Académique Documents
Professionnel Documents
Culture Documents
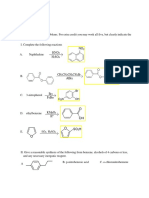
Afinidad Protónica y Sitios de Protonación en P-Nitroanilina
Transféré par
Fabian Loor CadenaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Afinidad Protónica y Sitios de Protonación en P-Nitroanilina
Transféré par
Fabian Loor CadenaDroits d'auteur :
Formats disponibles
REVISTA COLOMBIANA DE QUÍMICA, VOLUMEN 38, No.
1 DE 2 0 0 9
Moller Plesset (MP2). O efeito conjunto aniline is still a subject of debate: two
dos substituintes conduz a estabilidades protonated isomers have been found ex-
relativas para as espécies protonadas de perimentally (2); computationally, coe-
acordo com a seguinte escala: grupo nitro xistence of two isomers with slight
> grupo amino > carbono orto > car- energy preference towards protonation
bono para > carbono meta > carbono on the para carbon to the amino group is
ipso (posições relativas ao grupo amino). predicted (3). Regarding paranitroanili-
ne, experimental studies suggest that the
Palavras-chave: afinidad protónica, preferred protonation sites are the oxy¬
reactividad do anel aromático, efeitos in- gens belonging to the nitro group (4,5).
dutivos competitivos, p-Nitroanilina.
The various inductive effects on anili-
INTRODUCTION ne, nitrobenzene and paranitroaniline are
depicted in Figures 1-4. Atom charges
In Organic Chemistry, substituent effects (Figure 1) were calculated under the
are traditionally described in a qualitative CHELPG fitting scheme to the electrosta-
fashion. Substituent inductive effects are tic potential (6), resonant structures for
helpful in predicting the behavior and aniline (Figure 2), nitrobenzene (Figure
reactivity of many molecules, but they are 3) and p-nitroaniline (Figure 4) are drawn
not enough for a complete description in considering -NH as an electron donor
2
the cases where multiple substitution with group and -NO as an electron withdra¬
2
opposite effects are present. For exam¬ wing group. Charge distribution on the
ple, relative basicity criteria establish that ring in paranitroaniline resembles the
aniline protonation is favored on the ni¬ charge distribution in aniline, which sug¬
trogen atom of the amino group, predic¬ gests a dominant effect from the amino
tions that are in agreement with liquid group. The significant negative charges
phase experimental results (1). On the ot¬ on the nitrogen and oxygen atoms and on
her hand, gas phase protonation sites of the ortho carbon on p-nitroaniline makes
Figure 1. CHELPG charges on aniline, nitrobenzene and p-nitroaniline calculated at the B3LYP/6-31G*
level.
128
Vous aimerez peut-être aussi
- Lab Report - Thermodynamics and Kinetics of A Substitution Reaction of A Metal ComplexDocument16 pagesLab Report - Thermodynamics and Kinetics of A Substitution Reaction of A Metal ComplexValerie MangasarPas encore d'évaluation
- Spectrochimica Acta Part A: Molecular and Biomolecular SpectrosDocument15 pagesSpectrochimica Acta Part A: Molecular and Biomolecular SpectrosSergio Mauricio Betancur HincapiePas encore d'évaluation
- AnilineIJQC00 PDFDocument9 pagesAnilineIJQC00 PDFgunjaguptaPas encore d'évaluation
- Analysis of Results in Nuclear Magnetic Resonance (NMR) SpectrosDocument8 pagesAnalysis of Results in Nuclear Magnetic Resonance (NMR) SpectrostypodleePas encore d'évaluation
- AnilineIJQC00 PDFDocument9 pagesAnilineIJQC00 PDFStephen BrooksPas encore d'évaluation
- NMR interpretation key to structure determinationDocument16 pagesNMR interpretation key to structure determinationChandni SahaPas encore d'évaluation
- NMR Spectral Studies of Some Six-Membered and Seven-Membered Saturated Heterocyclic Compound T (3) - Ethyl-R (2), C (7) - Diphenylhomopiperazin-5-OneDocument3 pagesNMR Spectral Studies of Some Six-Membered and Seven-Membered Saturated Heterocyclic Compound T (3) - Ethyl-R (2), C (7) - Diphenylhomopiperazin-5-OneanitaPas encore d'évaluation
- Positive Inductive Effect of Methyl Groups in Nine Simple AlcoholsDocument12 pagesPositive Inductive Effect of Methyl Groups in Nine Simple Alcoholsmostafa barakatPas encore d'évaluation
- Content ServerDocument9 pagesContent ServerHadjer HadjerPas encore d'évaluation
- Wang 2001Document6 pagesWang 2001binaro8043Pas encore d'évaluation
- DownloadDocument8 pagesDownloadcesar8319Pas encore d'évaluation
- 8447 1384066805 Lectures.1-4.polyDocument50 pages8447 1384066805 Lectures.1-4.polyPragatiPas encore d'évaluation
- Generation, Structure and Reactivity of Arynes: A Theoretical StudyDocument12 pagesGeneration, Structure and Reactivity of Arynes: A Theoretical StudyUmar FarooqPas encore d'évaluation
- 304-Article Text-485-1-10-20220226Document5 pages304-Article Text-485-1-10-20220226MasoodPas encore d'évaluation
- The Characterization of α-PyrrolidinopentiophenoneDocument6 pagesThe Characterization of α-PyrrolidinopentiophenonetysonPas encore d'évaluation
- 10 2116analsci 18 997Document6 pages10 2116analsci 18 997Selma HassuonPas encore d'évaluation
- Manlio T20056443Document9 pagesManlio T20056443api-19793040Pas encore d'évaluation
- Key For Dec 2022 - Jan 2023 PaperDocument13 pagesKey For Dec 2022 - Jan 2023 PaperDivya ReddyPas encore d'évaluation
- Vidyalankar: IIT-202 3: ChemistryDocument18 pagesVidyalankar: IIT-202 3: ChemistrySwaroop NaikPas encore d'évaluation
- 31P NMR Spectroscopic Investigations of Low Cordinated Multiple Bonded PN Systemes - Niecke & GudatDocument16 pages31P NMR Spectroscopic Investigations of Low Cordinated Multiple Bonded PN Systemes - Niecke & GudatRAMONA RICLEAPas encore d'évaluation
- The CP-asymmetry in Resonant Leptogenesis - A. Anisimov 2005Document15 pagesThe CP-asymmetry in Resonant Leptogenesis - A. Anisimov 2005FRANK BULA MARTINEZPas encore d'évaluation
- Acidezdefenis JOC RepositoryDocument4 pagesAcidezdefenis JOC RepositoryUsama WaleedPas encore d'évaluation
- Chem 242 - Chapters 1&2 PDFDocument30 pagesChem 242 - Chapters 1&2 PDFKhaled AbeedPas encore d'évaluation
- Luminescence Probe Studies of Nafion PolyelectrolytesDocument5 pagesLuminescence Probe Studies of Nafion PolyelectrolytesLuis AlvarezPas encore d'évaluation
- Nuclear Chemistry BasicsDocument98 pagesNuclear Chemistry BasicsEGAS JAYSON RABEPas encore d'évaluation
- Aromatic AssignmentDocument5 pagesAromatic Assignmentowegibrian479Pas encore d'évaluation
- Molecular Structure HOMO LUMO MEP Natural Bond OrbDocument13 pagesMolecular Structure HOMO LUMO MEP Natural Bond OrbRakhel Dayanne SilvaPas encore d'évaluation
- NMR Caracterization - 2022-2023Document21 pagesNMR Caracterization - 2022-2023Paula ArmendárizPas encore d'évaluation
- PNAS 2008 Blanc 12123 7Document5 pagesPNAS 2008 Blanc 12123 7Le NhanPas encore d'évaluation
- Nuclear Magnetic Resonance SpectrosDocument40 pagesNuclear Magnetic Resonance SpectrosPragnesh ParmarPas encore d'évaluation
- 3 CML-101 - Determination of Reaction MechanismDocument53 pages3 CML-101 - Determination of Reaction MechanismChetram meenaPas encore d'évaluation
- Chjv01i04p0209 PDFDocument11 pagesChjv01i04p0209 PDFchemistryjournalPas encore d'évaluation
- Electrofugalities of 1,3-Diarylallyl Cations: Konstantin Troshin and Herbert MayrDocument12 pagesElectrofugalities of 1,3-Diarylallyl Cations: Konstantin Troshin and Herbert MayrSandipan SahaPas encore d'évaluation
- Geometry of N-BenzylideneanilineDocument5 pagesGeometry of N-BenzylideneanilineTheo DianiarikaPas encore d'évaluation
- Experimental and Theoretical Studies of The Photophysical Properties of 2-And 2,7-Functionalized Pyrene DerivativesDocument14 pagesExperimental and Theoretical Studies of The Photophysical Properties of 2-And 2,7-Functionalized Pyrene DerivativesBarry LorentzPas encore d'évaluation
- Article: Birgit Weber, F. Ann Walker, and Konstantin KaraghiosoffDocument6 pagesArticle: Birgit Weber, F. Ann Walker, and Konstantin KaraghiosoffArnau Dominguez ZoroaPas encore d'évaluation
- Pertemuan 11 LFER TutorialDocument11 pagesPertemuan 11 LFER TutorialSarah CipawPas encore d'évaluation
- Lai 1992Document7 pagesLai 1992Saurav PaulPas encore d'évaluation
- Quantum Mechanical Study of Regioselectivity of Radical Additions To Substituted OlefinsDocument17 pagesQuantum Mechanical Study of Regioselectivity of Radical Additions To Substituted Olefinssepot24093Pas encore d'évaluation
- Chemistry-Orgo II Exam 1 Version A (UD) Answer KeyDocument8 pagesChemistry-Orgo II Exam 1 Version A (UD) Answer KeyNesrine LaradjiPas encore d'évaluation
- Organic Chemistry-II SNAr MechanismDocument13 pagesOrganic Chemistry-II SNAr MechanismPackianathan SarathPas encore d'évaluation
- Herbert Brown and Edward N. Peters' : Abstract: 80% or (503 000)Document5 pagesHerbert Brown and Edward N. Peters' : Abstract: 80% or (503 000)Liz HansPas encore d'évaluation
- 10523-Etarjome EnglishDocument7 pages10523-Etarjome Englishtieum9761Pas encore d'évaluation
- Journal of Luminescence: SciencedirectDocument15 pagesJournal of Luminescence: SciencedirectRuan ReisPas encore d'évaluation
- Vac Hula 1986Document8 pagesVac Hula 1986KerryPas encore d'évaluation
- J Molstruc 2019 127104Document8 pagesJ Molstruc 2019 127104Mohammed OdayPas encore d'évaluation
- Polymers. Liquid Crystals: N. V. Agrinskaya and V. A. LukoshkinDocument4 pagesPolymers. Liquid Crystals: N. V. Agrinskaya and V. A. LukoshkinEdgar Blanco AcuñaPas encore d'évaluation
- Functional Groups Recap: Carboxylic Acids, Aldehydes, KetonesDocument34 pagesFunctional Groups Recap: Carboxylic Acids, Aldehydes, KetonesNoh MohamedPas encore d'évaluation
- Atomic Spectrometry OverviewDocument10 pagesAtomic Spectrometry Overviewzwei animePas encore d'évaluation
- Structural 2-Arylnorbornyl Other Nuclear Magnetic: Carbocations Increasing Electron DemandDocument9 pagesStructural 2-Arylnorbornyl Other Nuclear Magnetic: Carbocations Increasing Electron Demandyonadime922Pas encore d'évaluation
- Nuclear Chemistry LectureDocument12 pagesNuclear Chemistry LectureAMLU Law OfficesPas encore d'évaluation
- McMurry OC8e EV CH13 PDFDocument28 pagesMcMurry OC8e EV CH13 PDFCrizel Ricaro100% (1)
- Experimental and Theoretical IR and Raman Spectra of Picolinic, Nicotinic and Isonicotinic AcidsDocument7 pagesExperimental and Theoretical IR and Raman Spectra of Picolinic, Nicotinic and Isonicotinic AcidsJose GonzalezPas encore d'évaluation
- 2007 Peptide Nucleic AzcidsDocument4 pages2007 Peptide Nucleic Azcidsapi-19793040Pas encore d'évaluation
- Synthesis of Pyrimidine-Containing 3-AminobutenolidesDocument4 pagesSynthesis of Pyrimidine-Containing 3-Aminobutenolidesapi-19793040Pas encore d'évaluation
- Molecular Structure: To On 14N-14NDocument5 pagesMolecular Structure: To On 14N-14NAFrodita AzarPas encore d'évaluation
- Dr. Andrew Knox's Contact DetailsDocument45 pagesDr. Andrew Knox's Contact DetailskeatyPas encore d'évaluation
- Investigation 3 eDocument12 pagesInvestigation 3 ejilnaPas encore d'évaluation
- Stereoelectronic Effects: A Bridge Between Structure and ReactivityD'EverandStereoelectronic Effects: A Bridge Between Structure and ReactivityPas encore d'évaluation
- Sample Paper 1 (Solutions Only) - IsC Chemistry 2024Document17 pagesSample Paper 1 (Solutions Only) - IsC Chemistry 2024Dia SureshPas encore d'évaluation
- Review For FinalsDocument54 pagesReview For FinalsChristianAvelinoPas encore d'évaluation
- Covalent BondingDocument52 pagesCovalent BondingTanvir MatharuPas encore d'évaluation
- Chem 2412 Lecture 01Document28 pagesChem 2412 Lecture 01Jihee YoonPas encore d'évaluation
- PyridineDocument5 pagesPyridineMohini BajajPas encore d'évaluation
- Chapters 1, 2 and 3 Study Guide Organic ChemisryDocument34 pagesChapters 1, 2 and 3 Study Guide Organic ChemisryNeedBooksDontSuePas encore d'évaluation
- 1-Amino-3-Nitroguanidine (ANQ) in High-Performance Ionic Energetic MaterialsDocument16 pages1-Amino-3-Nitroguanidine (ANQ) in High-Performance Ionic Energetic MaterialsWaldyr NoronhaPas encore d'évaluation
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishorePas encore d'évaluation
- Ionic and Covalent Bondings - 48 SlidesDocument47 pagesIonic and Covalent Bondings - 48 SlidesanisghaniPas encore d'évaluation
- Chemical BondingDocument36 pagesChemical BondingMohitPas encore d'évaluation
- Organic ChemistryDocument67 pagesOrganic ChemistryOlga DeePas encore d'évaluation
- 2018 U.S. National Chemistry Olympiad ExamDocument13 pages2018 U.S. National Chemistry Olympiad Exam......Pas encore d'évaluation
- Organic Chemistry 9th Edition Carey Test BankDocument17 pagesOrganic Chemistry 9th Edition Carey Test Bankconalkeishaywx100% (25)
- Course 201N 1 Semester 2006-2007 Inorganic Chemistry Instructor: Jitendra K. BeraDocument11 pagesCourse 201N 1 Semester 2006-2007 Inorganic Chemistry Instructor: Jitendra K. BeraanoopPas encore d'évaluation
- Haloalkanes and Haloarenes-1Document34 pagesHaloalkanes and Haloarenes-1Subhransu Sekhar BarikPas encore d'évaluation
- Organic Chemistry Mechanistic Patterns Canadian 1st Edition Ogilvie Solutions Manual DownloadDocument64 pagesOrganic Chemistry Mechanistic Patterns Canadian 1st Edition Ogilvie Solutions Manual DownloadLaura Simpson100% (22)
- Kji NYISS5 M UDocument459 pagesKji NYISS5 M UAman PharmacyCollegePas encore d'évaluation
- Orbital and Bonding Concepts in Organic ChemistryDocument94 pagesOrbital and Bonding Concepts in Organic ChemistryDeither EdloyPas encore d'évaluation
- Group 6 - Lab 5 - Organic Reaction MechanismsDocument5 pagesGroup 6 - Lab 5 - Organic Reaction MechanismsCloudetteMendozaPas encore d'évaluation
- General Organic ChemistryDocument31 pagesGeneral Organic Chemistryshitaldr767Pas encore d'évaluation
- W2-CHM1052 2021 Lecture SlidesDocument34 pagesW2-CHM1052 2021 Lecture SlidesaqidahPas encore d'évaluation
- Physical Organic Chemistry Chapter ThreeDocument40 pagesPhysical Organic Chemistry Chapter ThreeMULUKEN TILAHUNPas encore d'évaluation
- Jarissa Banner Nitration of Bromobnzene LabDocument15 pagesJarissa Banner Nitration of Bromobnzene LabJuiloPas encore d'évaluation
- 6 C15 Notes CH4 Chemical BondsSTEM StudentsDocument12 pages6 C15 Notes CH4 Chemical BondsSTEM StudentsDONNA JEAN ACOJEDOPas encore d'évaluation
- Molecular Geometry and Bonding Theory GuideDocument2 pagesMolecular Geometry and Bonding Theory GuideDan McPas encore d'évaluation
- Cau Truc Cong HuongDocument35 pagesCau Truc Cong HuongAnonymous cgKtuWzPas encore d'évaluation
- Aromatic HydrocarbonDocument45 pagesAromatic HydrocarbonPrashantPas encore d'évaluation
- Chemical Bonding (F Only)Document28 pagesChemical Bonding (F Only)Raju SinghPas encore d'évaluation
- Jee (Advanced) Online Test Series: Class XIIDocument1 pageJee (Advanced) Online Test Series: Class XIINameet JainPas encore d'évaluation
- Resonance Exam Preparation Pack: Answer KeyDocument13 pagesResonance Exam Preparation Pack: Answer KeyBALAJI RAMPas encore d'évaluation