Académique Documents
Professionnel Documents
Culture Documents
CEA 11973223001 - en
Transféré par
ModestusTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
CEA 11973223001 - en
Transféré par
ModestusDroits d'auteur :
Formats disponibles
11973223001V15
CEA
Carcinoembryonic antigen
11731629 322 100 tests Application of a voltage to the electrode then induces chemiluminescent
emission which is measured by a photomultiplier.
• Indicates analyzers on which the kit can be used
• Results are determined via a calibration curve which is
MODULAR instrument-specifically generated by 2-point calibration and a
Elecsys 1010 Elecsys 2010 ANALYTICS cobas e 411 cobas e 601 master curve provided via the reagent barcode.
E170 a) Tris(2,2’-bipyridyl)ruthenium(II)-complex (Ru(bpy)2+
3 )
• • • • • Reagents - working solutions
M Streptavidin-coated microparticles (transparent cap), 1 bottle, 8 mL:
English Streptavidin-coated microparticles 0.72 mg/mL; preservative.
Please note R1 Anti-CEA-Ab~biotin (gray cap), 1 bottle, 10 mL:
The measured CEA value of a patient’s sample can vary depending on the Biotinylated monoclonal anti-CEA antibody (mouse/human) 3.0 mg/L;
testing procedure used. The laboratory finding must therefore always contain a phosphate buffer 100 mmol/L, pH 6.0; preservative.
statement on the CEA assay method used. CEA values determined on patient R2 Anti-CEA-Ab~Ru(bpy)2+ 3 (black cap), 1 bottle, 8 mL:
samples by different testing procedures cannot be directly compared with one Monoclonal anti-CEA antibody (mouse) labeled with ruthenium
another and could be the cause of erroneous medical interpretations. complex 4.0 mg/L; phosphate buffer 100 mmol/L, pH 6.5; preservative.
If there is a change in the CEA assay procedure used while monitoring therapy, Precautions and warnings
then the CEA values obtained upon changing over to the new procedure For in vitro diagnostic use.
must be confirmed by parallel measurements with both methods. Exercise the normal precautions required for handling all laboratory reagents.
For USA: Caution: US Federal law restricts this device to sale and Disposal of all waste material should be in accordance with local guidelines.
distribution by or on the order of a physician, or to a laboratory; and its Safety data sheet available for professional user on request.
use is restricted to, by or on the order of a physician. Avoid the formation of foam with all reagents and sample types
(specimens, calibrators, and controls).
Intended use
Immunoassay for the in vitro quantitative determination of carcinoembryonic Reagent handling
antigen in human serum and plasma. The Elecsys CEA assay The reagents in the kit have been assembled into a ready-for-use
is further indicated for serial measurement of CEA to aid in the unit that cannot be separated.
management of cancer patients. All information required for correct operation is read in via the
The electrochemiluminescence immunoassay “ECLIA” is intended for respective reagent barcodes.
use on Elecsys and cobas e immunoassay analyzers. Storage and stability
Summary Store at 2-8°C.
CEA is a monomeric glycoprotein (molecular weight approx. 180,000 daltons) Store the Elecsys CEA reagent kit upright in order to ensure complete
with a variable carbohydrate component of approx. 45-60%.1 availability of the microparticles during automatic mixing prior to use.
CEA, like AFP, belongs to the group of carcinofetal antigens that are produced Stability:
during the embryonic and fetal period. The CEA gene family consists of unopened at 2-8°C: up to the stated expiration date
about 17 active genes in two subgroups.2 The first group contains CEA after opening at 2-8°C: 12 weeks
and the Non-specific Cross-reacting Antigens (NCA); the second group on MODULAR ANALYTICS E170 and
contains the Pregnancy-Specific Glycoproteins (PSG). cobas e 601: 6 weeks
CEA is mainly found in the fetal gastrointestinal tract and in fetal serum. It on Elecsys 2010 and cobas e 411: 6 weeks
also occurs in slight quantities in intestinal, pancreatic, and hepatic tissue of
on Elecsys 1010: 4 weeks (stored alternately in the
healthy adults. The formation of CEA is repressed after birth, and accordingly
refrigerator and on the analyzer -
serum CEA values are hardly measurable in healthy adults.
ambient temperature 20-25°C; up to
High CEA concentrations are frequently found in cases of colorectal
20 hours opened in total)
adenocarcinoma.6,8 Slight to moderate CEA elevations (rarely > 10 ng/mL)
occur in 20-50% of benign diseases of the intestine, the pancreas, the liver, and Specimen collection and preparation
the lungs (e.g. liver cirrhosis, chronic hepatitis, pancreatitis, ulcerative colitis, Only the specimens listed below were tested and found acceptable.
Crohn’s Disease, emphysema).6,7 Smokers also have elevated CEA values. Serum collected using standard sampling tubes or tubes
The main indication for CEA determinations is the follow-up and containing separating gel.
therapy-management of colorectal carcinoma. Na-heparin, K3-EDTA, and sodium citrate plasma. When sodium heparin and
CEA determinations are not recommended for cancer-screening in the sodium citrate are used, the results obtained must be corrected by + 10%.
general population. CEA concentrations within the normal range do not Criterion: Recovery within 90-110% of serum value or slope 0.9-1.1 + intercept
exclude the possible presence of a malignant disease. within <± 2 x analytical sensitivity (LDL) + coefficient of correlation > 0.95.
The antibodies react with CEA and (as with almost all CEA methods) with Stable for 7 days at 2-8°C, 6 months at -20°C.9
the meconium antigen (NCA2).5 Cross-reactivity with NCA1 is 0.7%. The sample types listed were tested with a selection of sample collection tubes
The reactive epitopes of CEA have been characterized, and the available that were commercially available at the time of testing, i.e. not all available
monoclonal antibodies classified into 6 epitope groups.3,4 The antibodies tubes of all manufacturers were tested. Sample collection systems from
used in the Elecsys CEA assay react with epitopes 2 and 5. various manufacturers may contain differing materials which could affect
Test principle the test results in some cases. When processing samples in primary tubes
Sandwich principle. Total duration of assay: 18 minutes. (sample collection systems), follow the instructions of the tube manufacturer.
• 1st incubation: 10 µL of sample, a biotinylated monoclonal CEA-specific Centrifuge samples containing precipitates before performing the
antibody, and a monoclonal CEA-specific antibody labeled with a assay. Do not use heat-inactivated samples. Do not use samples
ruthenium complexa react to form a sandwich complex. and controls stabilized with azide.
Ensure the patients’ samples, calibrators, and controls are at ambient
• 2nd incubation: After addition of streptavidin-coated microparticles,
temperature (20-25°C) before measurement.
the complex becomes bound to the solid phase via interaction
Because of possible evaporation effects, samples, calibrators, and controls
of biotin and streptavidin.
on the analyzers should be measured within 2 hours.
• The reaction mixture is aspirated into the measuring cell where the
microparticles are magnetically captured onto the surface of the Materials provided
electrode. Unbound substances are then removed with ProCell. See “Reagents - working solutions” section for reagents.
2007-08, V 15 English 1/3 Elecsys and cobas e analyzers
CEA
Carcinoembryonic antigen
Materials required (but not provided) • after 7 days (ambient temperature 20-25°C)
• Cat. No. 11731645, CEA CalSet, 4 x 1 mL • after 3 days (ambient temperature 25-32°C)
• Cat. No. 11776452, PreciControl Tumor Marker, for 2 x 3 mL For all analyzers:
each of PreciControl Tumor Marker 1 and 2 • as required: e.g. quality control findings outside the specified limits
• Cat. No. 11732277, Diluent Universal, 2 x 16 mL sample diluent or
Cat. No. 03183971, Diluent Universal, 2 x 36 mL sample diluent Quality control
• General laboratory equipment For quality control, use Elecsys PreciControl Tumor Marker 1 and 2.
• Elecsys 1010/2010, MODULAR ANALYTICS E170 or cobas e analyzer Other suitable control material can be used in addition.
Controls for the various concentration ranges should be run as single
Accessories for Elecsys 1010/2010 and cobas e 411 analyzers: determinations at least once every 24 hours when the test is in use, once
• Cat. No. 11662988, ProCell, 6 x 380 mL system buffer per reagent kit, and after every calibration. The control intervals and
• Cat. No. 11662970, CleanCell, 6 x 380 mL measuring cell cleaning solution limits should be adapted to each laboratory’s individual requirements.
• Cat. No. 11930346, Elecsys SysWash, 1 x 500 mL washwater additive Values obtained should fall within the defined limits.
• Cat. No. 11933159, Adapter for SysClean Each laboratory should establish corrective measures to be taken
• Cat. No. 11706829, Elecsys 1010 AssayCup, 12 x 32 reaction vessels or if values fall outside the limits.
Cat. No. 11706802, Elecsys 2010 AssayCup, 60 x 60 reaction vessels
Calculation
• Cat. No. 11706799, Elecsys 2010 AssayTip, 30 x 120 pipette tips The analyzer automatically calculates the analyte concentration of
Accessories for MODULAR ANALYTICS E170 and cobas e 601 analyzers: each sample (either in ng/mL or µg/L).
• Cat. No. 04880340, ProCell M, 2 x 2 L system buffer 1 ng/mL CEA corresponds to 16.9 mIU/mL.
• Cat. No. 04880293, CleanCell M, 2 x 2 L measuring cell cleaning solution
Limitations - interference
• Cat. No. 12135027, CleanCell M, 1 x 2 L measuring cell The assay is unaffected by icterus (bilirubin < 1129 µmol/L or < 66 mg/dL),
cleaning solution (for USA) hemolysis (Hb < 1.4 mmol/L or < 2.2 g/dL), lipemia (Intralipid < 1500 mg/dL),
• Cat. No. 03023141, PC/CC-Cups, 12 cups to prewarm ProCell M and biotin < 491 nmol/L or < 120 ng/mL.
and CleanCell M before use Criterion: Recovery within ± 10% of initial value.
• Cat. No. 03005712, ProbeWash M, 12 x 70 mL cleaning solution for In patients receiving therapy with high biotin doses (i.e. > 5 mg/day), no sample
run finalization and rinsing during reagent change should be taken until at least 8 hours after the last biotin administration.
• Cat. No. 12102137, AssayTip/AssayCup Combimagazine M, 48 magazines No interference was observed from rheumatoid factors up to
x 84 reaction vessels or pipette tips, waste bags a concentration of 1500 IU/mL.
• Cat. No. 03023150, WasteLiner, waste bags There is no high-dose hook effect at CEA concentrations up to 200,000 ng/mL.
• Cat. No. 03027651, SysClean Adapter M In vitro tests were performed on 26 commonly used pharmaceuticals.
No interference with the assay was found.
Accessories for all analyzers:
As with all tests containing monoclonal mouse antibodies, erroneous findings
• Cat. No. 11298500, Elecsys SysClean, 5 x 100 mL system cleaning solution
may be obtained from samples taken from patients who have been treated with
Only available in the USA: monoclonal mouse antibodies or have received them for diagnostic purposes.
• Cat. No. 11776754 Elecsys CEA CalCheck, 3 concentration ranges In rare cases, interference due to extremely high titers of antibodies
to streptavidin and ruthenium can occur.
Assay The test contains additives which minimize these effects.
For optimum performance of the assay follow the directions given in For diagnostic purposes, the results should always be assessed in conjunction
this document for the analyzer concerned. Refer to the appropriate with the patient’s medical history, clinical examination and other findings.
operator manual for analyzer-specific assay instructions.
Resuspension of the microparticles takes place automatically before use. Measuring range
Read in the test-specific parameters via the reagent barcode. If in exceptional 0.200-1000 ng/mL (defined by the lower detection limit and the maximum
cases the barcode cannot be read, enter the 15-digit sequence of numbers. of the master curve). Values below the detection limit are reported as
< 0.200 ng/mL. Values above the measuring range are reported as
MODULAR ANALYTICS E170, Elecsys 2010 and cobas e analyzers: Bring the
> 1000 ng/mL (or up to 50,000 ng/mL for 50-fold diluted samples).
cooled reagents to approx. 20°C and place on the reagent disk (20°C) of the
analyzer. Avoid the formation of foam. The system automatically regulates Dilution
the temperature of the reagents and the opening/closing of the bottles. Samples with CEA concentrations above the measuring range can be diluted
Elecsys 1010 analyzer: Bring the cooled reagents to approx. 20-25°C and with Elecsys Diluent Universal. The recommended dilution is 1:50 (either
place on the sample/reagent disk of the analyzer (ambient temperature automatically by the MODULAR ANALYTICS E170, Elecsys 1010/2010 or
20-25°C). Avoid the formation of foam. Open bottle caps manually before cobas e analyzers or manually). The concentration of the diluted sample
use and close manually after use. Store at 2-8°C after use. must be > 20 ng/mL. After manual dilution, multiply the result by the dilution
factor. After dilution by the analyzers, the MODULAR ANALYTICS E170,
Calibration Elecsys 1010/2010 and cobas e software automatically takes the dilution
Traceability: This method has been standardized against the 1st into account when calculating the sample concentration.
IRP WHO Reference Standard 73/601.
Every Elecsys CEA reagent set has a barcoded label containing the specific Expected values
information for calibration of the particular reagent lot. The predefined master Studies with the Elecsys CEA assay were from performed on 352
curve is adapted to the analyzer by the use of Elecsys CEA CalSet. healthy subjects. The following results were obtained:
Calibration frequency: Calibration must be performed once per reagent lot Non-smokers Smokers
All subjects
using fresh reagent (i.e. not more than 24 hours since the reagent kit was (past/never smokers) (current)
registered on the analyzer). Renewed calibration is recommended as follows: Age (years) 20-69 ≥ 40 20-69 ≥ 40 20-69 ≥ 40
MODULAR ANALYTICS E170, Elecsys 2010 and cobas e analyzers: 95th percentile
(ng/mL) 4.7 5.2 3.8 5.0 5.5 6.5
• after 1 month (28 days) when using the same reagent lot
• after 7 days (when using the same reagent kit on the analyzer) N 352 203 242 154 110 49
Elecsys 1010 analyzer: Each laboratory should investigate the transferability of the expected values to
• with every reagent kit its own patient population and if necessary determine its own reference ranges.
Elecsys and cobas e analyzers 2/3 2007-08, V 15 English
11973223001V15
CEA
Carcinoembryonic antigen
Specific performance data References
Representative performance data on the analyzers are given below. 1. Gold P, Freedman SO. Demonstration of tumor-specific antigen in
Results obtained in individual laboratories may differ. human colonic carcinomata. J Exp Med 1965;121:(3)439.
2. Thompson JA. Molecular cloning and expression of carcinoembryonic
Precision antigen gene family members. Tumor Biol 1995;16:10-16.
Reproducibility was determined using Elecsys reagents, pooled human 3. Hammarström S, et al. Antigenic sites in carcinoembyronic antigen.
sera, and controls in a modified protocol (EP5-A) of the NCCLS (National Cancer Research 1989;49:4852-4858.
Committee for Clinical Laboratory Standards): 6 times daily for 10 days 4. Bormer OP, Thrane-Steen K. Epitope group specificity of six
(n = 60); within-run precision on MODULAR ANALYTICS E170 analyzer, immunoassays for carcino-embyronic antigen. Tumor Biol 1991;12:9-15.
n = 21. The following results were obtained: 5. Kuroki M, et al. Reaction profiles of seven enzyme immonoassay kits for
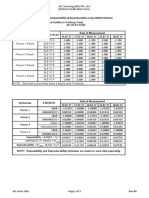
Elecsys 1010/2010 and cobas e 411 analyzers carcinoembyronic antigen (CEA) analyzed with purified preparations of
Within-run precision Total precision CEA and related normal antigens. Clin Biochem 1992;25:29-35.
Sample Mean SD CV SD CV 6. Stieber P, Fateh-Moghadam A. Sensible use of tumor markers.
ng/mL ng/mL ng/mL Boehringer Mannheim, Cat. No. 1536869 (Engl.), 1320947
% %
(German). ISBN 3-926725-07-9 German/English. Juergen Hartmann
Human serum 1 2.2 0.11 5.0 0.12 5.4 Verlag Marloffstein-Rathsberg (1993).
Human serum 2 19.6 0.32 1.6 0.44 2.3 7. Sell SS. Serological Cancer Markers. Humana Press 1992;
Human serum 3 528 6.82 1.3 10.6 2.0 ISBN 0-89603-209-4.
PreciControl TMb1 4.9 0.12 2.5 0.18 3.6 8. Ballesta AM, Molina R, Filella X, Jo J, Gimenez N. Carcinoembryonic
PreciControl TM2 34.1 0.58 1.7 1.02 3.0 Antigen in Staging and Follow-up of Patients with Solid
b) TM = Tumor Marker Tumors. Tumor Biol 1995;16:32-41.
9. Guder WG, Narayanan S, Wisser H, Zawta B. List of Analytes;
MODULAR ANALYTICS E170 and cobas e 601 analyzers Pre-analytical Variables. Brochure in: Samples: From the Patient to the
Within-run precision Total precision Laboratory. GIT-Verlag, Darmstadt 1996:10. ISBN 3-928865-22-6.
Sample Mean SD CV Mean SD CV 10. Bablok W, et al. A General Regression Procedure for Method
ng/mL ng/mL % ng/mL ng/mL % Transformation. J Clin Chem Clin Biochem 1988;26:783-790.
Human serum 1 3.32 0.05 1.3 3.90 0.18 4.7 For further information, please refer to the appropriate operator’s manual
Human serum 2 225 2.53 1.0 252 11.6 4.6 for the analyzer concerned, the respective application sheets, the product
Human serum 3 626 11.8 1.9 699 34.8 5.0 information, and the package inserts of all necessary components.
PreciControl TM1 4.38 0.10 2.5 4.74 0.24 5.1
FOR US CUSTOMERS ONLY: LIMITED WARRANTY
PreciControl TM2 33.8 0.73 2.0 34.9 1.71 4.9
Roche Diagnostics warrants that this product will meet the specifications
Analytical sensitivity (lower detection limit) stated in the labeling when used in accordance with such labeling and
0.20 ng/mL will be free from defects in material and workmanship until the expiration
The detection limit represents the lowest measurable analyte level that date printed on the label. THIS LIMITED WARRANTY IS IN LIEU OF ANY
can be distinguished from zero. It is calculated as the value lying two OTHER WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED
standard deviations above that of the lowest standard (master calibrator, WARRANTY OF MERCHANTABILITY OR FITNESS FOR PARTICULAR
standard 1 + 2 SD, within-run precision, n = 21). PURPOSE. IN NO EVENT SHALL ROCHE DIAGNOSTICS BE LIABLE FOR
INCIDENTAL, INDIRECT, SPECIAL OR CONSEQUENTIAL DAMAGES.
Method comparison
A comparison of the Elecsys CEA assay (y) with the Enzymun-Test CEA
method (x) using clinical samples gave the following correlations:
Number of samples measured: 108 COBAS, COBAS E, ELECSYS, ENZYMUN-TEST and MODULAR are trademarks of Roche. Other brand or
Passing/Bablok10 Linear regression product names are trademarks of their respective holders. INTRALIPID is a trademark of Fresenius Kabi AB.
Significant additions or changes are indicated by a change bar in the margin. Changes to reagent barcode
y = 0.91x + 0.06 y = 0.90x + 0.04 test parameters which have already been read in should be edited manually.
©2007 Roche Diagnostics.
τ = 0.913 r = 0.992
The sample concentrations were between approx. 0.7 and 52 ng/mL.
Analytical specificity Roche Diagnostics GmbH, D-68298 Mannheim
for USA: US Distributor:
For the monoclonal antibodies used, the following cross-reactivities were found: Roche Diagnostics, Indianapolis, IN
NCA1 < 0.7%, NCA2 72%. US Customer Technical Support 1-800-428-2336
No cross-reactivity with AFP and α1-acid glycoprotein.
No investigations into possible cross-reactivity with glycoproteins
from the lungs and liver have been performed.
2007-08, V 15 English 3/3 Elecsys and cobas e analyzers
Vous aimerez peut-être aussi
- A TpoDocument4 pagesA TpoJimboreanu György PaulaPas encore d'évaluation
- Insert - Elecsys Anti-HBs II - Ms - 05894816190.V2.EnDocument4 pagesInsert - Elecsys Anti-HBs II - Ms - 05894816190.V2.EnyantuPas encore d'évaluation
- Insert - Elecsys Total PSA - Ms - 04641655190.V13.EnDocument5 pagesInsert - Elecsys Total PSA - Ms - 04641655190.V13.Entawfiq MohammadPas encore d'évaluation
- Insert - Elecsys Syphilis - Ms 07802960190.V3.EnDocument5 pagesInsert - Elecsys Syphilis - Ms 07802960190.V3.EnGuneyden GuneydenPas encore d'évaluation
- Trouble Shooting Elecsys2 010v53Document142 pagesTrouble Shooting Elecsys2 010v53Hồ Thế NguyênPas encore d'évaluation
- Elecsys Hbsag Ii: A) Tris (2,2'-Bipyridyl) Ruthenium (Ii) - Complex (Ru (Bpy) )Document5 pagesElecsys Hbsag Ii: A) Tris (2,2'-Bipyridyl) Ruthenium (Ii) - Complex (Ru (Bpy) )Brian SamanyaPas encore d'évaluation
- Ft4 Ii: Free ThyroxineDocument4 pagesFt4 Ii: Free ThyroxinehairiPas encore d'évaluation
- Lab Policies Free Thyroxine FT4 Cobas E601 Lab 4045Document4 pagesLab Policies Free Thyroxine FT4 Cobas E601 Lab 4045TohăneanR.RomeliaPas encore d'évaluation
- PreciControl AMH - Ms 06709966190.V2.EnDocument2 pagesPreciControl AMH - Ms 06709966190.V2.EnARIF AHAMMED PPas encore d'évaluation
- ACTHDocument4 pagesACTHHassan GillPas encore d'évaluation
- COBAS Elecsys HBsAgDocument45 pagesCOBAS Elecsys HBsAgmeghnaPas encore d'évaluation
- Lab Policies Hemoglobin A1C - Cobas c501 Lab 4004Document6 pagesLab Policies Hemoglobin A1C - Cobas c501 Lab 4004yosefinPas encore d'évaluation
- Urea Nitrogen AnalyzerDocument16 pagesUrea Nitrogen AnalyzerSkywalker_92Pas encore d'évaluation
- Elecsys BRAHMS PCT: ProcalcitoninDocument5 pagesElecsys BRAHMS PCT: ProcalcitoninMilagrosLcPas encore d'évaluation
- Insert TRIGL 0020767107322COIN V11 enDocument4 pagesInsert TRIGL 0020767107322COIN V11 entechlabPas encore d'évaluation
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3Document26 pagesLyphochek Immunoassay Plus Control Levels 1, 2 and 3Aniket dubeyPas encore d'évaluation
- Calset TSHDocument2 pagesCalset TSHJimboreanu György Paula100% (1)
- ALTDocument10 pagesALTLiviu Athos Tamas0% (1)
- Elecsys Reference RangesDocument95 pagesElecsys Reference Rangescandy jmzPas encore d'évaluation
- PreciControl Anti CCP.05115680001.V3.EnDocument2 pagesPreciControl Anti CCP.05115680001.V3.EnARIF AHAMMED PPas encore d'évaluation
- B08179 IFU Gentian Cystatin C Reagent Kit AU Systems REF1103 7725-V09usaDocument9 pagesB08179 IFU Gentian Cystatin C Reagent Kit AU Systems REF1103 7725-V09usaAlberto Marcos100% (1)
- A1C-2 Whole Blood enDocument6 pagesA1C-2 Whole Blood enSyahdie FahlediePas encore d'évaluation
- TotalBhCG ARCDocument7 pagesTotalBhCG ARCLau GómezPas encore d'évaluation
- XN-L - Reference Interval From General Information 2017Document4 pagesXN-L - Reference Interval From General Information 2017widiawaty100% (1)
- ELISA Test For The Quantitative Determination of Total Thyroxine (T4) in Human SerumDocument2 pagesELISA Test For The Quantitative Determination of Total Thyroxine (T4) in Human SerumMaherPas encore d'évaluation
- LDLC3Document4 pagesLDLC3Michael HenzuPas encore d'évaluation
- Sysmex XW - 100: Instructions For Use ManualDocument32 pagesSysmex XW - 100: Instructions For Use ManualNahom BalchaPas encore d'évaluation
- Totalt4 ArcDocument6 pagesTotalt4 Arctesteste testePas encore d'évaluation
- Instructions For Use TSHDocument12 pagesInstructions For Use TSHBenjamin MannPas encore d'évaluation
- Elecsys T4: Cobas e 411 Cobas e 601 Cobas e 602 English System InformationDocument5 pagesElecsys T4: Cobas e 411 Cobas e 601 Cobas e 602 English System InformationIsmael CulquiPas encore d'évaluation
- EBX 041 192 - FT - EurobioPlex SARS CoV 2 Multiplex - EN - v4.00 - 20 04 20202 PDFDocument17 pagesEBX 041 192 - FT - EurobioPlex SARS CoV 2 Multiplex - EN - v4.00 - 20 04 20202 PDFAncapc AncapcPas encore d'évaluation
- ALP Testing SOPDocument6 pagesALP Testing SOPUMMID WashimPas encore d'évaluation
- Measure CRP Levels with Latex TurbidimetryDocument2 pagesMeasure CRP Levels with Latex TurbidimetryDharmesh Patel100% (1)
- Orac MethodDocument5 pagesOrac MethodPriscillaL.SilvaPas encore d'évaluation
- TSH Acculite Clia Rev 4Document2 pagesTSH Acculite Clia Rev 4ghumantuPas encore d'évaluation
- Multistix Reagent StripDocument3 pagesMultistix Reagent StripFrancis TorresPas encore d'évaluation
- Antihbs ArcDocument6 pagesAntihbs ArcYu YingPas encore d'évaluation
- Riqas QCDocument26 pagesRiqas QCCarlos Rodriguez GutierrezPas encore d'évaluation
- Competitive Evaluation of The GEM Premier 3000 With PDFDocument8 pagesCompetitive Evaluation of The GEM Premier 3000 With PDFEllya Latifah IlyasPas encore d'évaluation
- E Anti-TgDocument4 pagesE Anti-TgHassan GillPas encore d'évaluation
- GPE01006.004 ADVIA Chem Systems Operator Training ManualDocument440 pagesGPE01006.004 ADVIA Chem Systems Operator Training ManualОлександр100% (1)
- Creatinine - EnzymaticDocument2 pagesCreatinine - Enzymaticpsychejane100% (1)
- Lyphochek Assayed Chemistry Control Levels 1 and 2: MéthodeDocument1 pageLyphochek Assayed Chemistry Control Levels 1 and 2: MéthodeAnouar Nouioui100% (2)
- ETOHDocument4 pagesETOHARIF AHAMMED PPas encore d'évaluation
- Tests Affected by Haemolysed, Icteric and Lipemic Samples, W5-SOP-1-1-1Document2 pagesTests Affected by Haemolysed, Icteric and Lipemic Samples, W5-SOP-1-1-1Dejan BodetićPas encore d'évaluation
- CobasDocument3 pagesCobaskigm mkjPas encore d'évaluation
- 30 308 Vidas Anti-HCV: Summary and ExplanationDocument8 pages30 308 Vidas Anti-HCV: Summary and ExplanationHaider AlmothaferPas encore d'évaluation
- Assay SolutionsDocument1 pageAssay SolutionsAlex RamirezPas encore d'évaluation
- Evaluation of Antimicrobial AgentsDocument8 pagesEvaluation of Antimicrobial AgentsSureshCoolPas encore d'évaluation
- Alkaline Phosphatase Activity Test KitDocument2 pagesAlkaline Phosphatase Activity Test KitRury Darwa Ningrum100% (1)
- T4 IflashDocument4 pagesT4 IflashNIGHT tubePas encore d'évaluation
- hdlc4 2017-07 v2Document5 pageshdlc4 2017-07 v2Farid AbderahmanePas encore d'évaluation
- Totalt3 ArcDocument6 pagesTotalt3 ArcTanveerPas encore d'évaluation
- At HemoglobinDocument2 pagesAt HemoglobinzulfiPas encore d'évaluation
- Measure alpha-amylase levels with this single reagent kitDocument4 pagesMeasure alpha-amylase levels with this single reagent kitpetertrungPas encore d'évaluation
- Method Verification of TestDocument2 pagesMethod Verification of TestObak PrithibiPas encore d'évaluation
- 1.4. MonographsDocument2 pages1.4. MonographsArchanPas encore d'évaluation
- CREJ2Document4 pagesCREJ2ARIF AHAMMED PPas encore d'évaluation
- COCIIDocument6 pagesCOCIIARIF AHAMMED PPas encore d'évaluation
- Pharmaceutical and Biomedical Applications of Capillary ElectrophoresisD'EverandPharmaceutical and Biomedical Applications of Capillary ElectrophoresisPas encore d'évaluation
- T UptakeDocument3 pagesT UptakeModestus100% (1)
- CA 125 II 12176645001 - enDocument4 pagesCA 125 II 12176645001 - enModestusPas encore d'évaluation
- Cardiac Troponin TDocument2 pagesCardiac Troponin TModestusPas encore d'évaluation
- CA 19-9 11872141001 - enDocument3 pagesCA 19-9 11872141001 - enModestusPas encore d'évaluation
- C-Peptide 03724875001 - enDocument4 pagesC-Peptide 03724875001 - enModestusPas encore d'évaluation
- Calset CeaDocument1 pageCalset CeaModestusPas encore d'évaluation
- Anti HCVDocument4 pagesAnti HCVModestusPas encore d'évaluation
- AFP CalsetDocument1 pageAFP CalsetModestusPas encore d'évaluation
- FerritinDocument3 pagesFerritinModestus100% (1)
- Vitamin B12Document4 pagesVitamin B12ModestusPas encore d'évaluation
- CMV Igm: Igm Antibodies To Cytomegalovirus 04784618 190 100 TestsDocument4 pagesCMV Igm: Igm Antibodies To Cytomegalovirus 04784618 190 100 TestsModestusPas encore d'évaluation
- NIH Public Access: Author ManuscriptDocument13 pagesNIH Public Access: Author ManuscriptSyaiful ArifinPas encore d'évaluation
- 20 Urinary Incontinence LR1Document75 pages20 Urinary Incontinence LR1ModestusPas encore d'évaluation
- Bab IiDocument23 pagesBab IiModestusPas encore d'évaluation
- Hepatic Dysfunction FalciparumDocument8 pagesHepatic Dysfunction FalciparummodestusPas encore d'évaluation
- 20 Urinary Incontinence LR1Document75 pages20 Urinary Incontinence LR1ModestusPas encore d'évaluation
- 376 FullDocument13 pages376 FullModestusPas encore d'évaluation
- History shapes Philippine societyDocument4 pagesHistory shapes Philippine societyMarvin GwapoPas encore d'évaluation
- Department of Education Doña Asuncion Lee Integrated School: Division of Mabalacat CityDocument2 pagesDepartment of Education Doña Asuncion Lee Integrated School: Division of Mabalacat CityRica Tano50% (2)
- Book3 79 111000 0000100120 DAH MPL RPT 000005 - ADocument101 pagesBook3 79 111000 0000100120 DAH MPL RPT 000005 - ANassif Abi AbdallahPas encore d'évaluation
- Canterburytales-No Fear PrologueDocument10 pagesCanterburytales-No Fear Prologueapi-261452312Pas encore d'évaluation
- BBRC4103 - Research MethodologyDocument14 pagesBBRC4103 - Research MethodologySimon RajPas encore d'évaluation
- Neural Network For PLC PDFDocument7 pagesNeural Network For PLC PDFMarcelo Moya CajasPas encore d'évaluation
- Effect of Dust On The Performance of Wind Turbines PDFDocument12 pagesEffect of Dust On The Performance of Wind Turbines PDFJallal ArramachPas encore d'évaluation
- NewTrendsInLeadershipandManagement ArikkokDocument32 pagesNewTrendsInLeadershipandManagement Arikkoksocofem288Pas encore d'évaluation
- Economics and The Theory of Games - Vega-Redondo PDFDocument526 pagesEconomics and The Theory of Games - Vega-Redondo PDFJaime Andrés67% (3)
- Automotive E-Coat Paint Process Simulation Using FEADocument20 pagesAutomotive E-Coat Paint Process Simulation Using FEAflowh_100% (1)
- Direction: Read The Questions Carefully. Write The Letters of The Correct AnswerDocument3 pagesDirection: Read The Questions Carefully. Write The Letters of The Correct AnswerRomyross JavierPas encore d'évaluation
- History of English Prose PDFDocument21 pagesHistory of English Prose PDFMeisyita QothrunnadaPas encore d'évaluation
- Bolt Jul 201598704967704 PDFDocument136 pagesBolt Jul 201598704967704 PDFaaryangargPas encore d'évaluation
- Defining Public RelationsDocument4 pagesDefining Public RelationsKARTAVYA SINGHPas encore d'évaluation
- Lazo v. Judge TiongDocument9 pagesLazo v. Judge TiongKing BadongPas encore d'évaluation
- b2 Open Cloze - Western AustraliaDocument3 pagesb2 Open Cloze - Western Australiaartur solsonaPas encore d'évaluation
- Toki PonaDocument2 pagesToki PonaNicholas FletcherPas encore d'évaluation
- Oxy AcetyleneDocument43 pagesOxy Acetyleneregupathi100% (1)
- Kanavos Pharmaceutical Distribution Chain 2007 PDFDocument121 pagesKanavos Pharmaceutical Distribution Chain 2007 PDFJoao N Da SilvaPas encore d'évaluation
- F&B Data Analyst Portfolio ProjectDocument12 pagesF&B Data Analyst Portfolio ProjectTom HollandPas encore d'évaluation
- EINC ChecklistDocument3 pagesEINC ChecklistMARK JEFTE BRIONESPas encore d'évaluation
- Reading in Philippine History (Chapter 3)Document14 pagesReading in Philippine History (Chapter 3)AKIO HIROKIPas encore d'évaluation
- Assignment 2Document4 pagesAssignment 2maxamed0% (1)
- Teaching Support Untuk Managemen HRDocument102 pagesTeaching Support Untuk Managemen HRFernando FmchpPas encore d'évaluation
- Horizontal Vertical MarketDocument4 pagesHorizontal Vertical MarketVikasPas encore d'évaluation
- IntuitionDocument10 pagesIntuitionmailsonPas encore d'évaluation
- Graphic Organizers for Organizing IdeasDocument11 pagesGraphic Organizers for Organizing IdeasMargie Tirado JavierPas encore d'évaluation
- Test Fibrain RespuestasDocument2 pagesTest Fibrain Respuestasth3moltresPas encore d'évaluation
- Journal 082013Document100 pagesJournal 082013Javier Farias Vera100% (1)
- Socio-cultural influences on educationDocument4 pagesSocio-cultural influences on educationofelia acostaPas encore d'évaluation