Académique Documents
Professionnel Documents
Culture Documents
Worksheet 5
Transféré par
joanneDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Worksheet 5
Transféré par
joanneDroits d'auteur :
Formats disponibles
CHM1102 – Worksheet 5 Solutions
1. State Boyle’s Law in words and mathematically
2. State Charles’ Law in words and mathematically
3. State Avogadro’s Law in words and mathematically
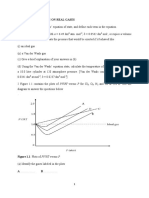
4. Which of the graphs below illustrate the gas laws?
Volume /dm3
Volume /cm3
Volume /m3
(i) (ii) (iii)
1
pressure/atm pressure/atm temperature /oC
Volume /cm3
Volume /m3
Volume /dm3
(iv) (v) (vi)
1 1
Temperature/K temperature/oC temperature/K
5. A chemist prepared a sample of hydrogen bromide and found that it occupied 255 ml
at 850C and 600 Torr. What volume would it occupy at 00C at the same pressure?
6. i.) Complete the following table for an ideal gas:
P (atm) Vol. (L) n (mol) Temp.
(a) 5.00 2.0 1550C
(b) 0.300 2.00 155K
(c) 4.47 25.0 2.01
(d) 2.25 10.5 750C
pV = nRT; R = 0.08206 LatmK-1mol-1; R = 8.314 JK-1mol-1
ii) If the sample of gas in (b) has a mass of 3.35g, calculate the molar mass of the gas.
This is a homonoid diatomic molecule. Deduce the name of the gas.
7. Methane reacts with oxygen according to the following equation:
CH4 (g) + 1 ½O2 (g) → CO(g) + 2H2O(g)
At a constant temperature of 373K and pressure of 1.00 atm, 100 cm3 of methane and
300 cm3 of oxygen are placed in a sealed container;
Calculate (a) the volumes of carbon monoxide and water vapour produced
(b) the total volume of gas in the container at the end of the process
(c) the partial pressure of oxygen at the end of the process.
8. Assume that you have a cylinder with a moveable piston. What would happen to the
gas volume of the cylinder if you were to do the following?
(a) Halve the Kelvin temperature while holding the pressure constant.
(b) Increase the amount of gas by 1/4 while holding the temperature and pressure
constant.
(c) Decrease the pressure by 75% at constant T.
(d) Double the Kelvin temperature and double the pressure.
Vous aimerez peut-être aussi
- Concrete Mix With CalculationsDocument12 pagesConcrete Mix With Calculationsarman malikPas encore d'évaluation
- Chang's Test Bank (Chapters 5, 7, 8, 9)Document27 pagesChang's Test Bank (Chapters 5, 7, 8, 9)asfaPas encore d'évaluation
- Welder Visual Inspection HandbookDocument77 pagesWelder Visual Inspection HandbookfaizalzolPas encore d'évaluation
- Naming Chemical Compounds WorksheetDocument4 pagesNaming Chemical Compounds WorksheetSam Jo100% (1)
- Chemistry-Gas Laws Multiple ChoiceDocument5 pagesChemistry-Gas Laws Multiple ChoiceGeorge Isaac McQuilesPas encore d'évaluation
- Gate 1993 PDFDocument11 pagesGate 1993 PDFVammsy Manikanta SaiPas encore d'évaluation
- Basic Technology JSS3Document28 pagesBasic Technology JSS3Timothy Clifford75% (4)
- EASA PART 66 GUIDE - EASA Part 66 - Material Question PDFDocument92 pagesEASA PART 66 GUIDE - EASA Part 66 - Material Question PDFimannur100% (1)
- OperationDocument11 pagesOperationmahboobiqbal09Pas encore d'évaluation
- Multiple Question CHM 101Document26 pagesMultiple Question CHM 101Emmanuella OffiongPas encore d'évaluation
- Union Universal RAWELTDocument4 pagesUnion Universal RAWELTLuisDonairesPas encore d'évaluation
- dtx33 PDFDocument10 pagesdtx33 PDFAiko Rowyena Constantino CruzPas encore d'évaluation
- CHM 1102 Worksheet 5 2023Document2 pagesCHM 1102 Worksheet 5 2023hemnarinesingh4Pas encore d'évaluation
- Chemistry 1st Year T-3Document2 pagesChemistry 1st Year T-3Amir HabibPas encore d'évaluation
- CHM 1102 Worksheet 5 2022Document2 pagesCHM 1102 Worksheet 5 2022kevin persaudPas encore d'évaluation
- Test Bank Chapter 5Document9 pagesTest Bank Chapter 5geenah111Pas encore d'évaluation
- 23.end Sem Question Paper - BMEL-405 2016-17Document2 pages23.end Sem Question Paper - BMEL-405 2016-17Manish SharmaPas encore d'évaluation
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandPas encore d'évaluation
- Lab RiportDocument7 pagesLab RiportKhamis MbaroukPas encore d'évaluation
- Date Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationDocument55 pagesDate Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationSickdanPas encore d'évaluation
- Gaseous State PDFDocument4 pagesGaseous State PDFramanji1021Pas encore d'évaluation
- Chem 1A Chapter5 Exercises PDFDocument5 pagesChem 1A Chapter5 Exercises PDFJoela Faith Ming GongPas encore d'évaluation
- Assignment 1Document3 pagesAssignment 1MaJasmineMayePas encore d'évaluation
- Topic 1 Quantitative SLHL Test ADocument9 pagesTopic 1 Quantitative SLHL Test APak Hei Marcus CHOWPas encore d'évaluation
- Exercise-1: Mcqs With One Correct Answer TypeDocument20 pagesExercise-1: Mcqs With One Correct Answer Typekiller heroPas encore d'évaluation
- A Level Chemistry Paper 2 Exam 14Document4 pagesA Level Chemistry Paper 2 Exam 14Anthony AndyPas encore d'évaluation
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyPas encore d'évaluation
- Revision STPM Term 1Document15 pagesRevision STPM Term 1Wong WengSiongPas encore d'évaluation
- PC Question Paper Nov 2021Document4 pagesPC Question Paper Nov 2021venkatesan sivaramuPas encore d'évaluation
- Ch-10, Ch-1 MCQDocument2 pagesCh-10, Ch-1 MCQanis.zamanPas encore d'évaluation
- Chemistry 1 Pre NectaDocument6 pagesChemistry 1 Pre NectabhaijanPas encore d'évaluation
- CPP of Gas LawsDocument2 pagesCPP of Gas LawsDivyansh Jain KingPas encore d'évaluation
- Previous Hse Questions From The Chapter "States of Matter"Document4 pagesPrevious Hse Questions From The Chapter "States of Matter"subiPas encore d'évaluation
- Sample Questions - Chapter 12Document7 pagesSample Questions - Chapter 12Rasel IslamPas encore d'évaluation
- 1.2 Moles, Molar Volume & Gas LawsDocument14 pages1.2 Moles, Molar Volume & Gas LawsShyamal DlrPas encore d'évaluation
- States of MatterDocument7 pagesStates of MatterMurali Karthik VPas encore d'évaluation
- CF Ph-1 Practice Paper CMM-3Document3 pagesCF Ph-1 Practice Paper CMM-3Divyansh Jain KingPas encore d'évaluation
- CH# 3 XI (Chem 11 Exam Task)Document5 pagesCH# 3 XI (Chem 11 Exam Task)Zeeshan Haider ChemistPas encore d'évaluation
- Gases (AP MC)Document11 pagesGases (AP MC)rejymolPas encore d'évaluation
- L-4/T-2/CE Date: 18/12/2012: Section ADocument55 pagesL-4/T-2/CE Date: 18/12/2012: Section ANafeesa KhanPas encore d'évaluation
- Test Bank Chapter 5Document7 pagesTest Bank Chapter 5Ahmed ZakiPas encore d'évaluation
- Tutorial Questions For CHME2201Document4 pagesTutorial Questions For CHME2201Peguy FotsoPas encore d'évaluation
- STP 112Document1 pageSTP 112seun DosunmuPas encore d'évaluation
- The Guiding Star Free of Charge Special Coaching Class Pre-Examination Chemistry Time Allowed: 3 HoursDocument5 pagesThe Guiding Star Free of Charge Special Coaching Class Pre-Examination Chemistry Time Allowed: 3 HoursPyae Sone KyawPas encore d'évaluation
- 21 - The Kinetic Theory of GasesDocument7 pages21 - The Kinetic Theory of GasesRaya Smagulova100% (1)
- JJKDocument11 pagesJJKAnonymous pa8pSCC15Pas encore d'évaluation
- Thermodynamics 2018 (Repaired)Document15 pagesThermodynamics 2018 (Repaired)carolPas encore d'évaluation
- 11 Chemistry PDFDocument9 pages11 Chemistry PDFkumar shivamPas encore d'évaluation
- ChE 12 CHE 111 2014-15Document4 pagesChE 12 CHE 111 2014-15aanika roshniPas encore d'évaluation
- Gujarat Technological UniversityDocument2 pagesGujarat Technological UniversityReniePas encore d'évaluation
- r7210305 ThermodynamicsDocument1 pager7210305 ThermodynamicssivabharathamurthyPas encore d'évaluation
- Gaseous State - QuizDocument4 pagesGaseous State - QuizMuffadal AlaviPas encore d'évaluation
- Unit VI One MarksDocument11 pagesUnit VI One MarksShanmugam RameshPas encore d'évaluation
- 02-JAD States of Matter-HWDocument2 pages02-JAD States of Matter-HWVijayPas encore d'évaluation
- Chemone 2Document4 pagesChemone 2pogi si mark leePas encore d'évaluation
- Chemistry PackageDocument6 pagesChemistry Packagepetermyonga3516Pas encore d'évaluation
- SQP 313 eDocument9 pagesSQP 313 eSangita SonwanePas encore d'évaluation
- MCQsDocument6 pagesMCQsKashan NoorPas encore d'évaluation
- Neet Full Test-2Document20 pagesNeet Full Test-2vasteducationalPas encore d'évaluation
- 3-17 Nervous HandoutDocument22 pages3-17 Nervous HandoutSeema ChaturvediPas encore d'évaluation
- Test Bank Chapter 5Document8 pagesTest Bank Chapter 5teafPas encore d'évaluation
- States of Matter - Entrance Exam Model Question Paper 2012Document3 pagesStates of Matter - Entrance Exam Model Question Paper 2012Vinod BhaskarPas encore d'évaluation
- University of LondonDocument6 pagesUniversity of LondonShootingStarPhotonsPas encore d'évaluation
- PM Office LensDocument1 pagePM Office LensSujal NepalPas encore d'évaluation
- Form 4 June 2023 Package ChemDocument3 pagesForm 4 June 2023 Package ChemTabithaPas encore d'évaluation
- Gas Hydrates 2: Geoscience Issues and Potential Industrial ApplicationsD'EverandGas Hydrates 2: Geoscience Issues and Potential Industrial ApplicationsLivio RuffinePas encore d'évaluation
- Conformations of AlkanesDocument3 pagesConformations of AlkanesjoannePas encore d'évaluation
- We Share A More Recent Relationship With Chimpanzees (Pan Troglodytes) and Bonobos (Pan Paniscus)Document4 pagesWe Share A More Recent Relationship With Chimpanzees (Pan Troglodytes) and Bonobos (Pan Paniscus)joannePas encore d'évaluation
- Sexual Selection and Evolution Experiments 67Document26 pagesSexual Selection and Evolution Experiments 67joannePas encore d'évaluation
- The Red Siskin Initiative Saving An Enda PDFDocument22 pagesThe Red Siskin Initiative Saving An Enda PDFjoannePas encore d'évaluation
- Worksheet 1Document1 pageWorksheet 1joannePas encore d'évaluation
- WS 1.5 AnswersDocument1 pageWS 1.5 AnswersjoannePas encore d'évaluation
- Worksheet 2018Document2 pagesWorksheet 2018joannePas encore d'évaluation
- Refractive Index of GlassDocument3 pagesRefractive Index of GlassjoannePas encore d'évaluation
- Toxins in The Foods We ConsumeDocument11 pagesToxins in The Foods We ConsumejoannePas encore d'évaluation
- Topic: Investigation Into Whether Sixth Form Students in St. Rose's High Eat Healthily or NotDocument9 pagesTopic: Investigation Into Whether Sixth Form Students in St. Rose's High Eat Healthily or NotjoannePas encore d'évaluation
- Mid Term PresentationDocument16 pagesMid Term PresentationSyed HusamPas encore d'évaluation
- Defects in Forming ProcessDocument7 pagesDefects in Forming ProcessDhruv BhandariPas encore d'évaluation
- Exp2 Coconut OilDocument3 pagesExp2 Coconut OilJane Guiron AballaPas encore d'évaluation
- 2010 Enviro Catalog 4-28-10Document60 pages2010 Enviro Catalog 4-28-10marald4432Pas encore d'évaluation
- How Connectors Get ManufacturedDocument4 pagesHow Connectors Get ManufacturedAisha IsaPas encore d'évaluation
- GST Charged DetailsDocument3 pagesGST Charged DetailsGaurav KPas encore d'évaluation
- Manara Plast PDFDocument2 pagesManara Plast PDFfarjana khatunPas encore d'évaluation
- Metals OL NotesDocument6 pagesMetals OL NotesHooria AminPas encore d'évaluation
- Gardex Primer: Technical Data SheetDocument3 pagesGardex Primer: Technical Data SheetTeknik GresikPas encore d'évaluation
- MMF & Texturing - UpdateDocument26 pagesMMF & Texturing - UpdateChamal JayasinghePas encore d'évaluation
- Parallel Session ICMIA Update 12 NovDocument4 pagesParallel Session ICMIA Update 12 NovnagatozzPas encore d'évaluation
- Thermal Degradation of ABSDocument10 pagesThermal Degradation of ABSdsqdPas encore d'évaluation
- About Welding Process 23Document1 pageAbout Welding Process 23XerexPas encore d'évaluation
- Tractor Emulsion AdvancedDocument2 pagesTractor Emulsion Advancedsatsanh_22100% (1)
- First Floor Beam & Slab Details PDFDocument1 pageFirst Floor Beam & Slab Details PDFRaghul ShangarthiyanPas encore d'évaluation
- EfkaPB2720 TDSDocument2 pagesEfkaPB2720 TDSSebastian GonzalezPas encore d'évaluation
- Wall-Wash Tests On Chemical TankersDocument6 pagesWall-Wash Tests On Chemical TankersMoe Win AungPas encore d'évaluation
- Washing Machines: Schedule - 12Document15 pagesWashing Machines: Schedule - 12muralisunPas encore d'évaluation
- Design A Composite Materials Landing GearDocument12 pagesDesign A Composite Materials Landing GearSoma VargaPas encore d'évaluation
- Elementis-Bentone GS - TDSDocument2 pagesElementis-Bentone GS - TDSmgamal1080Pas encore d'évaluation
- Pengaruh Penggunaan Limbah Kerak Tanur Cangkang Sawit Dengan Bahan Pengikat Retona Blend 55 Terhadap Campuran Laston Ac-WcDocument12 pagesPengaruh Penggunaan Limbah Kerak Tanur Cangkang Sawit Dengan Bahan Pengikat Retona Blend 55 Terhadap Campuran Laston Ac-WcBimmo Dwi HartonoPas encore d'évaluation
- Draft Rib Crbo1x1000mw TSB Ok Mhps Ok Hdec Og 180712 by CedhDocument1 658 pagesDraft Rib Crbo1x1000mw TSB Ok Mhps Ok Hdec Og 180712 by CedhZaki FauzanPas encore d'évaluation
- KAT0130-0004-E Cables For Reeling Systems PDFDocument76 pagesKAT0130-0004-E Cables For Reeling Systems PDFhino_kaguPas encore d'évaluation