Académique Documents
Professionnel Documents
Culture Documents
Relationship of Serum Uric Acid Level With Non-Alcoholic Fatty Liver Disease and Its in Ammation Progression in Non-Obese Adults
Transféré par
syifaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Relationship of Serum Uric Acid Level With Non-Alcoholic Fatty Liver Disease and Its in Ammation Progression in Non-Obese Adults
Transféré par
syifaDroits d'auteur :
Formats disponibles
Hepatology Research 2017; 47: E104–E112 doi: 10.1111/hepr.
12734
Original Article
Relationship of serum uric acid level with non-alcoholic fatty
liver disease and its inflammation progression in non-obese
adults
Jing Liu,1 Chengfu Xu,2 Limei Ying,3 Shufei Zang,4 Zhenjie Zhuang,5 Haifeng Lv,6 Wenjun Yang,7
Yan Luo,5 Xaojie Ma,1 Lei Wang,8 Yunhao Xun,9 Dewei Ye10 and Junping Shi1†
Departments of 1Liver Diseases, 4Endocrine Diseases, 5Center for Translational Medicine, and 7Pathology, The
Affiliated Hospital of Hangzhou Normal University, 2Department of Gastroenterology, The First Affiliated Hospital,
College of Medicine, Zhejiang University, 8Second Clinical Medical College, Zhejiang Chinese Medicine University,
and 9Department of Liver Diseases, Xixi Hospital of Hangzhou, Hangzhou, Zhejiang, 3Dalian University of Medicine,
Dalian, Liaoning and 10Department of Medicine, Faculty of Medicine, The University of Hong Kong, and 6Department
of Surgical Intensive Care Unit, The First Affiliated Hospital of Zhejiang University, China
Aim: This study aimed to evaluate the relationship between se- proven NAFLD, SUA levels were significantly higher in those with
rum uric acid (SUA) level and non-alcoholic fatty liver disease non-alcoholic steatohepatitis. The prevalence of non-alcoholic
(NAFLD) in non-obese adults. steatohepatitis and lobule inflammation tended to increase to
Methods: A cross-sectional study was carried out among 4098 57.58% and 66.67% as the SUA level increased to the fourth quar-
adults, including 1936 non-obese and 2162 obese individuals. An tile. Subjects with hyperuricemia had significantly higher NAFLD
additional 93 non-obese adults with biopsy-proven NAFLD were activity scores and more serious lobule inflammation than the
also included. normal group.
Results: The overall prevalence of NAFLD was 39.51% in the Conclusion: Non-obese adults have higher NAFLD risk with in-
study group, and 14.88% in non-obese adults. The NAFLD pa- creased SUA levels than obese individuals, and the inflammation
tients had significantly higher SUA levels than controls in both progression of NAFLD is associated with increased SUA level in
men and women. The non-obese group had a higher NAFLD risk non-obese subjects.
with increased SUA levels than the obese group, with odd ratios Key words: inflammation progression, non-obese adults, non-
(95% confidence interval) of 2.559 (1.870–3.503) and 1.692 alcoholic fatty liver disease, uric acid
(1.371–2.087), respectively. In 93 non-obese adults with biopsy-
INTRODUCTION Europe and North America, but also becoming increas-
ingly prevalent in the Asia-Pacific region, especially in
N ON-ALCOHOLIC FATTY LIVER disease (NAFLD) is
characterized by significant lipid deposition, usually
greater than 5% of the liver weight deposited as triglycer-
China with the prevalence of 15–30% in the general pop-
ulation.2 Non-alcoholic fatty liver disease mainly occurs in
obese individuals, but recent studies revealed that NAFLD
ide, in the liver parenchyma, without history of excessive
is also not rare in non-obese adults.3 The prevalence of
alcohol consumption. The NAFLD spectrum ranges from
NAFLD in non-obese adults was 15.2% in a Japanese study
simple steatosis (non-alcoholic fatty liver), to non-
group.4 So it is time to pay attention to NAFLD in non-
alcoholic steatohepatitis (NASH), to cirrhosis, ultimately
obese adults.
developing into hepatocellular carcinoma.1 Nowadays,
There is growing evidence that serum uric acid (SUA)
NAFLD is not only the most common liver disease in
level, the final product of purine degradation, is elevated
in metabolic syndrome (MetS) and strongly associated
Correspondence: Professor Jun-ping Shi, Department of Liver Diseases, The
with insulin resistance. The level of SUA is maintained
Affiliated Hospital of Hangzhou Normal University, 126 Wenzhou Road,
Hangzhou 310015, Zhejiang, China. Email: 13957121199@126.com by the balance between uric acid production and excre-
Conflict of interest: The authors have no conflict of interest. tion in physiological conditions. However, the preva-
Received 6 February 2016; revision 12 April 2016; accepted 26 April 2016. lence and incidence of hyperuricemia in the world
© 2016 The Japan Society of Hepatology E104
Hepatology Research 2017; 47: E104–E112 Serum uric acid level E105
population have steadily increased over the past to their examination. The examination consisted of a
40 years.5 Although uric acid is recognized as an antiox- physical examination and a health habit inventory by a
idant, hyperuricemia has been implicated in the develop- physician.
ment of gout, coronary artery disease, type 2 diabetes, Fasting blood samples were obtained from an
MetS, and NAFLD.6–9 Increased SUA has been linked to antecubital vein, and the samples were used for the
increased oxidative stress, chronic low grade inflamma- analysis of biochemical values. The values included al-
tion, and insulin resistance,10,11 which are the basic anine aminotransferase (ALT), aspartate aminotransfer-
pathophysiology of NAFLD. ase (AST), triglyceride (TG), total cholesterol (TC),
Although numerous studies have reported that SUA high-density lipoprotein cholesterol (HDL-C), low-
level is associated with NAFLD,12,13 no study has aimed density lipoprotein cholesterol (LDL-C), c-
to investigate the association of SUA level with NAFLD glutamyltransferase, SUA, and fasting plasma glucose
and its inflammation progression in non-obese adults. (FPG). All values were measured by an automatic bio-
This study aimed to investigate the clinical and histopath- chemical analyzer (model 7180; Hitachi, Tokyo, Japan)
ologic characteristics of NAFLD, and then analyze the rela- using standard methods.
tionships between SUA, NAFLD, and degree of
inflammation in non-obese adults. Ultrasonography
Hepatic ultrasonic examination was carried out in all
MATERIALS AND METHODS subjects by two trained ultrasound doctors who were
blinded to the clinical and laboratory data, using a
Study design and subjects Toshiba Nemio 20 sonography machine (Toshiba, To-
A CROSS-SECTIONAL STUDY was carried out to evalu-
ate the relationship between SUA level and NAFLD.
This study initially enrolled all of the adults who took their
kyo, Japan) with a 3.5-MHz probe. Hepatic steatosis
was diagnosed by characteristic echo patterns according
to conventional criteria, such as the evidence of diffuse
health examination at Xixi Hospital (Hangzhou, China) hyperechogenicity of the liver relative to the kidneys, ul-
and the Affiliated Hospital of Hangzhou Normal Univer- trasound beam attenuation, and poor visualization of
sity (Hangzhou, China) between January 1, 2013 and De- intrahepatic structures.14
cember 31, 2014. Subjects meeting the following criteria
were excluded: (i) those taking antihypertensive or antidi- Liver histology
abetic agents, lipid-lowering agents, or hypouricemic The histopathological diagnosis of NAFLD was
agents; (ii) those with alcohol consumption greater than established by two expert liver pathologist, blinded to
140 g/week for men and 70 g/week for women; (iii) those subjects’ details. Liver biopsy specimens were scored
with a history of other known causes of chronic liver dis- using the NASH CRN scoring system.15 In brief, the de-
ease, such as viral hepatitis or autoimmune hepatitis, and gree of steatosis, liver injury, and inflammatory activity
(iv) those using hepatotoxic medications. A total of 4098 were scored using an 8-point scale: steatosis, 0–3; lobular
eligible subjects were enrolled. An additional 93 non- inflammation, 0–3; ballooning hepatocyte degeneration,
obese adults with biopsy-proven NAFLD were also in- 0–2. The stage of fibrosis was scored using a 6-point
cluded. The study protocol was approved by the ethics scale: 1a, mild zone 3 perisinusoidal fibrosis; 1b, moder-
committee, complying with the ethical guidelines of the ate zone 3 perisinusoidal fibrosis; 1c, portal fibrosis only;
Declaration of Helsinki in 1995 (as revised in Edinburgh 2, zone 3 and portal/periportal fibrosis; 3, bridging fibro-
2000). And written informed consent was obtained from sis; 4, cirrhosis. In addition to determining the NAFLD
all subjects. activity score (NAS), an overall diagnostic categorization
was determined for each case as simple steatosis (NAS,
Clinical examination and biochemical analyses 0–2), indefinite NASH (NAS, 3–4), and NASH (NAS,
All participants underwent routine medical history and 5–8). According to the fibrosis stage, cases were divided
physical examination including blood pressure measure- into non-advanced fibrosis (stage of fibrosis, 0–2) and
ment, anthropometry, and laboratory assessments. Body advanced fibrosis.
mass index (BMI) was calculated as weight (kg) divided
by height (m) squared (kg/m2) and used as an index of Definitions and statistical analyses
body fat. Clinical examinations were undertaken in the Non-alcoholic fatty liver disease was diagnosed by abdom-
morning after an overnight fast, and subjects were also inal ultrasound following exclusion of alcohol consump-
instructed to refrain from exercise during the day prior tion and viral or autoimmune liver disease.16,17 A BMI of
© 2016 The Japan Society of Hepatology
E106 J. Liu et al. Hepatology Research 2017; 47: E104–E112
≥18.5 kg/m2 and <25 kg/m2 for non-obese subjects, and RESULTS
BMI ≥25 kg/m2 for obese subjects was defined according
Clinical and laboratory characteristics of
to the World Health Organization guidelines of categoriz-
subjects
ing obesity in Asian populations as proposed in 2000. Hy-
peruricemia was defined as an SUA level >420 μmol/L in
male subjects and >360 μmol/L in female subjects. For
men, quartiles (Q) were defined as: Q1,
O F 4098 SUBJECTS enrolled in this study, 1619
(39.51%) fulfilled NAFLD diagnostic criteria. An-
thropometric, clinical, and laboratory data of 4098 sub-
SUA ≤ 309 μmol/L; Q2, SUA 310–353 μmol/L; Q3, SUA jects are shown in Table 1. Compared with healthy
354–401 μmol/L; and Q4, SUA ≥ 402 μmol/L. For women, subjects, NAFLD patients were older, had significantly
quartiles were defined as: Q1, SUA ≤ 217 μmol/L; Q2, SUA higher BMI, FPG, TG, TC, LDL-C, ALT, AST, serum creati-
218–253 μmol/L; Q3, SUA 254–298 μmol/L; and Q4, nine (SCr), blood urea nitrogen (BUN), and SUA levels,
SUA ≤ 299 μmol/L. and lower HDL-C levels.
Statistical analyses were carried out using the SPSS There were 1936 non-obese subjects
software package version 13.0 for Windows (SPSS Inc., (18.5 ≤ BMI < 25 kg/m2) and 2162 obesity subjects
Chicago, IL, USA). Continuous variables are presented as (BMI ≥ 25 kg/m2). The prevalence of NAFLD was 14.88%
the mean and standard deviation (SD) or the median (288/1936) in the non-obese group, and 61.56% in the
and interquartile range (IQR), as appropriate. Student’s obese group. In the non-obese study group, NAFLD pa-
t-test or the Mann–Whitney U-test was used for two-group tients were older, and they had significantly higher BMI,
comparisons of continuous data; categorical variables were FPG, TG, TC, LDL-C, ALT, AST, SCr, and BUN levels, and
compared using the χ 2-test. One-way ANOVA followed by lower HDL-C levels than controls. Notably, significantly
Dunnett’s test was used for multiple group comparisons. higher SUA levels were observed in subjects with NAFLD
Linear correlation analysis was used to determine the rela- than in those without NAFLD in the non-obese group
tionship between SUA level and other parameters of (men, 368.00 ± 75.45 vs. 332.72 ± 72.82 μmol/L; women,
NAFLD patients. Multivariate logistic regression (back- 272.00 ± 71.84 vs. 232.46 ± 54.72 μmol/L) (Fig. 1a). Com-
ward Wald; entry, 0.05; removal, 0.10) was used to evalu- pared with NAFLD patients in the obese group, non-obese
ate the risk factors for NAFLD. P < 0.05 was considered patients were older and had significantly lower BMI, TG,
statistically significant. ALT, SCr, and SUA levels (Table 2). In female subjects,
Table 1 Clinical and laboratory characteristics of 4098 adults, including 1619 adults with non-alcoholic fatty liver disease (NAFLD)
Variable Total Healthy group NAFLD group P-value
n (women) 4098 (1515) 2479 (1096) 1619 (419) 0.000†
Age, years 41.73 ± 11.62 39.38 ± 11.34 45.52 ± 11.07 0.000
BMI, kg/m2 25.31 ± 3.94 23.62 ± 3.23 28.02 ± 3.45 0.000
SUA, μmol/L 316.63 ± 97.11 294.15 ± 93.94 353.08 ± 90.95 0.000
FPG, mmol/L 5.31 ± 1.32 5.12 ± 1.08 5.62 ± 1.60 0.000
TG, mmol/L 1.34 (0.88–2.08) 1.07 (0.75–163) 1.89 (1.31–2.82) 0.000‡
TC, mmol/L 4.69 ± 0.89 4.50 ± 0.92 5.00 ± 1.01 0.000
HDL-C, mmol/L 1.24 ± 0.37 1.31 ± 0.37 1.13 ± 0.35 0.000
LDL-C, mmol/L 2.88 ± 0.79 2.74 ± 0.75 3.11 ± 0.80 0.000
ALT, U/L 20.70 (14.90–30.40) 17.60 (13.00–24.90) 27.00 (19.00–39.00) 0.000‡
AST, U/L 19.00 (16.00–23.40) 18.00 (15.50–22.00) 21.00 (17.70–26.00) 0.000‡
BUN, mmol/L 5.09 ± 1.48 4.97 ± 1.43 5.29 ± 1.54 0.000
SCr, mmol/L 71.36 ± 19.58 69.80 ± 16.91 73.89 ± 23.04 0.000
Data are expressed as mean ± standard deviation or median (interquartile range).
‡P-value calculated using the Mann–Whitney U-test.
2
†P-value calculated using the χ -test.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; FPG, fasting plasma glucose;
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SCr, serum creatinine; SUA, serum uric acid; TC, total
cholesterol; TG, triglyceride.
© 2016 The Japan Society of Hepatology
Hepatology Research 2017; 47: E104–E112 Serum uric acid level E107
Figure 1 Serum uric acid (SUA) level distribution according to sex and age of study participants. (a) SUA levels in men and women.
*P < 0.05 versus non-obese non-alcoholic fatty liver disease (NAFLD) absent; ‡P < 0.05 versus non-obese NAFLD present. (b) SUA level
separated by age in female adults. *P < 0.05 versus their corresponding group aged <45 years; ‡P < 0.05 versus their corresponding
group aged >55 years. , Nonobese NAFLD Absent; , Nonobese NAFLD Present; , Obesity NAFLD Present; , Obesity NAFLD
Absent; , Nonobese NAFLD Absent; , Nonobese NAFLD Present; , Obesity NAFLD Present; , Obesity NAFLD Absent.
Table 2 Clinical and laboratory characteristics of adults with non-alcoholic fatty liver disease (NAFLD) according to body mass index (BMI)
Variable Non-obese subjects (n = 1936 ) Obese subjects (n = 2162 )
NAFLD absent NAFLD present P-value NAFLD absent NAFLD present P-value
n (women) 1648 ( 871 ) 288 ( 99 ) 0.001† 831 ( 225 ) 1331( 320 ) 0.026†
Age, years 37.89 ± 11.22 46.86 ± 10.99 0.000 42.50 ± 10.96 45.16 ± 11.03‡ 0.000
BMI, kg/m2 21.87 ± 1.99 23.56 ± 1.27 0.000 27.26 ± 2.04 28.93 ± 2.93‡ 0.000
SUA, μmol/L 279.13 ± 81.05 335.15 ± 87.03 0.000 326.12 ± 90.90 356.78 ± 90.90‡ 0.000
FPG, mmol/L 5.06 ± 1.07 5.53 ± 1.68 0.000 5.25 ± 1.09 5.64 ± 1.57 0.000
TG, mmol/L 0.96 (0.69–1.41) 1.77 (1.30–2.58) 0.001§ 1.41 (0.97–2.06) 1.93 (1.31–2.87)‡ 0.000§
TC, mmol/L 4.38 ± 0.90 5.01 ± 1.68 0.000 4.77 ± 0.94 5.00 ± 0.99 0.000
HDL-C, mmol/L 1.37 ± 0.37 1.16 ± 0.31 0.000 1.19 ± 0.33 1.13 ± 0.35 0.001
LDL-C, mmol/L 2.60 ± 0.72 3.01 ± 0.80 0.000 3.02 ± 0.74 3.11 ± 0.80 0.010
ALT, U/L 16.00 (12.00–21.85) 20.00 (17.00–23.40) 0.001§ 19.40 (16.20–23.62) 28.07 (20.00–40.00)‡ 0.000§
AST, U/L 17.80 (15.00–21.00) 23.65 (17.95–31.93) 0.001§ 22.35 (16.76–31.06) 21.20 (17.80–26.90)‡ 0.000§
BUN, mmol/L 4.88 ± 1.46 5.32 ± 1.48 0.000 5.14 ± 1.35 5.28 ± 1.55 0.033
SCr, mmol/L 67.59 ± 16.42 70.68 ± 16.37 0.009 75.03 ± 16.78 74.61 ± 26.36‡ 0.630
Data are expressed as mean ± standard deviation or median (interquartile range). Calculated by comparison of subjects with NAFLD within each
BMI group. Significant values appear in boldface type.
2
†P-value calculated using the χ -test.
‡For comparison of non-obese subjects and obese subjects with NALFD, P < 0.05.
§P-value calculated using the Mann–Whitney U-test.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FPG, fasting plasma glucose; HDL-C, high-density li-
poprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SCr, serum creatinine; SUA, serum uric acid; TC, total cholesterol; TG, triglyceride.
SUA level increased with age in both non-obese and obese parameters. Eleven variables including age, BMI, TG,
groups. Among non-obese NAFLD patients, women after TC, HDL-C, LDL-C, ALT, AST, FPG, SCr, and BUN were
menopause had significantly higher SUA levels than involved (Table 3). There were extremely significant pos-
women before menopause (286.44 ± 63.60 vs. 254.96 itive correlations between BMI, TG, TC, LDL-C, ALT, AST,
± 70.88 μmol/L) (Fig. 1b). SCr, BUN and SUA, whereas HDL-C had negative corre-
lations in both non-obese and obese subject groups. Re-
Association between SUA and other clinical markably, compared with the obese group, the
parameters correlation coefficients between BMI, TG, TC, LDL-C,
Linear correlation analysis was used to determine the re- HDL-C, ALT, SCr, BUN, and SUA were all higher in the
lationship between SUA level and other clinical non-obese group.
© 2016 The Japan Society of Hepatology
E108 J. Liu et al. Hepatology Research 2017; 47: E104–E112
Table 3 Association between serum uric acid (SUA) level and NAFLD in non-obese subjects. Remarkably, the non-obese
other parameters in non-obese and obese adults group had higher NAFLD risk with increased SUA levels
Variable Non-obese subjects Obese subjects than the obese group (odds ratio, 2.559 and 95% confi-
(n = 2038 ) (n = 2164 ) dence interval, 1.870–3.503 vs. odds ratio, 1.692 and
95% confidence interval, 1.371–2.087).
r P-value r P-value
Age, years 0.004 0.840 0.107 0.000 Correlation of SUA level with histological
BMI, kg/m2 0.218 0.000 0.059 0.007 severity in non-obese adults
FPG, mmol/L 0.061 0.006 0.098 0.000 To investigate the relationship between SUA level and dif-
TG, mmol/L 0.298 0.000 0.278 0.000
ferent stages of NAFLD in non-obese adults, 93 NAFLD pa-
TC, mmol/L 0.138 0.000 0.130 0.000
tients were divided into three groups according to biopsy
HDL-C, mmol/L 0.273 0.000 0.238 0.000
LDL-C, mmol/L 0.203 0.000 0.095 0.000
result (Table 5). An analysis using one-way ANOVA followed
ALT, U/L 0.185 0.000 0.129 0.000 by Dunnett’s test revealed that none of the variables had sig-
AST, U/L 0.093 0.000 0.186 0.000 nificant differences between non-NASH and NASH groups,
BUN, mmol/L 0.224 0.000 0.176 0.000 except for ALT and SUA. Notably, compared with the non-
SCr, mmol/L 0.421 0.000 0.320 0.000 NASH group, SUA levels were significantly higher in indefi-
nite NASH and NASH groups (344.53 ± 87.62 μmol/L vs.
Significant values appear in boldface type.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI,
397.71 ± 92.53 μmol/L and 403.21 ± 74.98 μmol/L, respec-
body mass index; BUN, blood urea nitrogen; FPG, fasting plasma tively). These results showed that non-obese adults had
glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- higher SUA levels as the inflammation advanced in NAFLD.
density lipoprotein cholesterol; r, correlation coefficient; SCr, serum
creatinine; TC, total cholesterol; TG, triglyceride.
Relationship between SUA level and
histopathological characteristics of NAFLD in
Risk factors analysis for NAFLD in non-obese non-obese adults
adults
The score distribution of NAS and its components accord-
Multivariate logistic regression analysis was undertaken to ing to SUA level is shown in Figure 2. The group with hy-
evaluate risk factors for NAFLD. Ten variables including peruricemia had significantly higher NAS scores and
male gender, age, TG, TC, HDL-C, LDL-C, FPG, SUA, more serious lobule inflammation than the normal group,
SCr, and BUN were entered into the original equation. whereas hepatic steatosis and ballooning degeneration
Our results showed that all variables except for male gen- had no significant difference between the two groups.
der, TC, SCr, and BUN remained in the final equation in To get a deeper understanding of the relationship be-
non-obese subjects. In obese subjects, male gender, age, tween SUA level and histopathological characteristics of
TG, FPG, and SUA remained in the final equation NAFLD patients in the non-obese group, the impact of
(Table 4). It was suggested that older age, hypertriglyc- SUA level on the prevalence rates of different NAS scores
eridemia, low HDL-C, elevated LDL-C and FPG, and hy- and its components were studied. Our results showed that
peruricemia are closely associated with the risk for the prevalence rates of high NAS and lobule inflammation
Table 4 Risk factors for non-alcoholic fatty liver disease in non-obese and obese adults
Variables Non-obese subjects Obese subjects
β P-value OR 95% CI β P-value OR 95% CI
Male gender – – – – 0.294 0.012 1.341 1.066–1.687
Older age 1.113 0.000 3.613 2.714–4.150 0.431 0.000 1.539 1.270–1.865
Elevated FPG 0.684 0.003 1.983 1.269–3.099 0.656 0.000 1.928 1.454–2.555
Hypertriglyceridemia 1.285 0.000 3.613 2.714–4.810 0.779 0.000 2.179 1.807–2.626
Low HDL-C 0.680 0.051 1.974 0.998–3.906 – – – –
Elevated LDL-C 0.684 0.000 1.981 1.483–2.648 – – – –
Hyperuricemia 0.940 0.000 2.559 1.870–3.503 0.526 0.000 1.692 1.371–2.087
–, none data; β, partial regression coefficient; CI, confidence interval; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol;
LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
© 2016 The Japan Society of Hepatology
Hepatology Research 2017; 47: E104–E112 Serum uric acid level E109
Table 5 Comparison of serum uric acid (SUA) level and other characteristics between non-alcoholic steatohepatitis (NASH), indefinite
NASH, and non-NASH in non-obese adults
Variable Total Non-NASH Indefinite NASH NASH P-value
n (male / female) 93 (67/26) 19 (15/4) 41 (28/13) 33 (24/9) 0.689
Age, years 36.84 ± 11.08 37.58 ± 9.71 35.83 ± 11.54 37.79 ± 11.59 0.738
BMI, kg/m2 23.44 ± 1.24 23.22 ± 1.23 23.62 ± 1.11 23.31 ± 1.41 0.469
TG, mmol/L 1.71 (1.27–2.79) 1.33(1.16–2.72) 1.62 (1.25–2.67) 1.94 (1.56–2.98) 0.307†
TC, mmol/L 5.06 ± 1.33 4.76 ± 0.99 5.08 ± 1.65 5.20 ± 1.01 0.324
HDL-C, mmol/L 1.27 ± 0.33 1.34 ± 0.29 1.20 ± 0.22 1.31 ± 0.43 0.193
LDL-C, mmol/L 3.08 ± 0.76 2.84 ± 0.70 3.25 ± 0.81 3.01 ± 0.69 0.295
FPG, mmol/L 5.26 ± 0.84 5.35 ± 1.15 5.20 ± 0.67 5.29 ± 0.82 0.835
Insulin, mU/mL 10.30 (8.77–15.30) 11.14 (7.38–18.19) 9.61 (8.77–15.30) 10.30 (8.00–12.30) 0.713†
HOMA-IR 2.20 (1.60–3.15) 2.30 (1.55–3.95) 2.05 (1.48–3.18) 2.30 (1.70–3.10) 0.615†
TNF-α, nmol/L 12.35 (8.48–17.85) 13.70 (9.70–17.02) 11.55 (6.00–18.24) 12.80 (8.85–19.55) 0.976†
AST, U/L 45.00 (30.00–69.5) 38.00 (24.00–55.00) 44.00 (28.00–62.50) 55.00 (33.00–80.50) 0.056†
ALT, U/L 75.00(42.00–116.50) 46.00 (28.00–98.00) 70.00 (44.00–96.5)‡ 100.00 (59.25–139.00)§ 0.009†
SUA, μmol/L 388.80 ± 87.72 344.53 ± 87.62 397.71 ± 92.53‡ 403.21 ± 74.98§ 0.030
Leptin, ng/mL 4.80 (2.60–9.30) 4.83 (2.53–15.6) 4.80 (2.68–9.30) 3.27 (1.90–10.00) 0.775†
Data are expressed as mean ± standard deviation or median (interquartile range). P-values calculated using one-way ANOVA, based on post hoc
multiple comparison between quartile groups.
†P-value was calculated using the Mann–Whitney U-test.
‡Compared with non-NASH, P < 0.05.
§Compared with indefinite NASH, P < 0.05.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density li-
poprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; TC, total
cholesterol; TG, triglyceride; TNF-α, tumor necrosis factor-α.
score tended to increase as the SUA level increased, increased SUA level is associated with an exacerbated risk
whereas this trend was not apparent in hepatic steatosis of NAFLD. This increased risk is probably independent of
and ballooning degeneration (Fig. 3). In Q4, the preva- conventional NAFLD risk factors.21 In our study, we also
lence rates of NAS ≥ 5 reached 57.58%, and the prevalence found hyperuricemia was an risk factor of NAFLD. Non-
rates of lobule inflammation score = 3 was 66.67% (Fig. 3). obese adults have higher NAFLD risk with increased SUA
It suggested that the inflammation of NAFLD tended to in- levels than obese adults, suggesting that increased SUA
crease as the SUA level increased in non-obese subjects. concentrations, even within the normal range, are associ-
ated with the presence of NAFLD in non-obese subjects.
Therefore, it is time to pay attention to the exact relation-
DISCUSSION
ship between SUA level and NAFLD in non-obese adults.
R ECENTLY, AN INCREASING number of studies have
examined the significance of NAFLD in non-obese
adults as NAFLD is increasingly prevalent in this group,
Large clinical studies have indicated that hyperuricemia
is associated with obesity, dyslipidemia, type 2 diabetes
mellitus, and MetS.22 There are also several studies suggest-
with reported prevalence from 11.5% to 16.1%.18,19 In this ing that SUA level is significantly associated with
study, we found that the prevalence of NAFLD was 14.88% NAFLD.12,13 However, few data are available about the re-
in non-obese adults. The NAFLD patients had higher SUA lationship between SUA level and inflammation associ-
levels than the control group. Previous studies showed that ated with NAFLD in non-obese adults. In 93 cases of
hyperuricemia is a significant and independent predictor non-obese biopsy-proven NAFLD we found that there
of MetS, and MetS risk increases with increased serum uric were no significant differences between non-NASH and
acid levels.20 Interestingly, the present study showed that NASH groups, except for ALT and SUA levels. The SUA
SUA levels were significantly and positively correlated with levels were significantly higher in indefinite NASH and
BMI, TG, TC, LDL-C, ALT, SCr, and BUN, and the correla- NASH groups. These results showed that higher SUA
tion coefficient in the non-obese group was higher than levels correlated with increased inflammation in NAFLD.
in the obese group. A meta-analysis suggested that One of the possible explanations for the relationship
© 2016 The Japan Society of Hepatology
E110 J. Liu et al. Hepatology Research 2017; 47: E104–E112
Figure 2 Score distribution of non-alcoholic fatty liver disease activity score (NAS) and its components according to serum uric acid (SUA)
level. (a) NAS. (b) Hepatic steatosis. (c) Ballooning degeneration. (d) Lobule inflammation. P-values calculated using the Mann–Whitney U-test.
Figure 3 Prevalence of different quartile (Q) levels of serum uric acid according to the non-alcoholic fatty liver disease activity score
(NAS) and its components. (a) NAS. (b) Hepatic steatosis. (c) Ballooning degeneration. (d) Lobule inflammation. P-values calculated
using one-way ANOVA.*P < 0.05 versus Q1; †P < 0.05 versus Q2; ‡P < 0.05 versus Q3, based on post hoc multiple comparison between
quartile groups. , Q1; , Q2; , Q3; , Q4.
© 2016 The Japan Society of Hepatology
Hepatology Research 2017; 47: E104–E112 Serum uric acid level E111
between SUA level and the inflammation progression REFERENCES
of NAFLD is that increased SUA is also linked to
1 Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Mo-
increased fat accumulation, hyperleptinemia, and insulin lecular mechanisms involved in NAFLD progression. J Mol
resistance.11,23 Med (Berl) 2009; 87: 679–95.
Uric acid becomes a strong oxidant in the environment of 2 Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver
MetS. Serum uric acid could also stimulate the release of disease in China. J Hepatol 2009; 50: 204–10.
oxidants to promote oxidative stress by stimulating nicotin- 3 Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and
amide adenine dinucleotide phosphate oxidase and then be risk factors for the development of nonalcoholic fatty
involved in the pathogenesis of NAFLD. More interestingly, liver disease in a nonobese Chinese population: the
in our study, patients with hyperuricemia had significantly Zhejiang Zhenhai Study. Am J Gastroenterol 2013; 108:
higher NAS scores than those without hyperuricemia, and 1299–304.
4 Nishioji K, Sumida Y, Kamaguchi M et al. Prevalence of and
lobule inflammation was also more serious. The prevalence
risk factors for non-alcoholic fatty liver disease in a non- obese
rates of high NAS and lobule inflammation tended to in-
Japanese population, 2011–2012. J Gastroenterol 2015; 50:
crease with increases in the SUA level. It suggested that the 95–108.
inflammation of NAFLD tended to increase as the SUA level 5 Edwards NL. The role of hyperuricemia in vascular disorders.
increased. Recent studies discovered a close association be- Curr Opin Rheumatol 2009; 21: 132–7.
tween the inflammation development of NAFLD and many 6 Mellen PB, Bleyer AJ, Erlinger TP et al. Serum uric acid predicts
pathogenic events, including hepatic macrophage recruit- incident hypertension in a biethnic cohort: the atherosclerosis
ment, activation of the innate immune system, and changes risk in communities study. Hypertension 2006; 48: 1037–42.
in lipid homeostasis.24 The innate immune system has 7 Rho YH, Choi SJ, Lee YH et al. The prevalence of metabolic
evolved mechanisms to detect the release of a subset of syndrome in patients with gout: a multicenter study. J Korean
these molecules, called damage-associated molecular pat- Med Sci 2005; 20: 1029–33.
8 Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyper-
terns (DAMPs).25,26 When macrophages sense the presence
uricemia in the US general population: NHANES 2007–2008.
of DAMPs they are stimulated to produce pro-
Am J Med 2012; 125: 679–87.
inflammatory cytokines that then induce inflammation.27 9 Xu C. Hyperuricemia and nonalcoholic fatty liver disease:
It has been confirmed that uric acid is one of the important from bedside to bench and back. Hepatol Int 2016; 10:
DAMPs.28,29 Does uric acid act as a DAMP by stimulating 286–93.
macrophages to produce pro-inflammatory cytokines and 10 Krishnan E, Pandya BJ, Chung L, Hariri A, Dabbous O. Hyper-
then induce inflammation progression in non-obese uricemia in young adults and risk of insulin resistance,
adults? Further research is needed. prediabetes, and diabetes: a 15-year follow-up study. Am J
In conclusion, the findings of the present study Epidemiol 2012; 176: 108–16.
suggest that hyperuricemia is an independent risk factor 11 Yoo HG, Lee SI, Chae HJ et al. Prevalence of insulin resistance
for NAFLD, and SUA level is significantly associated and metabolic syndrome in patients with gouty arthritis.
Rheumatol Int 2011; 31: 485–91.
with inflammation progression of NAFLD in non-obese
12 Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid
adults. However, the precise mechanism requires further
level with non-alcoholic fatty liver disease: a cross-sectional
study. Intervention trials are needed to investigate study. J Hepatol 2009; 50: 1029–34.
whether the reduction of SUA levels favorably impacts 13 Sertoglu E, Ercin CN, Celebi G et al. The relationship of serum
NAFLD. uric acid with non-alcoholic fatty liver disease. Clin Biochem
2014; 47: 383–8.
14 Targher G, Bertolini L, Poli F et al. Nonalcoholic fatty liver dis-
ACKNOWLEDGMENTS ease and risk of future cardiovascular events among type 2
diabetic patients. Diabetes 2005; 54: 3541–6.
T HIS WORK WAS supported by the Natural Science
Foundation of Zhejiang Province (Grant Nos.
LY14H070004 and LR15H030001), the National Natural
15 Kleiner DE, Brunt EM, Van Natta M et al. Design and valida-
tion of a histological scoring system for nonalcoholic fatty
liver disease. Hepatology 2005; 41: 1313–21.
Science Foundation of China (Grant Nos. 81100278 and
16 Cerda C, Pérez-Ayuso RM, Riquelme A et al. Nonalcoholic
81470838), International Science and Technology Coop-
fatty liver disease in women with polycystic ovary syndrome.
eration Projects of Zhejiang Province (Grant No. J Hepatol 2007; 47: 412–7.
2013C24010), Science Foundation of Health Bureau of 17 Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G,
Zhejiang Province (Grant No. 2012RCA026 and Bellentani S. Prevalence of and risk factors for nonalcoholic
2013C33187), and Wang Baoen Liver Fibrosis Foundation fatty liver disease: the Dionysos nutrition and liver study.
(Grant No. CFHPC20131041). Hepatology 2005; 42: 44–52.
© 2016 The Japan Society of Hepatology
E112 J. Liu et al. Hepatology Research 2017; 47: E104–E112
18 Chen CH, Huang MH, Yang JC et al. Prevalence and risk fac- 23 Rahmouni K, Haynes WG. Endothelial effects of leptin: impli-
tors of nonalcoholic fatty liver disease in an adult cations in health and diseases. Curr Diab Rep 2005; 5: 260–6.
population of Taiwan: metabolic significance of nonalcoholic 24 Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the
fatty liver disease in nonobese adults. J Clin Gastroenterol emerging view. J Hepatol 2009; 51: 212–23.
2006; 40: 745–52. 25 Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflamma-
19 Kim HJ, Kim HJ, Lee KE et al. Metabolic significance of nonal- tory response. Annu Rev Immunol 2010; 28: 321–42.
coholic fatty liver disease in nonobese, nondiabetic adults. 26 Rock KL, Lai JJ, Kono H. Innate and adaptive immune re-
Arch Intern Med 2004; 164: 2169–75. sponses to cell death. Immunol Rev 2011; 243: 191–205.
20 Nagahama K, Inoue T, Kohagura K, Ishihara A, Kinjo K, Ohya 27 Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of
Y. Hyperuricemia predicts future metabolic syndrome: a 4- the cellular sensor that stimulates the inflammatory response
year follow-up study of a large screened cohort in Okinawa. to sterile cell death. J Immunol 2010; 184: 4470–8.
Japan Hypertens Res 2014; 37: 232–8. 28 Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes
21 Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonal- an acute inflammatory response to sterile cell death in mice.
coholic fatty liver disease: a syst- ematic review and meta- J Clin Invest 2010; 120: 1939–49.
analysis. Clin Biochem 2016; 49: 636–42. 29 Shi Y, Evans JE, Rock KL. Molecular identification of a danger
22 Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and signal that alerts the immune system to dying cells. Nature
hyperuricemia. Curr Opin Rheumatol 2013; 25: 210–6. 2003; 425: 516–21.
© 2016 The Japan Society of Hepatology
Vous aimerez peut-être aussi
- Advances in Understanding The Progression of Non-Alcoholic Fatty Liver Disease To Non-Alcoholic Steatohepatitis: A Comprehensive ReviewDocument7 pagesAdvances in Understanding The Progression of Non-Alcoholic Fatty Liver Disease To Non-Alcoholic Steatohepatitis: A Comprehensive ReviewInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation
- Art 3 Non-Alcoholic Fatty Liver Disease ADocument10 pagesArt 3 Non-Alcoholic Fatty Liver Disease AAlexsander SarmientoPas encore d'évaluation
- Non Alcoholic Liver Disease PhilippinesDocument5 pagesNon Alcoholic Liver Disease PhilippinesMara Benzon-BarrientosPas encore d'évaluation
- Non-Alcoholic Fatty Liver DiseaseDocument15 pagesNon-Alcoholic Fatty Liver DiseaseKurnia pralisaPas encore d'évaluation
- Association Between Serum Uric Acid Level and Chronic Liver Disease in The United StatesDocument12 pagesAssociation Between Serum Uric Acid Level and Chronic Liver Disease in The United StatesAndy F Monroe100% (1)
- Clinical Spectrum of Non-Alcoholic Fatty Liver Disease in Patients With Diabetes MellitusDocument13 pagesClinical Spectrum of Non-Alcoholic Fatty Liver Disease in Patients With Diabetes Mellitusblume diaPas encore d'évaluation
- Systematic Review: The Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in AdultsDocument12 pagesSystematic Review: The Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in AdultsLays RosalPas encore d'évaluation
- Watanabe2015 - Article - Evidence-basedClinicalPracticeDocument14 pagesWatanabe2015 - Article - Evidence-basedClinicalPracticemariam.husseinjPas encore d'évaluation
- 1 s2.0 S1873959813000446 MainDocument5 pages1 s2.0 S1873959813000446 MainRob RobertPas encore d'évaluation
- Lipid and Glucose Profile in Non-Alcoholic Fatty Liver Disease: A Hospital Based StudyDocument8 pagesLipid and Glucose Profile in Non-Alcoholic Fatty Liver Disease: A Hospital Based StudyijasrjournalPas encore d'évaluation
- A Clinical Overview of Non-Alcoholic Fatty Liver Disease A GuideDocument12 pagesA Clinical Overview of Non-Alcoholic Fatty Liver Disease A GuideAnindya RosmaPas encore d'évaluation
- Higado GrasoDocument13 pagesHigado GrasoMarisol MolinaPas encore d'évaluation
- Clinical and Histologic Spectrum of Nonalcoholic Fatty Liver Disease Associated With Normal ALT ValuesDocument7 pagesClinical and Histologic Spectrum of Nonalcoholic Fatty Liver Disease Associated With Normal ALT ValuesndocPas encore d'évaluation
- Preamble: Podcast Interview: - Also Available On ItunesDocument18 pagesPreamble: Podcast Interview: - Also Available On ItuneswawanpecelPas encore d'évaluation
- Seminars in Pediatric Surgery: Nonalcoholic Fatty Liver Disease and Bariatric Surgery in AdolescentsDocument9 pagesSeminars in Pediatric Surgery: Nonalcoholic Fatty Liver Disease and Bariatric Surgery in AdolescentsDr Venkatachalapathy T S Ped SurgeonPas encore d'évaluation
- Nonalcoholic Fatty Liver in Diabetes Part I Epidemiology and NosisDocument15 pagesNonalcoholic Fatty Liver in Diabetes Part I Epidemiology and NosisFernando BorgesPas encore d'évaluation
- IntJMedPublicHealth32111-7924347 220043Document0 pageIntJMedPublicHealth32111-7924347 220043budi_26690Pas encore d'évaluation
- Hp165-02b-Nash (FF 050816v) ProtegidoDocument9 pagesHp165-02b-Nash (FF 050816v) ProtegidoAnonymous 4txA8N8etPas encore d'évaluation
- Cleveland Clinic Journal of Medicine 2008 KIMYOUNOSSI 721 8faal2Document8 pagesCleveland Clinic Journal of Medicine 2008 KIMYOUNOSSI 721 8faal2wira_meganPas encore d'évaluation
- Paper Insulin Resistance and Ferritin As Major Determinants of Nonalcoholic Fatty Liver Disease in Apparently Healthy Obese PatientsDocument7 pagesPaper Insulin Resistance and Ferritin As Major Determinants of Nonalcoholic Fatty Liver Disease in Apparently Healthy Obese PatientsNike Dwi Putri LestariPas encore d'évaluation
- JCTH 10 0979Document7 pagesJCTH 10 0979Svt Mscofficial2Pas encore d'évaluation
- S Uric Acid &NAFLD (US Popln)Document7 pagesS Uric Acid &NAFLD (US Popln)MalarPas encore d'évaluation
- Evaluation and Management of Obesity-Related Nonalcoholic Fatty Liver DiseaseDocument10 pagesEvaluation and Management of Obesity-Related Nonalcoholic Fatty Liver DiseaseVíctor V MayoralPas encore d'évaluation
- 1 s2.0 S0261561422002874 MainDocument12 pages1 s2.0 S0261561422002874 Maindr. Nur'aini HasanPas encore d'évaluation
- Epidemiology of Non-Alcoholic Fatty Liver Disease: Liver and Metabolic SyndromeDocument7 pagesEpidemiology of Non-Alcoholic Fatty Liver Disease: Liver and Metabolic SyndromeDidi Yudha TrisandyaPas encore d'évaluation
- Piis001650852200628x PDFDocument12 pagesPiis001650852200628x PDFAriana HurtadoPas encore d'évaluation
- Active Smoking, Passive Smoking, and Risk of Nonalcoholic Fatty Liver Disease (NAFLD) : A Population-Based Study in ChinaDocument7 pagesActive Smoking, Passive Smoking, and Risk of Nonalcoholic Fatty Liver Disease (NAFLD) : A Population-Based Study in ChinaYelvi Novita RozaPas encore d'évaluation
- A Placebo-Controlled Trial of Silymarin in Patients With Nonalcoholic Fatty Liver DiseaseDocument6 pagesA Placebo-Controlled Trial of Silymarin in Patients With Nonalcoholic Fatty Liver Diseasesnn123456Pas encore d'évaluation
- Pnpla2 rs693Document7 pagesPnpla2 rs693Vishnu Sai MokshagnaPas encore d'évaluation
- Reviews Advances in Pediatric Nonalcoholic Fatty Liver DiseaseDocument12 pagesReviews Advances in Pediatric Nonalcoholic Fatty Liver DiseasehansenpanjaitanPas encore d'évaluation
- 5135 17966 1 PBDocument6 pages5135 17966 1 PBDudiPas encore d'évaluation
- Epidemia HgnaDocument8 pagesEpidemia HgnaAntonio Alexis Bustos TorresPas encore d'évaluation
- 1.Dr. Hussnain Ali Shah GROWING ISSUES OF NAFLD Sri Lanka 2017Document27 pages1.Dr. Hussnain Ali Shah GROWING ISSUES OF NAFLD Sri Lanka 2017Hussain AliPas encore d'évaluation
- AACE NAFLD NASH 2020 Revision - Recd 0528 - FINALDocument20 pagesAACE NAFLD NASH 2020 Revision - Recd 0528 - FINALardhanputraPas encore d'évaluation
- Clinical Profile With Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome PatientsDocument7 pagesClinical Profile With Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome PatientsIJAR JOURNALPas encore d'évaluation
- Us Elastography in Hepatic Fibrosis Radiology in TrainingDocument8 pagesUs Elastography in Hepatic Fibrosis Radiology in TrainingValentina IorgaPas encore d'évaluation
- ResponseConsensusReportAntipsychoticsMetabolicIssuesLetter CITROME DiabCare2004Document5 pagesResponseConsensusReportAntipsychoticsMetabolicIssuesLetter CITROME DiabCare2004Leslie CitromePas encore d'évaluation
- Hepr 13321Document29 pagesHepr 13321Dolly JazmiPas encore d'évaluation
- Diagnosis Nafld 2016Document15 pagesDiagnosis Nafld 2016AfifMaulanaFirmansyahPas encore d'évaluation
- Am J Clin Nutr 2014 Chung Ajcn.114.086314Document17 pagesAm J Clin Nutr 2014 Chung Ajcn.114.086314Daniel Lee Eisenberg JacobsPas encore d'évaluation
- Wong 2018Document18 pagesWong 2018Mar CuchipartePas encore d'évaluation
- NAFLD in DM Banjarmasin Edit NEWDocument74 pagesNAFLD in DM Banjarmasin Edit NEWZayed NorwantoPas encore d'évaluation
- Non Alcoholic Fatty Liver Disease ThesisDocument5 pagesNon Alcoholic Fatty Liver Disease ThesisAshley Hernandez100% (2)
- Performance of Non-Invasive Models of Fibrosis in Predicting Mild To Moderate Fibrosis in Patients With Non-Alcoholic Fatty Liver DiseaseDocument8 pagesPerformance of Non-Invasive Models of Fibrosis in Predicting Mild To Moderate Fibrosis in Patients With Non-Alcoholic Fatty Liver DiseaseChausarPutraBenagilPas encore d'évaluation
- NafldDocument14 pagesNafldSrinivas PingaliPas encore d'évaluation
- Nonalcoholic Fatty Liver Disease (Nafld) Challenge To DiagnosisDocument31 pagesNonalcoholic Fatty Liver Disease (Nafld) Challenge To DiagnosisMohamed NaguibPas encore d'évaluation
- Clinical - Liver: Simple Noninvasive Systems Predict Long-Term Outcomes of Patients With Nonalcoholic Fatty Liver DiseaseDocument12 pagesClinical - Liver: Simple Noninvasive Systems Predict Long-Term Outcomes of Patients With Nonalcoholic Fatty Liver Disease走过一些路Pas encore d'évaluation
- HIgado GrasoDocument6 pagesHIgado GrasoWilson Suarez VenturaPas encore d'évaluation
- Non-Alchoholic Steatohepatitis (NASH) Advances in Evaluation and ManagementDocument52 pagesNon-Alchoholic Steatohepatitis (NASH) Advances in Evaluation and ManagementDr.Vivek AgarwalaPas encore d'évaluation
- MSB 20188793 PDFDocument16 pagesMSB 20188793 PDFAndreea RoxanaPas encore d'évaluation
- Complementary and Alternative Medical Lab Testing Part 8: UrologyD'EverandComplementary and Alternative Medical Lab Testing Part 8: UrologyÉvaluation : 3 sur 5 étoiles3/5 (1)
- NAFLD LancetDocument13 pagesNAFLD LancetSvt Mscofficial2Pas encore d'évaluation
- Alkohol Sirosis PDFDocument11 pagesAlkohol Sirosis PDFYashinta MaharaniPas encore d'évaluation
- Niveles de Catepsina D en Plasma - Una Nueva Herramienta para Predecir Pediátrica La Inflamación HepáticaDocument9 pagesNiveles de Catepsina D en Plasma - Una Nueva Herramienta para Predecir Pediátrica La Inflamación HepáticaLuz Guadalupe Cruz HuancaPas encore d'évaluation
- Resmetirom Phase 2 Clinical Trial ResultsDocument13 pagesResmetirom Phase 2 Clinical Trial ResultsRayPas encore d'évaluation
- The Evolving Landscape of Liver Cirrhosis ManagementD'EverandThe Evolving Landscape of Liver Cirrhosis ManagementHitoshi YoshijiPas encore d'évaluation
- Mechanism of NAFLD Dev & Therapeutic Strategies - Arun SanyalDocument15 pagesMechanism of NAFLD Dev & Therapeutic Strategies - Arun SanyalParul SoodPas encore d'évaluation
- NAFLD-dr. Masrul KuliahDocument103 pagesNAFLD-dr. Masrul KuliahAndrie WigunaPas encore d'évaluation
- Fatty Liver and MSDocument16 pagesFatty Liver and MSAljon Anies100% (1)
- Thesis Protocol: DNB General MedicineDocument30 pagesThesis Protocol: DNB General Medicinegattu santoshPas encore d'évaluation
- Perbandingan Kadar Kreatinin Dan Asam Urat Pada Serum Dan Urin Penderita Hipertensi Dan NormotensiDocument9 pagesPerbandingan Kadar Kreatinin Dan Asam Urat Pada Serum Dan Urin Penderita Hipertensi Dan NormotensisyifaPas encore d'évaluation
- 2194 3988 1 SMDocument7 pages2194 3988 1 SMmaritaPas encore d'évaluation
- 3905 KKKDocument7 pages3905 KKKMuhammad Khoirul SodiqPas encore d'évaluation
- Perbandingan Kadar Kreatinin Dan Asam Urat Pada Serum Dan Urin Penderita Hipertensi Dan NormotensiDocument9 pagesPerbandingan Kadar Kreatinin Dan Asam Urat Pada Serum Dan Urin Penderita Hipertensi Dan NormotensisyifaPas encore d'évaluation
- NancyDocument11 pagesNancyRizqa FajarPas encore d'évaluation
- 1924 4814 1 PBDocument10 pages1924 4814 1 PBDame rohanaPas encore d'évaluation
- AlkoholDocument11 pagesAlkoholNerissaPas encore d'évaluation
- Preventive MedicineDocument6 pagesPreventive MedicinesyifaPas encore d'évaluation
- Alcohol Consumption in Oliver Twist: Literature As Prevention of AlcoholismDocument6 pagesAlcohol Consumption in Oliver Twist: Literature As Prevention of AlcoholismsyifaPas encore d'évaluation
- Gender Differences in Problematic Alcohol Consumption in University ProfessorsDocument11 pagesGender Differences in Problematic Alcohol Consumption in University ProfessorssyifaPas encore d'évaluation
- Evaluation of Alcoholic Consumption On Serum Uric Acid, Urea, and Creatinine LevelsDocument3 pagesEvaluation of Alcoholic Consumption On Serum Uric Acid, Urea, and Creatinine LevelssyifaPas encore d'évaluation
- The Relation of Moderate Alcohol Consumption To Hyperuricemia in A Rural General PopulationDocument11 pagesThe Relation of Moderate Alcohol Consumption To Hyperuricemia in A Rural General PopulationsyifaPas encore d'évaluation
- DHF American JournalDocument3 pagesDHF American JournalMuhammad Rizki DmPas encore d'évaluation
- Effect of Alcohol Consumption On The Risk of Erectile DysfunctionDocument6 pagesEffect of Alcohol Consumption On The Risk of Erectile DysfunctionsyifaPas encore d'évaluation
- International Research Journal of PharmacyDocument4 pagesInternational Research Journal of PharmacysyifaPas encore d'évaluation
- Editorial Serum Uric Acid and Risk of Kidney Stones: Related Article, P. 173Document2 pagesEditorial Serum Uric Acid and Risk of Kidney Stones: Related Article, P. 173syifaPas encore d'évaluation
- Correspondence: Alcohol Intake, Serum Uric Acid Concentrations, and Risk of GoutDocument2 pagesCorrespondence: Alcohol Intake, Serum Uric Acid Concentrations, and Risk of GoutsyifaPas encore d'évaluation
- Inverse Association Between Coffee Drinking and Serum Uric Acid Concentrations in Middle-Aged Japanese MalesDocument6 pagesInverse Association Between Coffee Drinking and Serum Uric Acid Concentrations in Middle-Aged Japanese MalessyifaPas encore d'évaluation
- Relationship Between Dietary Beef, Fat, and Pork and Alcoholic CirrhosisDocument9 pagesRelationship Between Dietary Beef, Fat, and Pork and Alcoholic CirrhosissyifaPas encore d'évaluation
- Jurnal InternasionalDocument15 pagesJurnal InternasionalsyifaPas encore d'évaluation
- Ijerph 12 02411 PDFDocument26 pagesIjerph 12 02411 PDFsyifaPas encore d'évaluation
- Serum Uric Acid In: HypertensiveDocument6 pagesSerum Uric Acid In: HypertensivesyifaPas encore d'évaluation
- Research Article: The Relation of Coffee Consumption To Serum Uric Acid in Japanese Men and Women Aged 49-76 YearsDocument8 pagesResearch Article: The Relation of Coffee Consumption To Serum Uric Acid in Japanese Men and Women Aged 49-76 YearssyifaPas encore d'évaluation
- The Association Between Alcohol Consumption and - Cell Function and Insulin Sensitivity in Korean PopulationDocument11 pagesThe Association Between Alcohol Consumption and - Cell Function and Insulin Sensitivity in Korean PopulationsyifaPas encore d'évaluation
- Nitte University Journal June 2012-18-23Document11 pagesNitte University Journal June 2012-18-23syifaPas encore d'évaluation
- Ijerph 11 12700 PDFDocument16 pagesIjerph 11 12700 PDFsyifaPas encore d'évaluation
- Ijerph 07 04023Document14 pagesIjerph 07 04023syifaPas encore d'évaluation
- Ijerph 12 02411 PDFDocument26 pagesIjerph 12 02411 PDFsyifaPas encore d'évaluation
- A Multilevel Study of Students in Vietnam: Drinking Motives and Drinking Context As Predictors of Alcohol ConsumptionDocument13 pagesA Multilevel Study of Students in Vietnam: Drinking Motives and Drinking Context As Predictors of Alcohol ConsumptionsyifaPas encore d'évaluation
- Effects of Beverages On Alcohol Metabolism: Potential Health Benefits and Harmful ImpactsDocument12 pagesEffects of Beverages On Alcohol Metabolism: Potential Health Benefits and Harmful ImpactssyifaPas encore d'évaluation
- Umibilical Cord - ProlapsDocument22 pagesUmibilical Cord - ProlapsAtikah PurnamasariPas encore d'évaluation
- Can Mung Beans Cause GoutDocument1 pageCan Mung Beans Cause GoutAINA NAJWA BINTI ABDULLAH MoePas encore d'évaluation
- Jurnal PterigiumDocument6 pagesJurnal PterigiumMonica Lauretta Sembiring IIPas encore d'évaluation
- GMU Immunization FormDocument5 pagesGMU Immunization FormAurora Kitt McGowanPas encore d'évaluation
- Refractive Surgery Standards Dec 2004Document7 pagesRefractive Surgery Standards Dec 2004dr_jrcPas encore d'évaluation
- Cps-Pg-Admission-2019 First Selection Round of Neet Eligible Candidates For The State of MaharashtraDocument93 pagesCps-Pg-Admission-2019 First Selection Round of Neet Eligible Candidates For The State of MaharashtrarohankananiPas encore d'évaluation
- Acute Renal FailureDocument1 pageAcute Renal FailureSonia Letran Singson100% (1)
- GMMMC Sukkur MbbsDocument6 pagesGMMMC Sukkur MbbsSherazAhmedPas encore d'évaluation
- Jubilant Life Sciences Research Report PDFDocument50 pagesJubilant Life Sciences Research Report PDFAbhiroop DasPas encore d'évaluation
- Salubris Medical Center: National Highway, Brgy. Roxas, Solano, Nueva VizcayaDocument10 pagesSalubris Medical Center: National Highway, Brgy. Roxas, Solano, Nueva Vizcayajulie ann afanPas encore d'évaluation
- Approach To Nursing Assessment 1Document5 pagesApproach To Nursing Assessment 1Taiye OkondoPas encore d'évaluation
- Neri, Angela - Act. 1,2,3Document5 pagesNeri, Angela - Act. 1,2,3Angela NeriPas encore d'évaluation
- International Wound Journal Volume 7 Issue 4 2010 (Doi 10.1111/j.1742-481x.2010.00682.x) Christine A Chrisman - Care of Chronic Wounds in Palliative Care and End-Of-Life Patients PDFDocument22 pagesInternational Wound Journal Volume 7 Issue 4 2010 (Doi 10.1111/j.1742-481x.2010.00682.x) Christine A Chrisman - Care of Chronic Wounds in Palliative Care and End-Of-Life Patients PDFNovaPas encore d'évaluation
- Activity Journal Mobile Blood Donation Advocacy 1Document3 pagesActivity Journal Mobile Blood Donation Advocacy 1Cherrymae BenzonPas encore d'évaluation
- AJHP Pharmacy Forecast 2018-3Document32 pagesAJHP Pharmacy Forecast 2018-3tpatel0986Pas encore d'évaluation
- Features of Visualization of Chronic Obstructive Pulmonary Disease in Diabetes MellitusDocument4 pagesFeatures of Visualization of Chronic Obstructive Pulmonary Disease in Diabetes MellitusCentral Asian StudiesPas encore d'évaluation
- Slow Contact Tracing' Blamed For Spread of New CoronavirusDocument6 pagesSlow Contact Tracing' Blamed For Spread of New CoronavirusProdigal RanPas encore d'évaluation
- Delirium in Critical Care, 2nd EditionDocument248 pagesDelirium in Critical Care, 2nd EditionDuk Han KimPas encore d'évaluation
- Quitnet Presentation-Csu StanislausDocument14 pagesQuitnet Presentation-Csu StanislausMaria Carmela CabalquintoPas encore d'évaluation
- Nursing Care Plans For Renal CalculiDocument3 pagesNursing Care Plans For Renal CalculiRaveen mayi77% (22)
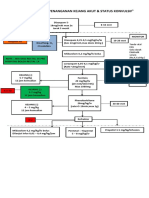
- Algoritma Penanganan Kejang AkutDocument1 pageAlgoritma Penanganan Kejang AkutEwa ClaudiaPas encore d'évaluation
- Slide Paparan - Dicky - Potensi Genomik DM BGSIDocument27 pagesSlide Paparan - Dicky - Potensi Genomik DM BGSIFebrian DewiPas encore d'évaluation
- How To Prepare For ExamDocument4 pagesHow To Prepare For ExamJaspreet KaurPas encore d'évaluation
- Health Guard Brochure Print76Document22 pagesHealth Guard Brochure Print76fhnPas encore d'évaluation
- Angiokeratoma of FordyceDocument2 pagesAngiokeratoma of FordycenivasshaanPas encore d'évaluation
- 1st Floor No 105 Above Raymonds Opp Medical College Koti: SHARMA Bpo SupportDocument4 pages1st Floor No 105 Above Raymonds Opp Medical College Koti: SHARMA Bpo SupportMK Musthafa GudalurPas encore d'évaluation
- NHB Renewal Application 2022Document4 pagesNHB Renewal Application 2022Chandran OchathevarPas encore d'évaluation
- Unicef: General InformationDocument2 pagesUnicef: General InformationjobPas encore d'évaluation
- Louis Kuhne - Neo-Naturopathy (New Science of Healing) (1917)Document313 pagesLouis Kuhne - Neo-Naturopathy (New Science of Healing) (1917)Școala Solomonară / The Solomonary School100% (16)
- SedativesDocument4 pagesSedativesalghashm001Pas encore d'évaluation