Académique Documents
Professionnel Documents
Culture Documents
IR Absorption Table PDF
Transféré par
David QuinteroTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
IR Absorption Table PDF
Transféré par
David QuinteroDroits d'auteur :
Formats disponibles
24/10/2018 IR Absorption Table
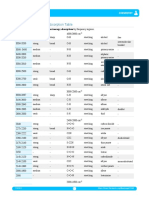
Table of IR Absorptions
Characteristic Absorption(s)
Functional Group Notes
(cm-1)
Alkane C-H
bonds are
fairly
ubiquitous
Alkyl C-H Stretch 2950 - 2850 (m or s) and therefore
usually less
useful in
determining
structure.
Absorption
peaks above
Alkenyl C-H Stretch 3100 - 3010 (m) 3000 cm-1
Alkenyl C=C Stretch 1680 - 1620 (v) are
frequently
diagnostic of
unsaturation
Alkynyl C-H Stretch ~3300 (s)
Alkynyl C=C Stretch 2260 - 2100 (v)
Aromatic C-H Stretch ~3030 (v)
Aromatic C-H Bending 860 - 680 (s)
Aromatic C=C Bending 1700 - 1500 (m,m)
See "Free vs.
Hyrdogen-
Bonded
Hydroxyl
Groups" in
Alcohol/Phenol O-H Stretch 3550 - 3200 (broad, s)
the
Introduction
to IR Spectra
for more
information
Carboxylic Acid O-H Stretch 3000 - 2500 (broad, v)
https://webspectra.chem.ucla.edu//irtable.html 1/3
24/10/2018 IR Absorption Table
Primary
amines
produce two
N-H stretch
Amine N-H Stretch 3500 - 3300 (m) absorptions,
secondary
amides only
one, and
tetriary none.
Nitrile C=N Stretch 2260 - 2220 (m)
The carbonyl
stretching
absorption is
one of the
strongest IR
absorptions,
and is very
useful in
structure
Aldehyde C=O Stretch 1740 - 1690 (s)
determination
Ketone C=O Stretch 1750 - 1680 (s)
as one can
Ester C=O Stretch 1750 - 1735 (s)
determine
Carboxylic Acid C=O Stretch 1780 - 1710 (s)
both the
Amide C=O Stretch 1690 - 1630 (s)
number of
carbonyl
groups
(assuming
peaks do not
overlap) but
also an
estimation of
which types.
Amide N-H Stretch 3700 - 3500 (m) As with
amines, an
amide
produces
zero to two
N-H
absorptions
depending on
its type.
https://webspectra.chem.ucla.edu//irtable.html 2/3
24/10/2018 IR Absorption Table
All figures are for the typical case only -- signal positions and intensities may vary
depending on the particular bond environment.
Introduction to IR Spectra Back to WebSpectra Home Page
https://webspectra.chem.ucla.edu//irtable.html 3/3
Vous aimerez peut-être aussi
- List GoodDocument2 pagesList GoodSumonPas encore d'évaluation
- IR Spectrum Table & Chart - Sigma-AldrichDocument6 pagesIR Spectrum Table & Chart - Sigma-Aldrichnouman ashraf50% (2)
- Bảng tra cứu các dao động của nhóm chức trong Phổ IRDocument17 pagesBảng tra cứu các dao động của nhóm chức trong Phổ IRntrangthu1409Pas encore d'évaluation
- IR Spectrum TableDocument22 pagesIR Spectrum TableJanno MallariPas encore d'évaluation
- DKO (Spektroskopi IR) : Arif Fadlan Gasal 2018/2019Document43 pagesDKO (Spektroskopi IR) : Arif Fadlan Gasal 2018/2019Hanan HakimPas encore d'évaluation
- IR Spectrum Table & Chart - Sigma-AldrichDocument6 pagesIR Spectrum Table & Chart - Sigma-AldrichPhysical Chemist100% (2)
- IR Spectrum Table and ChartDocument9 pagesIR Spectrum Table and ChartBožidarka TripkovićPas encore d'évaluation
- IR SpectrumDocument8 pagesIR SpectrumRizki100% (1)
- IR Spectrum TableDocument18 pagesIR Spectrum Table노래하는?서효민100% (1)
- Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM NotesDocument2 pagesTable of IR Absorptions: Functional Group Characteristic Absorption(s) (CM NotesaexmooPas encore d'évaluation
- IR Spectrum Table by Frequency Range: Frequency Range Absorption (CM) Appearance Group Compound Class CommentsDocument10 pagesIR Spectrum Table by Frequency Range: Frequency Range Absorption (CM) Appearance Group Compound Class CommentsNegru Laura MariaPas encore d'évaluation
- IR TableDocument1 pageIR TableMohamed DahmanePas encore d'évaluation
- IR Spectrum Table by Frequency Range: Frequency Range Absorption (CM) Appearance Group Compound Class CommentsDocument7 pagesIR Spectrum Table by Frequency Range: Frequency Range Absorption (CM) Appearance Group Compound Class CommentsRyo CaesarPas encore d'évaluation
- Loudon 5 Ech 12 Sec 04Document6 pagesLoudon 5 Ech 12 Sec 04Amylase NguyenPas encore d'évaluation
- Https WWW - Sigmaaldrich.com Technical-Documents Articles Biology Ir-Spectrum-Table - Printerview.htmlDocument1 pageHttps WWW - Sigmaaldrich.com Technical-Documents Articles Biology Ir-Spectrum-Table - Printerview.htmlnissa0% (1)
- Infra Red Infra Red: Atmanto Heru WDocument15 pagesInfra Red Infra Red: Atmanto Heru WSetya Dhana Santika AjiPas encore d'évaluation
- IR Absorption TableDocument2 pagesIR Absorption TablefikrifazPas encore d'évaluation
- Bảng phổ IRDocument5 pagesBảng phổ IRĐan KhanhPas encore d'évaluation
- IR Spectrum TableDocument17 pagesIR Spectrum TableAndres Felipe MPas encore d'évaluation
- FT-IR Spectrum TableDocument5 pagesFT-IR Spectrum TableMike Dinh100% (3)
- ASS Instrumental OrganicDocument17 pagesASS Instrumental OrganicMohamed SakrPas encore d'évaluation
- IR Spectroscopy Problem Set 4 Answer Key PDFDocument6 pagesIR Spectroscopy Problem Set 4 Answer Key PDFHuy Đặng AnhPas encore d'évaluation
- If You Already Know The Frequency Range Use This IR Spectrum Chart. If You Already Know The Frequency Use The AboveDocument11 pagesIf You Already Know The Frequency Range Use This IR Spectrum Chart. If You Already Know The Frequency Use The AboveAdza LuailikPas encore d'évaluation
- IR Spectrum Table by Frequency RangeDocument5 pagesIR Spectrum Table by Frequency RangeDiego RodriguezPas encore d'évaluation
- 6-IR Spectroscopy of Alkane, Alkene and Carbonyl CompoundsDocument8 pages6-IR Spectroscopy of Alkane, Alkene and Carbonyl Compoundsbloodhound13042005Pas encore d'évaluation
- Lecture 4 IR Spectrum AnalysisDocument43 pagesLecture 4 IR Spectrum AnalysiskhadijahhannahPas encore d'évaluation
- Advanced PH Analytics Lec 4Document32 pagesAdvanced PH Analytics Lec 4knowlegebook6Pas encore d'évaluation
- 16 40 Which Diagram Shows The Infrared Spectrum of A Compound That Contains Both A C O and AnDocument1 page16 40 Which Diagram Shows The Infrared Spectrum of A Compound That Contains Both A C O and AnZianPas encore d'évaluation
- 5 IR Analisis KualitatifDocument38 pages5 IR Analisis KualitatifPAHTMA PURNAMA SARI DEWIPas encore d'évaluation
- Other Phenomena Reduce The No. of Bands IncludingDocument37 pagesOther Phenomena Reduce The No. of Bands IncludingFatima AhmedPas encore d'évaluation
- Infrared SpectrosDocument110 pagesInfrared SpectrosBHARTI GAURPas encore d'évaluation
- Study Notes-IR SpectrosDocument24 pagesStudy Notes-IR SpectrosakshantratwanPas encore d'évaluation
- IR Spectroscopy TutorialDocument36 pagesIR Spectroscopy TutorialreddygrPas encore d'évaluation
- Aplikasi Infra Merah Dalam Bidang Farmasi: Abdul Rohman Laboratorium Kimia Analisis Fakultas Farmasi UGMDocument38 pagesAplikasi Infra Merah Dalam Bidang Farmasi: Abdul Rohman Laboratorium Kimia Analisis Fakultas Farmasi UGMKhumairah MohtarPas encore d'évaluation
- Module 2: Infrared SpectrosDocument5 pagesModule 2: Infrared SpectrosAngela ReyesPas encore d'évaluation
- Interpretation of Spectra of Different CompoundsDocument15 pagesInterpretation of Spectra of Different Compoundsmariam nawabPas encore d'évaluation
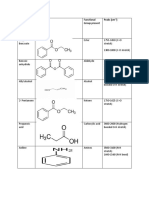
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANPas encore d'évaluation
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANPas encore d'évaluation
- BÀI TẬP MẪUDocument13 pagesBÀI TẬP MẪUMinh Tân LêPas encore d'évaluation
- Spektro IRDocument64 pagesSpektro IRAnonymous NSK4nvH4ufPas encore d'évaluation
- BP701T Ima IiDocument36 pagesBP701T Ima Iidwivedishrishti18Pas encore d'évaluation
- Fourier Transform Infrared Spectroscopy: Shimadzu (Asia Pacific) Pte LTD SingaporeDocument56 pagesFourier Transform Infrared Spectroscopy: Shimadzu (Asia Pacific) Pte LTD SingaporeBenjamín Málaga EspichánPas encore d'évaluation
- Aplikasi Infra Merah Dalam Bidang Farmasi: Muhammad Taupik Laboratorium Kimia Analisis Farmasi UNGDocument35 pagesAplikasi Infra Merah Dalam Bidang Farmasi: Muhammad Taupik Laboratorium Kimia Analisis Farmasi UNGVsten MramisPas encore d'évaluation
- HW Ir 2020Document5 pagesHW Ir 2020iris glzPas encore d'évaluation
- Aplikasi Spek IR Untuk FarmasiDocument38 pagesAplikasi Spek IR Untuk FarmasiHifdzurRashifRija'iPas encore d'évaluation
- Infrared Spectroscopy Absorption TableDocument7 pagesInfrared Spectroscopy Absorption TableMaria Yolanda GilPas encore d'évaluation
- IR Analisis KualitatifDocument38 pagesIR Analisis KualitatifRetno SulistyaningrumPas encore d'évaluation
- B.Tech Spectroscopy Module 5.4: Applications of IR SpectrosDocument16 pagesB.Tech Spectroscopy Module 5.4: Applications of IR SpectrosKAMALAPas encore d'évaluation
- Applications of Infra Red SpectrosDocument5 pagesApplications of Infra Red SpectrosEditor IJTSRDPas encore d'évaluation
- Interpretation of IR Compounds BY KhanDocument7 pagesInterpretation of IR Compounds BY KhanShamsheer KhanPas encore d'évaluation
- 08 - Infrared Spectroscopy ManualDocument5 pages08 - Infrared Spectroscopy ManualShubham BobadePas encore d'évaluation
- MASS SPECTROMETRY Only Fragmentation Pattern - OCT.2024Document23 pagesMASS SPECTROMETRY Only Fragmentation Pattern - OCT.2024aimaananwarPas encore d'évaluation
- Chapter 7 IR, NMR and Mass SpectrosDocument53 pagesChapter 7 IR, NMR and Mass SpectrosEsuendalew DebebePas encore d'évaluation
- Empirical Metallogeny: Depositional Environments, Lithologic Associations and Metallic OresD'EverandEmpirical Metallogeny: Depositional Environments, Lithologic Associations and Metallic OresPas encore d'évaluation
- Borehole ProblemsDocument10 pagesBorehole ProblemsMuh Andika Pratama WarisPas encore d'évaluation
- Method For Ultra High Purity Gold - Gold Refining ForumDocument11 pagesMethod For Ultra High Purity Gold - Gold Refining Forummladen lakic100% (1)
- Solutios, Solutions of Non Electrolyte - 2019-2020 v2Document80 pagesSolutios, Solutions of Non Electrolyte - 2019-2020 v2hazo hazPas encore d'évaluation
- Endothermic and Exothermic Reactions WorksheetDocument4 pagesEndothermic and Exothermic Reactions Worksheetabdulhakim100% (1)
- Minimum Pipe Thickness - B31.1 - PG1Document10 pagesMinimum Pipe Thickness - B31.1 - PG1ravivarmadatla2011Pas encore d'évaluation
- CRYOGENIC MORTAR C-1 - PRODUCT DATA SHEET Ed. 2Document4 pagesCRYOGENIC MORTAR C-1 - PRODUCT DATA SHEET Ed. 2ANIBAL LOPEZPas encore d'évaluation
- HTT 45 CableDocument1 pageHTT 45 CableKoel DeyPas encore d'évaluation
- OR Water TreatmentDocument13 pagesOR Water Treatmentafif ginandarPas encore d'évaluation
- Component in Making Fiber Board As Substitute For HardiflexDocument9 pagesComponent in Making Fiber Board As Substitute For Hardiflexxiniac_1Pas encore d'évaluation
- 410 Stainless Steel: Form of SupplyDocument3 pages410 Stainless Steel: Form of SupplyDeepak SinghPas encore d'évaluation
- Embuild BrochureDocument38 pagesEmbuild BrochureKawish TamourPas encore d'évaluation
- RNA Structure, Functions WebDocument25 pagesRNA Structure, Functions WebEmad ManniPas encore d'évaluation
- GUIDE-MQA-017-006 (Good Manufacturing Practice For Assemblers of Medicinal Products)Document15 pagesGUIDE-MQA-017-006 (Good Manufacturing Practice For Assemblers of Medicinal Products)William ChandraPas encore d'évaluation
- Problemario MFCDocument80 pagesProblemario MFCBassaldua AlfreedPas encore d'évaluation
- Stepan Pol Ps 2352Document2 pagesStepan Pol Ps 2352A MahmoodPas encore d'évaluation
- Stainless EnstainlessDocument27 pagesStainless EnstainlessVinaya Almane DattathreyaPas encore d'évaluation
- DR FCC PDFDocument7 pagesDR FCC PDFAle SanzPas encore d'évaluation
- Distortions: M. Vedani Failure and Control of Metals - AY 2020/21Document7 pagesDistortions: M. Vedani Failure and Control of Metals - AY 2020/21MarcoPas encore d'évaluation
- Hemodialysis Medical Supply and MedicationDocument10 pagesHemodialysis Medical Supply and Medicationsiuagan.jayveePas encore d'évaluation
- B.tech Labmanual - FinalDocument99 pagesB.tech Labmanual - FinalSumathi JeganathanPas encore d'évaluation
- Praxis GuideDocument43 pagesPraxis GuideRyan NegadPas encore d'évaluation
- Galvaspan G450Document2 pagesGalvaspan G450khurshedlakhoPas encore d'évaluation
- Experilab Breinwave: Bubble BlowersDocument4 pagesExperilab Breinwave: Bubble BlowersHirenkumarPas encore d'évaluation
- Titanium WeldingDocument16 pagesTitanium WeldingMuhammad IrdhamPas encore d'évaluation
- Basic Concepts of ChemistryDocument31 pagesBasic Concepts of ChemistryMohammadHussainKhan100% (1)
- Hazen - William Coeficiente FBEDocument10 pagesHazen - William Coeficiente FBEOrlandoPas encore d'évaluation
- Energy Systems Worksheet 7-12: Summary QuestionsDocument2 pagesEnergy Systems Worksheet 7-12: Summary Questionsapi-341899824Pas encore d'évaluation
- The Big Picture: EnzymesDocument54 pagesThe Big Picture: EnzymesSoumiya SrinivasanPas encore d'évaluation
- SigmazincDocument8 pagesSigmazincHaresh BhavnaniPas encore d'évaluation
- A Clear & Present Danger 2 - The Use of QT or TMT Rebars in Seismic Zone 4Document12 pagesA Clear & Present Danger 2 - The Use of QT or TMT Rebars in Seismic Zone 4friends_y2k5Pas encore d'évaluation