Académique Documents
Professionnel Documents
Culture Documents
Bacte 3RD Qee
Transféré par
Thea GonzalesTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Bacte 3RD Qee
Transféré par
Thea GonzalesDroits d'auteur :
Formats disponibles
FUNGAL SMEAR AND CULTURE
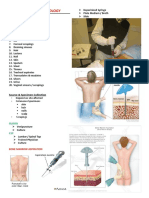
Preparation of KOH from the following specimen:
Blood Wright’s or Giemsa’s stain; India ink or direct antigen test for
Cryptococcus; 5 ml blood to 20 ml broth
Bone marrow aspirate 0.5 ml of marrow with sterile syringe in heparin
anticoagulant
CSF Collect in sterile tubes. If volume is more than 2 ml,
centrifugation and membrane filtration are recommended.
Use sediment for slide and plating if volume is less than 2 ml,
prepare smears and plates from uncentrifuged specimen.
Cutaneous hair Pluck by roots with sterile forceps. Select hairs that fluoresce
or are broken and scally
Skin scraping Clean skin with 70% alcohol. Scrape outer edge of ring in
cases of suspected ringworm; scrape area of active infection if
no ring. Place in sterile petri plate, & examine microscopically
using KOH.
Prepare a glass slide, a few drops of KOH will be
added to the skin sample, then a coverslip will be placed over
the top. Examine microscopically.
o 10% KOH – soft tissues (skin scrapings)
o 20% KOH – hard tissues (hairs, nails)
Nail Clean nails with 70% alcohol. Scrape area; dispose of outer
layer and collect inner, infected mail; may also collect using
nail clipper. Place nail in sterile petri plate. Cut nail into small
pieces and examine microscopically using KOH
Sputum, bronchial Should be collected during early morning on 3 consecutive
washings, morning days. Avoid 24-hour collections because of
transtracheal contamination and overgrowth. Expectorated sputum. Collect
aspirates in sterile saline aspirate.
Throat Collect with 2 sterile swabs. Scrape off and collect material
with tongue depressor
Urine Clean catch midstream of first morning void into a sterile
container. Centrifuge and use sediment for microscopic
examination. Process within 2 hours or refrigerate to avoid
bacterial overgrowth
Vaginal, cervical Collect with 2 swabs and place in transport media. Prepare
slide from one swab, and examine microscopically.
SITE BLOOD BORNE PATHOGENS
Blood/bone marrow Histoplasma capsulatum, Cryptococcus neoformans, Candida
albicans, Blastomyces dermatitidis,
Malassezia furfur
CSF Cryptococcus neoformans, Coccidioides immitis, Histoplasma
capsulatum, Candida spp
Hair Trichophyton, Microsporum
Skin Candida, Trichophyton, Microsporum, Epidermophyton
Nail Trichophyton, Epidermophyton, Candida, Aspergillus
Sputum, bronchial Candida, Aspergillus, Rhizopus, Mucor, Penicillium, Blastomyces
washings, dermatitidis, Coccidioides immitis,
transtracheal Paracoccidioides brasiliensis, Histoplasma capsulatum, Sporothrix
aspirates schenckii
Throat Candida albicans, Geotrichum candidum
Urine Candida, Blastomyces dermatitidis, Coccidioides immitis,
Histoplasma capsulatum, Cryptococcus
neoformans
Cervical, vaginal Candida
Common structures in Mycology
Hyphae – tubelike structures; fundamental units of fungus; many hyphae join to
form the mycelium, which forms the colony of the fungus
o Septate Hyphae – contains cross-walls or septa
o Aseptate Hyphae – lacks cross-walls
Yeast cells – clusters of round cells, budding forms
Arthoconidia – presence of spores, asexual spores formed by fragmentation of
mycelia into rectangular, barrel-shaped or cask-shaped, thick walled spores
Fusiform – spindle-shaped conidium that is wider in the middle & narrows
toward either end
Pseudohyphae – chain of cells produced by budding that may resemble true
hyphae. Constricted at the septa & form branches that begin at the septation.
Mass of pseudohyphae is pseudomycelium
Ascopores – small spores produced in a round, saclike ascus that contains 2-8
ascospores
Germ tube – no constriction. 3-4 times the length of the original yeast cell
Piriform – pear-shaped conidia
Spherule – large, round, thick walled
Tuberculate – with knoblike projections
Vous aimerez peut-être aussi
- Mycology: Specimen Collection & HandlingDocument46 pagesMycology: Specimen Collection & Handlingbetty kassawPas encore d'évaluation
- Mycology Specimen CollectionDocument6 pagesMycology Specimen CollectionMaam ShaPas encore d'évaluation
- Lab. Activity MycologyDocument61 pagesLab. Activity Mycologyuci marleyPas encore d'évaluation
- Types of Mycoses According To SiteDocument8 pagesTypes of Mycoses According To SiteAlyanna ManguerraPas encore d'évaluation
- Identification of FungiDocument44 pagesIdentification of FungiGianPas encore d'évaluation
- Methods of Studying Fungi: Dr. Alice Alma C. BungayDocument74 pagesMethods of Studying Fungi: Dr. Alice Alma C. BungayKaycee Gretz LorescaPas encore d'évaluation
- Collection of Cervical MucusDocument6 pagesCollection of Cervical MucusSurya AdhikariPas encore d'évaluation
- Sample Collection&TransportationDocument6 pagesSample Collection&TransportationGuna ChaitanyaPas encore d'évaluation
- Residents' Page: Methods of Specimen Collection For Diagnosis of SuperfiDocument4 pagesResidents' Page: Methods of Specimen Collection For Diagnosis of SuperfiasfwegerePas encore d'évaluation
- Germ Tube TestDocument4 pagesGerm Tube TestJericho SingcoPas encore d'évaluation
- Methods of Specimen Collection For Diagnosis of Superficial and Subcutaneous Fungal InfectionsDocument5 pagesMethods of Specimen Collection For Diagnosis of Superficial and Subcutaneous Fungal InfectionssaadmcsPas encore d'évaluation
- 9.3rd June - Mycology - Culture and StainingDocument41 pages9.3rd June - Mycology - Culture and StainingUmme RubabPas encore d'évaluation
- (OS 217 - IDS) LEC 04 Diagnostic MycologyDocument6 pages(OS 217 - IDS) LEC 04 Diagnostic MycologyErtyWitalayaL.ToruanPas encore d'évaluation
- Stool Examination (4)Document29 pagesStool Examination (4)محمد رحيم حسن محمودPas encore d'évaluation
- Lecture 1 FungiDocument23 pagesLecture 1 FungiDragon Gie30Pas encore d'évaluation
- Skin Scraping and A Potassium Hydroxide MountDocument8 pagesSkin Scraping and A Potassium Hydroxide MountbayoesunaryoPas encore d'évaluation
- SELF STUDY Specimen Collection and TransportDocument3 pagesSELF STUDY Specimen Collection and TransportAngelic AngelesPas encore d'évaluation
- Lec 15mycology SPPDocument35 pagesLec 15mycology SPPbujalkanPas encore d'évaluation
- Mycology Specimen CollectionDocument2 pagesMycology Specimen CollectionFuture TrekingPas encore d'évaluation
- Lecture 1 MycoDocument85 pagesLecture 1 MycoRona SalandoPas encore d'évaluation
- Flora of Skin by SwabDocument2 pagesFlora of Skin by SwabDevlina SenguptaPas encore d'évaluation
- Cabison - Mycology ReviewerDocument10 pagesCabison - Mycology ReviewerPatricia Cabison100% (1)
- Blood Culture CollectionDocument2 pagesBlood Culture CollectionNestor PlasabasPas encore d'évaluation
- Lab Diagnosis and TreatmentDocument22 pagesLab Diagnosis and TreatmentDavid lufafaPas encore d'évaluation
- Anaerobe ModuleDocument6 pagesAnaerobe ModuleKevin J. PizarroPas encore d'évaluation
- Laboratory Diagnosis of Fungal Infections: by Prof. Suzan El-FikyDocument56 pagesLaboratory Diagnosis of Fungal Infections: by Prof. Suzan El-FikyShama Al-Shadidi100% (1)
- NoneDocument4 pagesNoneMaw BerryPas encore d'évaluation
- Mycology Part 1Document191 pagesMycology Part 1Patricia Jean RodriguezPas encore d'évaluation
- BSC 204 Notes MidtermsDocument25 pagesBSC 204 Notes MidtermsWil LynPas encore d'évaluation
- Fungal Cultivation and Identification (Side Notes)Document8 pagesFungal Cultivation and Identification (Side Notes)Reca Marie FRIAS100% (1)
- 1-Collection, Storage and Transportataion of MicrobiologicalDocument49 pages1-Collection, Storage and Transportataion of MicrobiologicalSummi NizPas encore d'évaluation
- Sop Od DermatophytosisDocument10 pagesSop Od DermatophytosisAnanyaPas encore d'évaluation
- Lab Diagnosis of Fungal InfectionDocument31 pagesLab Diagnosis of Fungal InfectionVysakh P RPas encore d'évaluation
- Parasitology PDFDocument38 pagesParasitology PDFTofa IriePas encore d'évaluation
- Lab 9. Sample CollectionDocument23 pagesLab 9. Sample CollectionSuzan AbdPas encore d'évaluation
- 1ST - Microbiology and Parasitology (Laboratory)Document11 pages1ST - Microbiology and Parasitology (Laboratory)Krianne Chris DimaanoPas encore d'évaluation
- Mycology Lab Procedures Summer 2012Document23 pagesMycology Lab Procedures Summer 2012leoPas encore d'évaluation
- Additional Notes For CM AscpDocument4 pagesAdditional Notes For CM AscpAry OuiPas encore d'évaluation
- Capillary Tube Centrifugation (CTC)Document22 pagesCapillary Tube Centrifugation (CTC)MegbaruPas encore d'évaluation
- Práctica4.Cultivo de Exudado ÓticoDocument5 pagesPráctica4.Cultivo de Exudado ÓticoLuis RamirezPas encore d'évaluation
- Stool Routine ExaminationDocument38 pagesStool Routine Examinationharrykumbhar31Pas encore d'évaluation
- LESSON 7 Capillary Puncture Equipment and ProcedureDocument3 pagesLESSON 7 Capillary Puncture Equipment and ProcedureAlthea EspirituPas encore d'évaluation
- Agar-Gel Immunodiffusion (AGID) : EquipmentDocument6 pagesAgar-Gel Immunodiffusion (AGID) : Equipmenta教授Pas encore d'évaluation
- Skin Scraping and A Potassium Hydroxide Mount, KOHDocument4 pagesSkin Scraping and A Potassium Hydroxide Mount, KOHKuldeepSinghBanaPas encore d'évaluation
- Sample Collection, Transport & Processing - BloodDocument39 pagesSample Collection, Transport & Processing - BloodchambhaPas encore d'évaluation
- Capillary Puncture Equipment and Procedures: Topic 7Document39 pagesCapillary Puncture Equipment and Procedures: Topic 7Angelica Camille B. AbaoPas encore d'évaluation
- Wet MountDocument25 pagesWet MountJuan Marcos Martinez ZevallosPas encore d'évaluation
- Veterinary Laboratory ManualDocument44 pagesVeterinary Laboratory Manualmmohammed seidPas encore d'évaluation
- Presumptive DX: Pneumonia (Pneumococcal Pneumonia) (Usually Sudden, With Fever, ChillsDocument4 pagesPresumptive DX: Pneumonia (Pneumococcal Pneumonia) (Usually Sudden, With Fever, ChillsPaulene RiveraPas encore d'évaluation
- PPT4 Reviewer MycoViro DIAGNOSTIC MYCOLOGYDocument8 pagesPPT4 Reviewer MycoViro DIAGNOSTIC MYCOLOGYKaye Levine VaronaPas encore d'évaluation
- Ma. Minda Luz M. Manuguid, M.DDocument47 pagesMa. Minda Luz M. Manuguid, M.Djulo_05100% (1)
- MicrobiologySampleCollectionandTransport PDFDocument16 pagesMicrobiologySampleCollectionandTransport PDFWINDA YANIPas encore d'évaluation
- 13.introduction To MycologyDocument77 pages13.introduction To MycologyAzli GhadinPas encore d'évaluation
- Protocol-MYCOLOGY SPECIMEN COLLECTION091614Document3 pagesProtocol-MYCOLOGY SPECIMEN COLLECTION091614windaPas encore d'évaluation
- First Professional Questions and Answers by Hon. Ukpor IyangDocument86 pagesFirst Professional Questions and Answers by Hon. Ukpor IyangRhindaPas encore d'évaluation
- Unit 1 - 1.2 Techniques Oriented Examination of Specimens-Blood, CSF, Body Fluids, Pus and Aspirates, Throat Swab, StoolDocument47 pagesUnit 1 - 1.2 Techniques Oriented Examination of Specimens-Blood, CSF, Body Fluids, Pus and Aspirates, Throat Swab, StoolProvince Public Health Laboratory JanakpurPas encore d'évaluation
- Lecture 11 SCI 8007SEF Medical Microbiology & Virology Topic 5 - Introduction of Mycology - 12 Nov 2023Document80 pagesLecture 11 SCI 8007SEF Medical Microbiology & Virology Topic 5 - Introduction of Mycology - 12 Nov 2023YY CheungPas encore d'évaluation
- Sputum ExaminationDocument31 pagesSputum ExaminationMalliga Sundareshan100% (1)
- Clinical MicrobiologyDocument91 pagesClinical MicrobiologyDr. Jayesh Patidar67% (3)
- The Microscope. Its History, Construction, and Application 15th ed: Being a familiar introduction to the use of the instrument, and the study of microscopical scienceD'EverandThe Microscope. Its History, Construction, and Application 15th ed: Being a familiar introduction to the use of the instrument, and the study of microscopical sciencePas encore d'évaluation
- BB NotesDocument15 pagesBB NotesThea Gonzales100% (3)
- Part2MICRO (COMPILED)Document9 pagesPart2MICRO (COMPILED)Thea GonzalesPas encore d'évaluation
- AR-THEA Aug1-15Document1 pageAR-THEA Aug1-15Thea GonzalesPas encore d'évaluation
- Gonzales - Toxic Granulation Hema ReportDocument5 pagesGonzales - Toxic Granulation Hema ReportThea GonzalesPas encore d'évaluation
- "Arrowhead" ("Bowtie"/"butterfly-Like: Pin Head Colonies", Soft Butter-Like, Oil Paint, Grapelike ClustersDocument3 pages"Arrowhead" ("Bowtie"/"butterfly-Like: Pin Head Colonies", Soft Butter-Like, Oil Paint, Grapelike ClustersThea GonzalesPas encore d'évaluation
- Humble and Generous ServiceDocument5 pagesHumble and Generous ServiceThea Gonzales100% (1)
- Ascaris Lumbricoides: Ascariasis. Infections Most Often Seen in Young Children byDocument7 pagesAscaris Lumbricoides: Ascariasis. Infections Most Often Seen in Young Children byThea GonzalesPas encore d'évaluation
- Categories: Staphylococcus Aureus Escherichia ColiDocument2 pagesCategories: Staphylococcus Aureus Escherichia ColiThea GonzalesPas encore d'évaluation
- Gonzales, Thea Izza T. Mls IiicDocument6 pagesGonzales, Thea Izza T. Mls IiicThea GonzalesPas encore d'évaluation
- Form:: Form and Matter Ministers EffectsDocument9 pagesForm:: Form and Matter Ministers EffectsThea GonzalesPas encore d'évaluation
- Augustinian ChurchesDocument2 pagesAugustinian ChurchesThea GonzalesPas encore d'évaluation
- PhilLit MidtermsDocument49 pagesPhilLit MidtermsThea GonzalesPas encore d'évaluation
- Republic Act No 8981Document9 pagesRepublic Act No 8981Thea GonzalesPas encore d'évaluation
- Material ManagementDocument37 pagesMaterial ManagementThea GonzalesPas encore d'évaluation
- Kidney: Activity 14 The Urinary SystemDocument3 pagesKidney: Activity 14 The Urinary SystemThea GonzalesPas encore d'évaluation
- Activity 15 The Endocrine System Thyroid: Capayan, Mer Conrad L. Mls 3-CDocument5 pagesActivity 15 The Endocrine System Thyroid: Capayan, Mer Conrad L. Mls 3-CThea GonzalesPas encore d'évaluation
- Heart and Layers of The Heart Purkinje Fiber EndocardiumDocument2 pagesHeart and Layers of The Heart Purkinje Fiber EndocardiumThea GonzalesPas encore d'évaluation
- Activity 11 The Digestive System EsophagusDocument2 pagesActivity 11 The Digestive System EsophagusThea GonzalesPas encore d'évaluation
- Olfactory Mucosa: Activity 12 The Respiratory SystemDocument3 pagesOlfactory Mucosa: Activity 12 The Respiratory SystemThea GonzalesPas encore d'évaluation
- Responses To Drug Administration:: Factors Affecting Drug ResponseDocument4 pagesResponses To Drug Administration:: Factors Affecting Drug ResponseThea GonzalesPas encore d'évaluation
- Trematodes MidtermsDocument20 pagesTrematodes MidtermsThea GonzalesPas encore d'évaluation
- Trematodes MidtermsDocument20 pagesTrematodes MidtermsThea GonzalesPas encore d'évaluation
- Augustinian ChurchesDocument4 pagesAugustinian ChurchesThea GonzalesPas encore d'évaluation
- Chickenwings Restaurant Owned and Managed by Krizzy (Customer's Name: Chriza Jem S. Suello)Document18 pagesChickenwings Restaurant Owned and Managed by Krizzy (Customer's Name: Chriza Jem S. Suello)Thea GonzalesPas encore d'évaluation
- PhilLit MidtermsDocument55 pagesPhilLit MidtermsThea Gonzales0% (1)
- IQ TestDocument11 pagesIQ TestThea GonzalesPas encore d'évaluation
- 107 Reaction PaperDocument1 page107 Reaction PaperKL Ea100% (1)
- Vaginal Discharge in DogsDocument5 pagesVaginal Discharge in DogsDarmawan Prastyo100% (1)
- Breathing and RespirationDocument5 pagesBreathing and RespirationPriyansh PatelPas encore d'évaluation
- This Study Resource WasDocument3 pagesThis Study Resource WasCarlito AglipayPas encore d'évaluation
- Psych-K Practical ApplicationsDocument10 pagesPsych-K Practical Applicationsdeluca4482% (17)
- Seizure Updated ILAE ClassificationDocument12 pagesSeizure Updated ILAE ClassificationNasheei RadjaPas encore d'évaluation
- SAOB AS OF December 31 2011Document5 pagesSAOB AS OF December 31 2011bacsecPas encore d'évaluation
- Assessment Standard 4 Traits Employability Skills TESTDocument5 pagesAssessment Standard 4 Traits Employability Skills TESTnurse1990Pas encore d'évaluation
- Case Study GerdDocument3 pagesCase Study Gerdapi-287249002Pas encore d'évaluation
- Nursing Note Sample 17Document6 pagesNursing Note Sample 17Lanzen Dragneel100% (1)
- OPD Schedule (November 2021)Document32 pagesOPD Schedule (November 2021)Ajay DherwaniPas encore d'évaluation
- Intensive Behavioral Therapy For Autism Spectrum Disorders: Coverage RationaleDocument14 pagesIntensive Behavioral Therapy For Autism Spectrum Disorders: Coverage Rationalediana100% (1)
- Serotonin: Perception of Resource AvailabilityDocument3 pagesSerotonin: Perception of Resource AvailabilityStregattoPas encore d'évaluation
- Anaphy (Lab) ReviewerDocument3 pagesAnaphy (Lab) ReviewerAgatha Cristie AndradaPas encore d'évaluation
- Hospital and Clinical Pharmacist PDFDocument23 pagesHospital and Clinical Pharmacist PDFmajd67% (6)
- Work Comp C4Document5 pagesWork Comp C4ijustwanawritePas encore d'évaluation
- 2022 - ACLS - Handbook 1 30 13 30 PDFDocument18 pages2022 - ACLS - Handbook 1 30 13 30 PDFJefferson MoraPas encore d'évaluation
- GoniometryDocument22 pagesGoniometryDuppala Sateesh KumarPas encore d'évaluation
- Benign Diseases of ThyroidDocument70 pagesBenign Diseases of ThyroidMounica MekalaPas encore d'évaluation
- Implant PanaceaDocument2 pagesImplant PanaceaKarina OjedaPas encore d'évaluation
- PlaintDocument20 pagesPlaintSaige DriverPas encore d'évaluation
- PSAvs PsadDocument6 pagesPSAvs PsadRaga ManduaruPas encore d'évaluation
- FastDocument10 pagesFastAnonymous Lxho3IPas encore d'évaluation
- Nejme 2305752Document2 pagesNejme 23057525fqkqkcdhtPas encore d'évaluation
- DC-TMD SQ Shortform 2013-05-12Document2 pagesDC-TMD SQ Shortform 2013-05-12Luz MendozaPas encore d'évaluation
- Hizon, DrugsDocument4 pagesHizon, DrugsDan HizonPas encore d'évaluation
- Case Study - Seizuring DogDocument8 pagesCase Study - Seizuring Dogapi-301746262Pas encore d'évaluation
- Sumithion 40 WPDocument7 pagesSumithion 40 WPIzzat RusliPas encore d'évaluation
- Drug Tariff July 2014 PDFDocument784 pagesDrug Tariff July 2014 PDFGisela Cristina MendesPas encore d'évaluation
- What Is Permanent Make-Up?: First in Looks That LastDocument4 pagesWhat Is Permanent Make-Up?: First in Looks That LastNatural Enhancement0% (1)