999, 1993. 12. Mensinger, A. F., J. P. Carey, R. Boyle, and S. M. High-
2. Baird, R.A., G. Desmadryl, C. Fernandez, and J. M. Gold- stein. Differential central projections of physiologically
berg. The vestibular nerve of the chinchilla. II. Relation characterized horizontal semicircular canal vestibular
between afferent response properties and peripheral nerve afferents in the toadfish, Opsanus tau. J. Comp.
innervation patterns in the semicircular canals. J. Neuro- Neurol. 384: 71–85, 1997.
physiol. 60: 182–203, 1988. 13. Minor, L. B., and J. M. Goldberg. Vestibular-nerve inputs
3. Boyle, R., J. P. Carey, and S. M. Highstein. Morphological to the vestibulo-ocular reflex: a functional-ablation study
correlates of response dynamics and efferent stimulation in the squirrel monkey. J. Neurosci. 11: 1636–1648,
in horizontal semicircular canal afferents of the toadfish, 1991.
Opsanus tau. J. Neurophysiol. 66: 1504–1521, 1991. 14. Peterson, E. H., J. R. Cotton, and J. W. Grant. Structural
4. Boyle, R., J. M. Goldberg, and S. M. Highstein. Inputs variation in ciliary bundles of the posterior semicircular
from regularly and irregularly discharging vestibular canal. Quantitative anatomy and computational analysis.
nerve afferents to secondary neurons in squirrel monkey Ann. NY Acad. Sci. 781: 85–102, 1996.
vestibular nuclei. III. Correlation with vestibulospinal and 15. Sato, F., and H. Sasaki. Morphological correlations
vestibuloocular output pathways. J. Neurophysiol. 68: between spontaneously discharging primary vestibular
471–496, 1992. afferents and vestibular nucleus neurons in the cat. J.
5. Brichta, A. M., and J. M. Goldberg. Afferent and efferent Comp. Neurol. 333: 554–566, 1993.
In Forthcoming Issue
Calcium Channels Formed by Mammalian Trp Homologues
Xi Zhu and Lutz Birnbaumer
Do Cellular Heat Acclimation Responses Modulate
Central Thermoregulatory Activity?
Michal Horowitz
Two Subgroups of Gonadotropin-Releasing Hormone
Neurons Control Gonadotropin Secretion in Rats
Fukuko Kimura and Toshiya Funabashi
News Physiol. Sci. • Volume 13 • August 1998 201
Downloaded from www.physiology.org/journal/physiologyonline by ${individualUser.givenNames} ${individualUser.surname} (082.242.110.027) on May 15, 2018.
Copyright © 1998 American Physiological Society. All rights reserved.
Vous aimerez peut-être aussi
- Neurobiology of Sleep and MemoryD'EverandNeurobiology of Sleep and MemoryJames McGaughPas encore d'évaluation
- Serotonin Neurons, Neuroplasticity, and Homeostasis of Neural TissueDocument13 pagesSerotonin Neurons, Neuroplasticity, and Homeostasis of Neural TissueFuyumi RiaPas encore d'évaluation
- Extra Credit Neuroscience TwoDocument3 pagesExtra Credit Neuroscience TwoCaroline SummerPas encore d'évaluation
- The Neural Mechanisms of Gustation: A Distributed Processing CodeDocument12 pagesThe Neural Mechanisms of Gustation: A Distributed Processing CodesylPas encore d'évaluation
- Morphological and Functional Diversity of First-Order Somatosensory NeuronsDocument10 pagesMorphological and Functional Diversity of First-Order Somatosensory NeuronsgiorgiocrucittiPas encore d'évaluation
- Sleep, Emotions, and Visceral Control: I. N. Pigarev and M. L. PigarevaDocument12 pagesSleep, Emotions, and Visceral Control: I. N. Pigarev and M. L. PigarevaMariaPas encore d'évaluation
- Walsh 2000 PDFDocument7 pagesWalsh 2000 PDFChrysoula GkaniPas encore d'évaluation
- 2008 - Ehninger, Kempermann - Neurogenesis in The Adult HippocampusDocument8 pages2008 - Ehninger, Kempermann - Neurogenesis in The Adult HippocampusNityananda PortelladaPas encore d'évaluation
- Generalized Potential of Adult Neural Stem Cells: EportsDocument5 pagesGeneralized Potential of Adult Neural Stem Cells: EportsmisterxPas encore d'évaluation
- Currie, 2016 PDFDocument18 pagesCurrie, 2016 PDF22gaefPas encore d'évaluation
- Plant Development: Brassinosteroids Go Out of Bounds: PTN 1 PTN 2 ADocument3 pagesPlant Development: Brassinosteroids Go Out of Bounds: PTN 1 PTN 2 AAle LuPas encore d'évaluation
- Papanicolaou - Brain Mechanisms For Reading in Children With and Without Dyslexia A Review of Studies of Normal Development and PlasticityDocument21 pagesPapanicolaou - Brain Mechanisms For Reading in Children With and Without Dyslexia A Review of Studies of Normal Development and PlasticityBrittany Caroline BlackPas encore d'évaluation
- Review Article TekuDocument9 pagesReview Article Tekushubhamatilkar04Pas encore d'évaluation
- Neurogenese AdultosDocument17 pagesNeurogenese AdultosDenise GarciaPas encore d'évaluation
- Sleep Memory Processing The Sequential Hypothesis (Theoretical Lens)Document8 pagesSleep Memory Processing The Sequential Hypothesis (Theoretical Lens)Sebastian CrayPas encore d'évaluation
- Study of Neurosecretory Cells in OdonataDocument16 pagesStudy of Neurosecretory Cells in Odonatashubhamatilkar04Pas encore d'évaluation
- Patzke 2013Document23 pagesPatzke 2013Kamilla souzaPas encore d'évaluation
- The Functional Utility of Consciousness Depends On Content As Well As On StateDocument73 pagesThe Functional Utility of Consciousness Depends On Content As Well As On StatePusat Bahasa NasionalPas encore d'évaluation
- Organelos en Sueño PDFDocument14 pagesOrganelos en Sueño PDFLEISLY TATIANA OVIEDO GOMEZPas encore d'évaluation
- 幹細胞與前瞻Document14 pages幹細胞與前瞻lunamedinaPas encore d'évaluation
- References: Section I / GeneralDocument10 pagesReferences: Section I / GeneralAndreea LăzăroiuPas encore d'évaluation
- Posner Attentional NetworksDocument5 pagesPosner Attentional NetworksBarnabyDPas encore d'évaluation
- Cognitive Map NobelDocument2 pagesCognitive Map NobelSakditad SaowapaPas encore d'évaluation
- Pain and Its Control in R 2018 Veterinary Clinics of North America Exotic ADocument16 pagesPain and Its Control in R 2018 Veterinary Clinics of North America Exotic AYaiza Garcia CasadoPas encore d'évaluation
- Thalamus Modulates Consciousness Via Layer Specific Control of Cortex Michelle J Redinbaugh Jessica M Phillips Niranjan A Kambi Sounak Mohanta Samantha Andryk Gaven L Full ChapterDocument46 pagesThalamus Modulates Consciousness Via Layer Specific Control of Cortex Michelle J Redinbaugh Jessica M Phillips Niranjan A Kambi Sounak Mohanta Samantha Andryk Gaven L Full Chapterronald.kenworthy366100% (7)
- Thalamic Neuron TheoryDocument18 pagesThalamic Neuron TheorybazediPas encore d'évaluation
- Types of Neurons in The Enteric Nervous SystemDocument10 pagesTypes of Neurons in The Enteric Nervous SystemErnesto Ochoa MonroyPas encore d'évaluation
- Miller2012 Braqin TeasersDocument1 pageMiller2012 Braqin Teasersiulia andreeaPas encore d'évaluation
- Neuroanatomic Connectivity of The Human Ascending ArousalDocument16 pagesNeuroanatomic Connectivity of The Human Ascending ArousalBritany SantanaPas encore d'évaluation
- Evidence For A Life-Span Theory of Socioemotional SelectivityDocument7 pagesEvidence For A Life-Span Theory of Socioemotional SelectivityNadia Oyarzo MillarPas encore d'évaluation
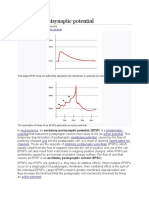
- Excitatory Postsynaptic Potential: Jump To Navigation Jump To SearchDocument6 pagesExcitatory Postsynaptic Potential: Jump To Navigation Jump To SearchJennie KimPas encore d'évaluation
- Mr. Phinoj K Abraham (Moth) : Assistant Professor, SRM College of Occupational TherapyDocument34 pagesMr. Phinoj K Abraham (Moth) : Assistant Professor, SRM College of Occupational TherapyKazi AijazPas encore d'évaluation
- Cereb. Cortex 2003 Duan 950 61Document12 pagesCereb. Cortex 2003 Duan 950 61AlfonsoVillarroelCalistoPas encore d'évaluation
- Autonomic NeuroscienceDocument13 pagesAutonomic NeuroscienceReila KnbsPas encore d'évaluation
- Sonic HedgehogDocument11 pagesSonic HedgehogPaola Rivera ÁvilaPas encore d'évaluation
- Referencia 39 2021 Kitazawa Mutacion Adrb1-A187vDocument20 pagesReferencia 39 2021 Kitazawa Mutacion Adrb1-A187vKaren RoblesPas encore d'évaluation
- MicrosDocument11 pagesMicrosEka JuliantaraPas encore d'évaluation
- Journal Pbio 3002242Document37 pagesJournal Pbio 3002242Raymundo Lopez NPas encore d'évaluation
- Gallup Contagious Yawning Systematic ReviewDocument13 pagesGallup Contagious Yawning Systematic ReviewShaelynPas encore d'évaluation
- Ajbsr MS Id 000960Document14 pagesAjbsr MS Id 000960TrishaPas encore d'évaluation
- Natural Waking and Sleep State A View From Inside Neocortical NeuronsDocument17 pagesNatural Waking and Sleep State A View From Inside Neocortical NeuronsLeslie AcevesPas encore d'évaluation
- Sleep 1Document40 pagesSleep 1Sst YadavPas encore d'évaluation
- Homer1a Is A Core Brain Molecular Correlate of Sleep LossDocument6 pagesHomer1a Is A Core Brain Molecular Correlate of Sleep LosswestPas encore d'évaluation
- Blume Et Al - 2018 - Standing Sentinel During Human SleepDocument11 pagesBlume Et Al - 2018 - Standing Sentinel During Human SleepChandan ShandilPas encore d'évaluation
- Vivar 2016 Running Rewires The Neuronal Network of Adult-Born DentateDocument14 pagesVivar 2016 Running Rewires The Neuronal Network of Adult-Born DentateJosé Luis García MezaPas encore d'évaluation
- Latest Abstract and Researches About MotivationDocument2 pagesLatest Abstract and Researches About MotivationJoymi ClairePas encore d'évaluation
- Sistem Saraf 2Document7 pagesSistem Saraf 2Neza SepteviaPas encore d'évaluation
- The Midbrain Periaqueductal Gray As An Integrative and Interoceptive Neural Structure For BreathingDocument10 pagesThe Midbrain Periaqueductal Gray As An Integrative and Interoceptive Neural Structure For BreathingAlessandra LacerdaPas encore d'évaluation
- Synapse and MemoryDocument19 pagesSynapse and Memorysomya mathurPas encore d'évaluation
- Forebrain Engraftment by Human Glial Progenitor Cells Enhances Synaptic Plasticity and Learning in Adult MiceDocument22 pagesForebrain Engraftment by Human Glial Progenitor Cells Enhances Synaptic Plasticity and Learning in Adult MiceAylin Lidsay Feria GPas encore d'évaluation
- (Advances in Cell Aging and Gerontology 17) Mark P. Mattson (Eds.) - Sleep and Aging-Academic Press, Elsevier (2005)Document192 pages(Advances in Cell Aging and Gerontology 17) Mark P. Mattson (Eds.) - Sleep and Aging-Academic Press, Elsevier (2005)Marcos R Galvão BatistaPas encore d'évaluation
- Neonate Movement Is Synchronized With Adult SpeechDocument3 pagesNeonate Movement Is Synchronized With Adult SpeechMarie Christine laznikPas encore d'évaluation
- Anafi Kayser and Raizen Nature Reviews Neuroscience 2018Document8 pagesAnafi Kayser and Raizen Nature Reviews Neuroscience 2018Francisco MtzPas encore d'évaluation
- Spinal Interneurons and Preganglionic Neurons in Sacral Autonomic Reflex PathwaysDocument15 pagesSpinal Interneurons and Preganglionic Neurons in Sacral Autonomic Reflex PathwaysNurRahMatPas encore d'évaluation
- The Neurologyof Sleep 2012 Published FormDocument18 pagesThe Neurologyof Sleep 2012 Published FormNeurologie PediatricaPas encore d'évaluation
- Communication From The Cerebellum To The Neocortex During Sleep SpindlesDocument1 pageCommunication From The Cerebellum To The Neocortex During Sleep SpindlesDavid NazarovPas encore d'évaluation
- Nihms 710720Document32 pagesNihms 710720Estigma Universidad Del RosarioPas encore d'évaluation
- Henn FA, Biol Psych 56, 2004Document5 pagesHenn FA, Biol Psych 56, 2004etaylorbensonPas encore d'évaluation
- Edwards - Neck Muscle Afferents Influence Oromotor and Cardiorespiratory CircuitsDocument16 pagesEdwards - Neck Muscle Afferents Influence Oromotor and Cardiorespiratory CircuitssbachorickPas encore d'évaluation
- UC San Diego Previously Published WorksDocument4 pagesUC San Diego Previously Published WorksGokushimakPas encore d'évaluation
- 12 ReferencesDocument26 pages12 ReferencesramopavelPas encore d'évaluation
- The Glymphatic System: A Novel Component of Fundamental NeurobiologyDocument14 pagesThe Glymphatic System: A Novel Component of Fundamental NeurobiologyramopavelPas encore d'évaluation
- Appendix Rotations in 3-D: A.1 Coordinate FramesDocument5 pagesAppendix Rotations in 3-D: A.1 Coordinate FramesramopavelPas encore d'évaluation
- Chapter Six Experimental Protocol: Study 1A 1B 2A 2B Subjects Stimulus TargetDocument15 pagesChapter Six Experimental Protocol: Study 1A 1B 2A 2B Subjects Stimulus TargetramopavelPas encore d'évaluation
- Chapter Two Ocular Motor System: Class Main Function Fixations Vestibular OptokineticDocument17 pagesChapter Two Ocular Motor System: Class Main Function Fixations Vestibular OptokineticramopavelPas encore d'évaluation
- Chapter Five Data Acquisition: 5.2a Search CoilsDocument7 pagesChapter Five Data Acquisition: 5.2a Search CoilsramopavelPas encore d'évaluation
- Aerobic Exerc Myotrophiesvoet2010Document34 pagesAerobic Exerc Myotrophiesvoet2010ramopavelPas encore d'évaluation
- Herbert Marcuse - Aggressiveness in Advanced Industrial SocietyDocument17 pagesHerbert Marcuse - Aggressiveness in Advanced Industrial SocietyHagay BarPas encore d'évaluation
- Chapter Eight Study OneDocument30 pagesChapter Eight Study OneramopavelPas encore d'évaluation
- Of Immune-Neuroendocrine Interactions: NetworkDocument12 pagesOf Immune-Neuroendocrine Interactions: NetworkramopavelPas encore d'évaluation
- Hell and Heaven, Nature and PersonDocument24 pagesHell and Heaven, Nature and PersonAlex ZammitPas encore d'évaluation
- Bosanquet Etal DMCN 2013Document9 pagesBosanquet Etal DMCN 2013ramopavelPas encore d'évaluation
- Chapter Three Vestibular SystemDocument22 pagesChapter Three Vestibular SystemramopavelPas encore d'évaluation
- MERS Coronavirus: Diagnostics, Epidemiology and TransmissionDocument21 pagesMERS Coronavirus: Diagnostics, Epidemiology and TransmissionramopavelPas encore d'évaluation
- Vestibulr NP Jn.00009.2016Document9 pagesVestibulr NP Jn.00009.2016ramopavelPas encore d'évaluation
- Loadin Startle ResponsDocument5 pagesLoadin Startle ResponsramopavelPas encore d'évaluation
- Journal of Clinical Microbiology-2020-Chan-JCM.00310-20.full PDFDocument33 pagesJournal of Clinical Microbiology-2020-Chan-JCM.00310-20.full PDFDwi Ratna KarimPas encore d'évaluation
- Hess Vest Phys Physiologyonline.2001.16.5.234Document5 pagesHess Vest Phys Physiologyonline.2001.16.5.234ramopavelPas encore d'évaluation
- Hess Vest Phys Physiologyonline.2001.16.5.234Document17 pagesHess Vest Phys Physiologyonline.2001.16.5.234ramopavelPas encore d'évaluation
- Escape From Homeostasis: Spinal Microcircuits and Progression of Amyotrophic Lateral SclerosisDocument13 pagesEscape From Homeostasis: Spinal Microcircuits and Progression of Amyotrophic Lateral SclerosisramopavelPas encore d'évaluation
- Einspieler Etal JPsychol 2008Document7 pagesEinspieler Etal JPsychol 2008ramopavelPas encore d'évaluation
- Alexandre Dumas 1845 Familia DeMediciDocument7 pagesAlexandre Dumas 1845 Familia DeMediciramopavelPas encore d'évaluation
- Vestibular Jn.01049.2015Document16 pagesVestibular Jn.01049.2015ramopavelPas encore d'évaluation
- Development of Postural Adjustments During Reaching in Infants at Risk For Cerebral Palsy From 4 To 18 MonthsDocument9 pagesDevelopment of Postural Adjustments During Reaching in Infants at Risk For Cerebral Palsy From 4 To 18 MonthsramopavelPas encore d'évaluation
- Alexandre Dumas 1845 Familia DeMediciDocument7 pagesAlexandre Dumas 1845 Familia DeMediciramopavelPas encore d'évaluation
- Vest jn.00078.2014Document11 pagesVest jn.00078.2014ramopavelPas encore d'évaluation
- Hess Vest Phys Physiologyonline.2001.16.5.234Document5 pagesHess Vest Phys Physiologyonline.2001.16.5.234ramopavelPas encore d'évaluation
- Konrad Lorenz The Analogy As A Source of KnowledgeDocument17 pagesKonrad Lorenz The Analogy As A Source of KnowledgeramopavelPas encore d'évaluation
- Cervical SpineDocument69 pagesCervical Spineveravero100% (3)
- Sci10 LM U3Document131 pagesSci10 LM U3Romel B Agno86% (7)
- Cns PathologyDocument18 pagesCns Pathologysunnyorange8Pas encore d'évaluation
- Assessing Sciatic Nerve Glide, Part II (Myofascial Techniques)Document4 pagesAssessing Sciatic Nerve Glide, Part II (Myofascial Techniques)Advanced-Trainings.com100% (7)
- All Type Essays - Biology emDocument27 pagesAll Type Essays - Biology emjoker boy100% (1)
- Human Nervous SystemDocument28 pagesHuman Nervous SystemMuhammad HaqPas encore d'évaluation
- Unifying Themes in The Study of LifeDocument7 pagesUnifying Themes in The Study of LifeKyla Renz de Leon100% (5)
- Chapter 17 Biology 2nd Year - Prof. Ijaz Ahmed Khan Abbasi (Lecturer Biology PGC) Notes - MDCAT by FUTURE DOCTORS - Touseef Ahmad Khan - 03499815886Document36 pagesChapter 17 Biology 2nd Year - Prof. Ijaz Ahmed Khan Abbasi (Lecturer Biology PGC) Notes - MDCAT by FUTURE DOCTORS - Touseef Ahmad Khan - 03499815886Suffa Academy100% (3)
- Geoffrey Jefferson (Contra Turing) - The Mind of Mechanical Man (CFR J Bruner)Document6 pagesGeoffrey Jefferson (Contra Turing) - The Mind of Mechanical Man (CFR J Bruner)Anonymous JIc7R6Pas encore d'évaluation
- Which of The Following Is The Component of Central Nervous System?Document28 pagesWhich of The Following Is The Component of Central Nervous System?nisaPas encore d'évaluation
- ALL DR Robert O. Becker: Silver Iontophoresis (Regeneration by Silver Ions)Document28 pagesALL DR Robert O. Becker: Silver Iontophoresis (Regeneration by Silver Ions)StellaEstel100% (1)
- Hes 029 Lab Sas 2Document2 pagesHes 029 Lab Sas 2Crislyn Jilou Bugas AdlawanPas encore d'évaluation
- The Main Parts of The Eye and Their FunctionDocument5 pagesThe Main Parts of The Eye and Their FunctionJoshuaPas encore d'évaluation
- Marcus Jacobson (Auth.) - Foundations of Neuroscience (1993)Document394 pagesMarcus Jacobson (Auth.) - Foundations of Neuroscience (1993)patata100% (1)
- 2301-Human Brain Apparent BoonDocument103 pages2301-Human Brain Apparent BoonSubramanya Seshagiri0% (1)
- CH 8 Nervous SystemPractice Test and KeyDocument13 pagesCH 8 Nervous SystemPractice Test and KeyvpcpharmdPas encore d'évaluation
- A. Multiple Choice. Answer TheDocument1 pageA. Multiple Choice. Answer TheJoeric CarinanPas encore d'évaluation
- Tissues - McqsDocument5 pagesTissues - McqsDocumentSharerPas encore d'évaluation
- PDF - Sat Practice Test 10 Answers PDFDocument50 pagesPDF - Sat Practice Test 10 Answers PDFAryan ShahPas encore d'évaluation
- The MELT Method by Sue HitzmannDocument20 pagesThe MELT Method by Sue HitzmannHarperOne (an imprint of HarperCollins)67% (6)
- Definition of Medical EtymologyDocument2 pagesDefinition of Medical EtymologyOny Hertriandita APas encore d'évaluation
- 06a - Introduction To Spinal Cord - NeuroanatomyDocument9 pages06a - Introduction To Spinal Cord - Neuroanatomyhiba jasimPas encore d'évaluation
- Science6 q2 Mod3 Explain How The Different Organ System v2Document28 pagesScience6 q2 Mod3 Explain How The Different Organ System v2Mark James VallejosPas encore d'évaluation
- Neurotoxin Protocol Jan06Document19 pagesNeurotoxin Protocol Jan06mary eng100% (2)
- Sistem SarafDocument141 pagesSistem SarafPei YiingPas encore d'évaluation
- Brain NeuroanatomyDocument227 pagesBrain NeuroanatomyNkechukwu OgakwuPas encore d'évaluation
- SUSAN R - JOHNSON, M - D - On Television and Our Children's MindsDocument11 pagesSUSAN R - JOHNSON, M - D - On Television and Our Children's MindsSusan HoekstraPas encore d'évaluation
- 2014 Neurogenic NeuroinflammationDocument11 pages2014 Neurogenic NeuroinflammationrendyjiwonoPas encore d'évaluation
- Neural Mobilizationw10Document33 pagesNeural Mobilizationw10manjukumard2007Pas encore d'évaluation
- Meningitis (Deadly Diseases and Epidemics) (PDFDrive)Document121 pagesMeningitis (Deadly Diseases and Epidemics) (PDFDrive)Islamiyatul Hayati MiswanPas encore d'évaluation
- Biological Basis of PersonalityDocument58 pagesBiological Basis of PersonalityAldrian Maulion EvangelistaPas encore d'évaluation