Académique Documents
Professionnel Documents
Culture Documents
Application From For Recruitment of Constable-2017
Transféré par
Sunil RatheeDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Application From For Recruitment of Constable-2017
Transféré par
Sunil RatheeDroits d'auteur :
Formats disponibles
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
NEAR IR SPECTROSCOPY
Infra red spectrum is an important record which gives sufficient information about the
structure of a compound. In recent years, NIR spectroscopy has become so widespread in
process analysis and within pharmaceutical industry for raw material testing, product
quality control and process monitoring. Not only in the pharmaceutical industry it has
gained wide acceptance in biotechnology, genomics analysis, proteomic analysis,
interactomics research, inline textile monitoring, food analysis, plastics, textiles, insect
detection, forensic lab application, crime detection, various military applications, and is a
major branch of astronomical spectroscopy and so on. NIR absorption bands are typically
broad, overlapping and 10–100 times weaker than their corresponding fundamental mid-
IR absorption bands. NIR spectroscopy is a vibrational spectroscopic method belongs to
the infrared light spectrum which is very close to the visible region (from about 750 to
2500 nm), where the most of organic and some inorganic compounds shows good
reflectance or transmission properties. That means they are exhibiting good absorption of
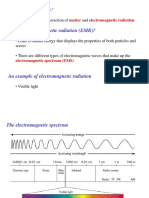
light at the NIR region. Figure 1 express the range of electromagnetic radiation.
Figure 1: Range of Electromagnetic radiation
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 1
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
Theory & Principle of near-infrared (NIR) spectroscopy
The American Society of Testing and Materials (ASTM) defines the NIR region of the
electromagnetic spectrum as the wavelength range of 780– 2526 nm corresponding to the
wave number range 12820–3959 cm-1. The most prominent absorption bands occurring in
the NIR region are related to overtones and combinations of fundamental vibrations of –
CH, –NH, –OH (and –SH) functional groups. Combinations arise by interaction of two or
more vibrations taking place simultaneously. For a given molecule, a normal mode of
vibration corresponds to internal atomic motions in which all atoms move in phase with
same frequency but with different amplitude. Additionally to these normal vibrations
transitions corresponds to be called overtones. Such transitions are forbidden by the
selection rules of quantum mechanics. As a result the molar absorptivity in the near IR
region is very small. There are two laws which govern the basics of vibrational
spectroscopy, they are Hooke’s law and Frank condon principle. Hooke’s law states that,
for two body harmonic oscillator, the frequency of vibration is
Where, C = speed of light, K = force constant (5 × 105 dynes/cm).
Hooke’s law can be used to calculate the fundamental vibrations for diatomic molecules
in IR. Transition from the ground state to the first excited state absorbs light strongly in
IR region and give rise to intense bands called the fundamental bands. Transition from
the ground state to the second excited state with the absorption of NIR give rise to weak
bands called 1st overtone in NIR. Transition from the ground state to the third excited
state with the absorption of NIR give rise to weak bands called 2nd overtone in NIR. Like
wise 3rd and 4th overtone bands will occur based on the transition to the fourth and fifth
excited state with the absorption of NIR.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 2
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
Franck Condon Principle: When a molecule vibrates the probability of finding of given
atom at a certain point is inversely proportional to its velocity. Therefore the atoms in a
vibrating molecule spend most of their time in configuration in which the kinetic energy
is low, that is the configuration in which potential energy is nearly identical with total
energy or at intersection of the vibrational energy level with the potential energy surface
of the molecule. Thus the photon is most likely to be absorbed when the nuclei are
stationary or moving slowly. The excitation resulting from the absorption of photon can
not be transferred immediately to the nuclei. The nuclei will therefore tend to continue
moving slowly after absorption. The nuclear configuration also tends to be close to
intersection of the vibrational energy level with the potential energy surface of the
molecule. Therefore transitions tend to take place between vibrational levels in which
nuclear configurations are same in both states. Thus these small variations give rise to
anharmonicity which causes the combination and over tone bands. The spectrum can be
measured based on the sample nature either in transmission or reflection. Transparent
materials are usually measured in transmittance, solids and turbid, semi solid solutions
measured in diffuse reflection, transflection or transmission. In the 1100–2500 nm region,
the amount of scattering makes the path length so great that transmittance through 1 cm
of most samples, such as wheat meal, flour or milk powder, is negligible. This situation is
called diffuse reflectance because most of the incident radiation is reflected. Hybrid mode
of transmittance and reflectance called transflectance, where the radiation is transmitted
through the sample reflected from a ceramic tile beneath the sample and then transmitted
back through the sample before finally reaching the detector.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 3
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
Instrumentation
A NIR spectrometer is generally composed of a light source, a monochromator, a sample
holder or a sample presentation interface, and a detector, allowing for transmittance or
reflectance measurements (Fig. 2).
Detector
Diffuse Reflectance
Light Source Monochromator Sample Detector
Transmittance
Fig. 2. Basic NIR spectrometer configurations.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 4
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
Source: The light source is usually a tungsten halogen lamp, which produce heat up to
1100k. These lamps emit visible radiation along with considerable NIR radiation. So
filters are needed in order to eliminate visible radiation which helps in prevention of
unnecessary sample heating. The advantages of this source are cheap in cost and are
readily available and these lamps can not increase their energy out put as the voltage of
source is increased.
Most of the applications requires only selected wavelength. LEDs emit radiation at
specific wavelength, but it is relatively broad energy emitted. LEDs combined with filters
are using as wavelength selector and these are very efficient, cheap and ideal for portable,
so low cost instruments can be produced without having moving parts.
Grating:
Gratings comprises metal or glass engraved surface with many fine parallel lines. When
the light beam strikes the surface it divides into various wavelengths by diffraction. In
interferometer wavelength selector, the light beam is split into two beams with a beam
splitter. The two separated beams strikes the fixed and moving mirrors respectively and
they are reflected back to beam splitter. They are then recombined and exit the
interferometer in the direction of sample. Based on the material ( KBr, CaF2 or quartz)
used for the beam splitter interferometer operate at specific wavelength.
Sample Holder
Sample holder Cells made up of quartz or glass to perform transmission with liquids.
Depending on the design of the instrument the cells are of varied sizes and designs. Some
instruments use round sample cups for dry solid and grained samples. Transport cells are
designed for analyzing the bulk samples. Round cups are often rotated in order to scan
maximum amount of sample and to eliminate the lack of sample homogeneity. Transport
cells move vertically where the scanning beam remains stationary.
Detector
Detector The choice of detectors depends on Wavelength range, Spectrometer design
characteristics, detector characteristics such as photosensitivity (responsivity), noise
equivalent power (NEP), detectivity. Photosensitivity measures the voltage output per
unit of incident radiant at a particular wavelength when noise is not considerable. NEP
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 5
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
measures the quantity of light when the signal to noise ratio is 1. Detectivity is a
parameter used to compare the performance of different detectors. Best detector should
possess the higher in the signal of detectivity. Detectity is the signal to noise ratio at
particular electrical frequency and in a 1HZ bandwidth when 1 watt of radiant on a 1cm2
active area detector.
Table 1: Detectors used in NIR spectroscopy
Detector Wavelength Region Responsivity/Detectivity Remark
Range
PbS 1100-2500 NIR Intermediate/Intermediate PbS sandwiched
400-2600 UV-NIR with silicon
1100-4500 NIR-MIR photodiodes are
often used for
VIS-NIR
PbSe 1100-5000 NIR-MIR Fast/High The detector
must be cooled
with liquid
nitrogen
InGa As 700-1700 NIR Fast/ Very High Linear arrays
NIR Raman High sensitivity,
dynamic range,
signal to noise
performance and
stability FT-NIR
Diode arrays
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 6
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
spectrometer
InSb/In As 1000-5500 NIR Fast/ Very High High quality
IR detector,
NIR-MIR Detector
Photodiodes
CCD 800-2200 NIR Fast/ High High
performance
detector,
Applied in
cameras, Diode
arrays
spectrometer
Detectors using in NIR spectrometers are Lead sulphide detectos (PbS), Lead selenide
detectors (PbSe), Silicon detectors, Indium antimonide detectors, InGaAs, InSb, Common
Charged Coupled Devices (CCD).Silicon detectors are fast, low noise, small and highly
sensitive from the visible region to 1100 nm. PbS detectors are slower, but very popular
since they are sensitive from 1100 to 2500 nm and provide good signal-to-noise
properties. The most expensive InGaAs detector combines the speed and size
characteristics of the silicon detector with the wavelength range of the PbS detector
Types of instrument used in NIR Spectroscopy
Modern NIR instruments are classified in terms of the technology employed for
wavelength selection. These are Filter based instruments, LED based instruments, AOTF
based instruments, Dispersive optics-based instrument, Fourier-transform based
instruments.
Filter based instruments
These instruments employing filters as wavelength selectors are commercially available
for dedicated applications. For example, an instrument for determination of the quality
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 7
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
parameters of gasoline (Zeltex Inc.) employs interference (Fabri-Perrot) filters and LED
(Light Emitting Diode) sources in the NIR region. Variable (scanning) interference filters
based on the variation of the dielectric layer thickness by using piezoelectric drivers have
recently been used.
LED based instruments
Recently a tendency to reduce the price and size of the instruments, aiming for use in the
field, has taken advantage of the use of Light Emitting Diodes (LED). These devices can
produce NIR radiation with a band width of about 30 - 50 nm, centred in any wavelength
of the spectral region. The instruments can employ a set of LEDs as sources of narrow
bands of near infrared radiation or use them to produce a polychromatic, highly stable
source whose radiation is dispersed by using common monochromator devices such as
those based on gratings or filter optics.
AOTF based instruments
The instruments based on Acousto-Optical Tunable Filters (AOTF) are modern scan
spectrophotometers employing a technology that allows constructing instruments with no
moving parts, capable of reaching very high scan speeds over a broad range of the NIR
spectral region. Scan speed is fast and up to 2000 wavelengths can be selected per
second. The scan speed is usually limited by the detector response time.
The AOTF operating in a non-collinear configuration (acoustic wave and radiation beam
propagating at almost perpendicular angles), is a device made of a birefringent crystal of
TeO2, cut in a special angle. Other materials can be used but the characteristics of TeO2
are suitable for the NIR regions and has been chosen by all instrument manufacturers.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 8
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
A piezoelectric material (usually LiNiO4) is attached to one end of the crystal which,
under excitation from an external radio frequency signal (rf), produces a mechanical
(acoustic) wave which propagates through the crystal. The acoustic wave produces a
periodic variation of the refractive index of the crystal in a frequency determined by the
Rf signal, in the range of 50 to 120 MHz. The interaction of the electromagnetic wave
and the acoustic wave causes the crystal to refract selectively a narrow wavelength band.
The birefringence of the TeO2 crystal leads to the production of two monochromatic
beams whose angular separation is about 7o . Both or only one diffracted beam can be
used by NIR instruments. Dual beam (with a reference beam produced by splitting one of
the beams) or single beam instruments can be found. Alternatively, the two
monochromatic beams can be employed for the construction of a dual beam instrument
with an optimised use of the radiation throughput.
The non-moving parts concept of the AOTF-based NIR spectrophotometers impart to
them some unbeatable qualifying characteristics for use in the field or on the factory
floor, aiming at in-line monitoring. The wavelength precision is about ±0.05 nm and the
resolution is dependent on the wavelength, with typical values in the range 5 to 15 nm for
the wavelength in the range 1000 to 2500 nm.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 9
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
Dispersive optics-based instruments
Dispersive instruments based on diffraction gratings were employed in the early days of
NIR spectroscopy and were responsible for the research initially developed to consolidate
NIR spectroscopy as an analytical tool. This technology has continued through the 90's
and is still being used, with many manufacturers offering instruments with performance
suitable for many practical applications.
The first commercial Brazilian-made NIR spectrophotometer, manufactured by Femto
Ltda., was launched in 1999 and is based on a monochromator employing a moving
diffraction grating and Czerny-Turner optics. Its performance suffices for many
transmitance measurement-based analytical methods employed in the control of sugar
and alcohol production, a very important Brazilian industrial sector . The instruments
based on grating monochromators present the advantage of a relatively low cost when
compared with other scanning instruments employing modern technologies. The main
disadvantages of dispersive instruments are the slow scan speed and a lack of wavelength
precision, which deteriorates for long term operation due to mechanically driven
mechanism fatigue. This can be a negative regarding maintenance of multivariate models.
Also, the presence of moving parts limits the use of dispersive instruments in the field
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 10
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
and in more aggressive environments. On the other hand, recent evolution in sensor
production technology gives dispersive optics a longer life. That is because it is now
possible to construct linear arrays of PbS and InGaAs sensors containing up to 256
independent elements. Placed in the focal plane of plane or concave grating optics, the
sensor array allows to scan an entire spectra in a few milliseconds.
Fourier-transform based instruments
Spectrophotometers based on the use of interferometers and Fourier transform to recover
the intensities of individual wavelengths in the NIR region are, undoubtedly, the
instruments combining most of the best characteristics in terms of wavelength precision
and accuracy, high signal-to-noise ratio and scan speed (although slower than AOTF
based instruments). These instruments add to the previously mentioned Fellget gain the
Jaquinot gain, which is not attainable with dispersive Hadamard instruments. The
Jaquinot gain arises from the high radiation throughput achieved for a Fourier instrument,
due the fact that it does not employ entrance or exit slits to limit the radiation intensity
reaching the detector. Typical wavelength accuracy is better than 0.05 nm and the
resolution can achieve values below 1 nm in the NIR region, at cost of decreasing the
scan speed.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 11
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
These advantages turn the NIR spectrophotometer based on interferometric measurement
and Fourier transform into an unbeatable research instrument. On the other hand, the
spectrophotometer is not as robust as an AOTF-based instrument which is assembled
without any moving parts. The robustness of the Fourier spectrophotometer has been
improved by using a "wishbone" type of interferometer, such as the one found in the
Bomem instruments. This type of interferometer employs half cubic mirrors to
compensate for beam travelling distance changes arising from non uniform mirror
movement during data acquisition. The result is a Fourier-based spectrophotometer that
can tolerate some environmental vibrations without losing wavelength precision and
photometric reproducibility. The price of a Fourier based instrument is comparable with
the AOTF-based spectrophotometer and, therefore, both are considered expensive
relative to other options.
Pharmaceutical applications
NIR spectroscopy combined with multivariate data analysis opens many interesting
perspectives in pharmaceutical analysis, both qualitatively and quantitatively.
Identification and qualification of raw materials and intermediates
The chemical identity of a active ingredients and excipients are usually confirmed by
NIR techniques with a spectral library approach.
Analysis of intact dosage forms
The nondestructive and multivariate nature of NIR techniques opens new perspectives in
the pharmaceutical analysis of intact dosage forms, including chemical, physical and
related biopharmaceutical aspects. Fast and nondestructive identification of active
ingredients and excipients in whole tablets, even through the blister packaging, is
certainly a domain of NIR spectroscopy.
Analysis of polymorphs
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 12
SHRI GURU RAM RAI INSTITUTE OF TECHNOLOGY AND SCIENCE
NIR spectroscopy for the quantitative determination of polymorphs in a formulation
matrix. Most polymorphs exhibit spectral differences in the mid-IR, since NIR spectra
arise from overtones and combinations of mid-IR absorbances, NIR was also reasoned to
be suitable for the analysis of polymorphs.
Determination of Adulteration of Essential Oils
Near-infrared (NIR) technologies using for the assessment of essential oil components
and in the identification of individual essential oils. NIR can be used to discriminate
between the ravintsara(adultrant) and ravensara(authentic) essential oils.
Protein quantification within lipid implants
Lipid implants have been proposed as promising sustained release devices for the
parenteral application of pharmaceutical proteins. Near infrared spectroscopy (NIRS) as
tool for drug quantification within controlled release matrix systems based on poly-
(lactic-co-glycolic) acid (PLGA).
References
Williams, P.; Norris, K., eds.; Near-Infrared Technology, 2nd ed. , American Association
of Cereal Chemistry, Inc.: St. Paul, MN, USA, 2001.
Reddy, P.H; Kumar,D.S; Near Infra Red Spectroscopy- An Overview, International
Journal of ChemTech Research,2011, Vol. 3, No.2, pp 825-836
Gabriele, R.; Near-infrared spectroscopy and imaging: Basic principles and
pharmaceutical applications, Advanced Drug Delivery Reviews ,2005, 57, 1109– 1143
E.W. Ciurczak, Principles of near-infrared spectroscopy, in: D.A. Burns, E.W. Ciurczak
(Eds.), Handbook of Near- Infrared Analysis, 2nd edition, Marcel Dekker Inc., New
York/Basel, 2001, pp. 7 –18.
Dr. ALKA CHOUDHARY, Asso. Prof, Division of Pharmaceutical Sciences Page 13
Vous aimerez peut-être aussi
- SpectrosDocument37 pagesSpectrosKiruthick DonPas encore d'évaluation
- Ftir PDFDocument40 pagesFtir PDFWahyuni EkaPas encore d'évaluation
- Presentation 1Document38 pagesPresentation 1Kiruthick DonPas encore d'évaluation
- Applications of IRDocument29 pagesApplications of IRRESHMA RANJANPas encore d'évaluation
- IR SpectrosDocument17 pagesIR SpectrosRaman AgarwalPas encore d'évaluation
- Presentation FTIR SpectDocument62 pagesPresentation FTIR SpectYogi IndrawanPas encore d'évaluation
- Presentation FTIR SpectDocument62 pagesPresentation FTIR SpectQaishanafis Dheiyana Shafa0% (1)
- Introduction To IR SpectrosDocument119 pagesIntroduction To IR SpectrosAvinash100% (3)
- Analisa Fisikokimia Ir FriardiDocument52 pagesAnalisa Fisikokimia Ir FriardiDityaPas encore d'évaluation
- FTIRDocument25 pagesFTIRAnubhav ShuklaPas encore d'évaluation
- Hospital Management AppDocument12 pagesHospital Management AppAnu VinodPas encore d'évaluation
- Fourier Transform Infra Red (FTIR) : Group 3 Dwi Yuliantiningsih Wifqul Laili Riski Ayu Candra Rina RukaenahDocument19 pagesFourier Transform Infra Red (FTIR) : Group 3 Dwi Yuliantiningsih Wifqul Laili Riski Ayu Candra Rina RukaenahWifqul LailyPas encore d'évaluation
- KK-CHP 2.4 (IR) Part 4 of 4Document33 pagesKK-CHP 2.4 (IR) Part 4 of 4ShafiqahFazyaziqahPas encore d'évaluation
- Ir PresentationDocument24 pagesIr PresentationMuneebPas encore d'évaluation
- FTIR (Things We Need To Know)Document3 pagesFTIR (Things We Need To Know)Keith Jaszer AgarradoPas encore d'évaluation
- Modes of Vibration Basic Principles OfIR SpectrosDocument9 pagesModes of Vibration Basic Principles OfIR SpectrosMuhammad Hussnain100% (1)
- Explainer - The Difference Between IR, FTIR, and ATR-FTIRDocument5 pagesExplainer - The Difference Between IR, FTIR, and ATR-FTIRArt AuthenticationPas encore d'évaluation
- SpectrosDocument25 pagesSpectrosanuj phalswalPas encore d'évaluation
- Shining a Light on Cool Standards: Infrared Spectroscopy Theory Explored.D'EverandShining a Light on Cool Standards: Infrared Spectroscopy Theory Explored.Pas encore d'évaluation
- Overview of Lasers: Uddhav A. Patil, Lakshyajit D. DhamiDocument13 pagesOverview of Lasers: Uddhav A. Patil, Lakshyajit D. Dhamizidan ojaPas encore d'évaluation
- IR Spectroscopy: Dr. M. Saqlain TahirDocument19 pagesIR Spectroscopy: Dr. M. Saqlain TahirAsif AliPas encore d'évaluation
- 3 CC1Document6 pages3 CC1marianPas encore d'évaluation
- Lasers in Pediatric Dentistry - 2Document82 pagesLasers in Pediatric Dentistry - 2Shameena KnPas encore d'évaluation
- F Spectroscopic InstrumentationDocument21 pagesF Spectroscopic Instrumentationmaxwell amponsahPas encore d'évaluation
- IR SpectrosDocument21 pagesIR SpectroskushalPas encore d'évaluation
- IR QualitativeDocument4 pagesIR QualitativeredaelwanPas encore d'évaluation
- Chapter-2 11th Class P and EDocument51 pagesChapter-2 11th Class P and ErajindertelecomnsqfPas encore d'évaluation
- 8 Infra RedDocument41 pages8 Infra RedMykyl Dominic Duremdes LariosaPas encore d'évaluation
- FTIR - Polymer AnalysisDocument20 pagesFTIR - Polymer AnalysisberkahPas encore d'évaluation
- Kuliah Infra MerahDocument110 pagesKuliah Infra Merahmuhlisun azimPas encore d'évaluation
- Lasers PDFDocument41 pagesLasers PDFPalanimani PGPas encore d'évaluation
- 8.2. EMW-source, Frequency Range, UsesDocument3 pages8.2. EMW-source, Frequency Range, UsesRamananPas encore d'évaluation
- Infra Red Absorption SpectrosDocument18 pagesInfra Red Absorption SpectrosSukumar PaniPas encore d'évaluation
- FTIRDocument6 pagesFTIRAnubhav ShuklaPas encore d'évaluation
- Infrared: Infrared (IR) Light Is Electromagnetic Radiation With A Wavelength Longer Than That of VisibleDocument8 pagesInfrared: Infrared (IR) Light Is Electromagnetic Radiation With A Wavelength Longer Than That of VisibleSyazwan ZulhilmiPas encore d'évaluation
- IR Spectrometers: AbbreviationsDocument9 pagesIR Spectrometers: AbbreviationsAlex DodonovPas encore d'évaluation
- Xray FundamentalsDocument27 pagesXray FundamentalsABISHKAR SARKARPas encore d'évaluation
- Color Wavelength NM Violet Blue Cyan Green Yellow Orange RedDocument4 pagesColor Wavelength NM Violet Blue Cyan Green Yellow Orange RedJhenard John Lansangan BeltranPas encore d'évaluation
- Examen Ambrosio 2 InglesDocument12 pagesExamen Ambrosio 2 InglesLadhy Guadalupe Feliciano FuentesPas encore d'évaluation
- Ir Music Transmitter and Reciever Project ReportDocument40 pagesIr Music Transmitter and Reciever Project ReportVaddi Vishwanath Kaushik100% (1)
- Infra Red 1Document18 pagesInfra Red 1Sukumar PaniPas encore d'évaluation
- Infra Red SpectrophotometryDocument5 pagesInfra Red SpectrophotometryKhem BhattaraiPas encore d'évaluation
- Particle Size AnalyzerDocument14 pagesParticle Size AnalyzermmmonmissionPas encore d'évaluation
- Chapter One: Introduction To Optical Communication Systems 1.1Document7 pagesChapter One: Introduction To Optical Communication Systems 1.1Odoch HerbertPas encore d'évaluation
- 2 FT IrDocument25 pages2 FT Irsana siddiquePas encore d'évaluation
- Spectro - NMR 2016Document54 pagesSpectro - NMR 2016Syafiqah SuhaimiPas encore d'évaluation
- Infrared Radiation PDFDocument10 pagesInfrared Radiation PDFAjay Pal NattPas encore d'évaluation
- Infrared Spectroscopy HomeworkDocument4 pagesInfrared Spectroscopy Homeworkafnkdjwhaedkbq100% (1)
- A Tutorial On Near Infrared Spectroscopy and Its CalibrationDocument16 pagesA Tutorial On Near Infrared Spectroscopy and Its CalibrationFabio OliveiraPas encore d'évaluation
- I R SpectrosDocument20 pagesI R Spectrosbiswajit_baruah6487Pas encore d'évaluation
- InfraRed SpectrosDocument7 pagesInfraRed SpectrosGanesh V GaonkarPas encore d'évaluation
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument17 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsJonathan YepesPas encore d'évaluation
- FTIRDocument79 pagesFTIRshruti shahPas encore d'évaluation
- Theoryofirspectroscopy 160622080111Document50 pagesTheoryofirspectroscopy 160622080111ArshPas encore d'évaluation
- IR Spectroscopy Review ArticalDocument5 pagesIR Spectroscopy Review ArticalInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation
- Introduction To SpectrosDocument24 pagesIntroduction To SpectrosPIRZADA TALHA ISMAIL100% (1)
- Fourier Transform Infrared (FT-IR) Spectroscopy: Theory and PrinciplesDocument28 pagesFourier Transform Infrared (FT-IR) Spectroscopy: Theory and PrinciplesmaopacificPas encore d'évaluation
- CHY 66 - Spectroscopic Methods - Optical Instruments - PPTDocument24 pagesCHY 66 - Spectroscopic Methods - Optical Instruments - PPTNathaniel Jay SumalinogPas encore d'évaluation
- Infrared and Raman Spectroscopy: Principles and Spectral InterpretationD'EverandInfrared and Raman Spectroscopy: Principles and Spectral InterpretationÉvaluation : 3 sur 5 étoiles3/5 (1)
- Intermolecular ForcesDocument7 pagesIntermolecular ForcesHarold Nalla HusayanPas encore d'évaluation
- Compressible Gas Flow in PipelinesDocument3 pagesCompressible Gas Flow in PipelinesRahul ChandrawarPas encore d'évaluation
- Jee Main 2021 Mar 18 First Shift PaperDocument15 pagesJee Main 2021 Mar 18 First Shift Papershivam singhPas encore d'évaluation
- Chem 31 NotesDocument4 pagesChem 31 NotesEvernim OmpacanPas encore d'évaluation
- Electrolysis of Water: AA Battery Tap Water Salt HydrogenDocument12 pagesElectrolysis of Water: AA Battery Tap Water Salt HydrogenFlorin AndreiPas encore d'évaluation
- Unsteady State Heat TransferDocument4 pagesUnsteady State Heat Transfernaser hasan fauziPas encore d'évaluation
- UV - Vis Spectros PDFDocument25 pagesUV - Vis Spectros PDFNelson BarriosPas encore d'évaluation
- MEP HyperCel InsertDocument2 pagesMEP HyperCel InsertChopra SinghPas encore d'évaluation
- Preparation of An Alum From Scrap Aluminium (2) New One 4Document12 pagesPreparation of An Alum From Scrap Aluminium (2) New One 4Savita SinghPas encore d'évaluation
- Environmental Green Chemistry Applications of Nanoporous CarbonsDocument24 pagesEnvironmental Green Chemistry Applications of Nanoporous CarbonsIvan AlcomendrasPas encore d'évaluation
- Fiber Optic Chemical Sensors Biosensors PDFDocument2 pagesFiber Optic Chemical Sensors Biosensors PDFKatreenaPas encore d'évaluation
- Determining Molar Mass Using CryosDocument6 pagesDetermining Molar Mass Using CryosValentin-AngeloUzunovPas encore d'évaluation
- Board-Exam May2223242019Document11 pagesBoard-Exam May2223242019Jonnah Faye MojaresPas encore d'évaluation
- CALORIMETRYDocument3 pagesCALORIMETRYqueenjosePas encore d'évaluation
- Properties of LNGDocument2 pagesProperties of LNGdilloosePas encore d'évaluation
- FinalDocument4 pagesFinalSimge DemirPas encore d'évaluation
- Lynch - 2012 - Hydrogen Embrittlement Phenomena and MechanismsDocument19 pagesLynch - 2012 - Hydrogen Embrittlement Phenomena and MechanismsIgor FernandoPas encore d'évaluation
- Problems On RadiationDocument3 pagesProblems On RadiationSuraj KumarPas encore d'évaluation
- Concept of PH and BufferDocument27 pagesConcept of PH and BufferRolling Coast100% (1)
- SOLUTION Test 2023Document3 pagesSOLUTION Test 2023साहिल PandeyPas encore d'évaluation
- Akash PDFDocument22 pagesAkash PDFry886450Pas encore d'évaluation
- Coolant and Your EngineDocument78 pagesCoolant and Your Enginedummy accountPas encore d'évaluation
- Lecture - 2&3 - Direct DyesDocument41 pagesLecture - 2&3 - Direct Dyesmlg54100% (1)
- 156-EG-100 Rev1Document44 pages156-EG-100 Rev1Tony StatelovPas encore d'évaluation
- Booklet 5, CHG 806Document7 pagesBooklet 5, CHG 806ONAFUWA AyodelePas encore d'évaluation
- Week7Quiz AnswersDocument4 pagesWeek7Quiz AnswersChin AliciaPas encore d'évaluation
- Calculating Core Temperature (PAPER)Document6 pagesCalculating Core Temperature (PAPER)Giovanni Hernández DecaniniPas encore d'évaluation
- Numerical Analysis For Energy Performance Optimization of Hollow Bricks For Roofing. Case Study - Hot Climate of AlgeriaDocument10 pagesNumerical Analysis For Energy Performance Optimization of Hollow Bricks For Roofing. Case Study - Hot Climate of Algeriamah0809Pas encore d'évaluation
- W5 Source ModelsDocument51 pagesW5 Source ModelsFakhrulShahrilEzaniePas encore d'évaluation
- MI - 1748 Rev FDocument21 pagesMI - 1748 Rev FSudarshan Dhumal100% (2)