Académique Documents
Professionnel Documents
Culture Documents
Experiment Reactants Condition of Reaction: SMK Kepong Kimia Tingkatan 5 Cube 1
Transféré par
Klvin TeeDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Experiment Reactants Condition of Reaction: SMK Kepong Kimia Tingkatan 5 Cube 1
Transféré par
Klvin TeeDroits d'auteur :
Formats disponibles
SMK KEPONG
KIMIA TINGKATAN 5
CUBE 1
1 Three experiments, I, II and III are carried out to investigate the factors affecting the rate of reaction. Table 1 shows
the reactants and the conditions of reaction involved.
Experiment Reactants Condition of reaction
I Excess zinc 50 cm3 of 0.5 mol dm-3 hydrochloric acid Room temperature
3 -3
II Excess zinc 50 cm of 0.5 mol dm sulphuric acid Room temperature
III Excess zinc 50 cm3 of 0.5 mol dm-3 sulphuric acid 60 °C
Table 1
(a) (i) Refering to experiments I, II and III, state:
The meaning of the rate of reaction
Two factors that affect the rate of reaction [3 marks]
ii) Write a balanced chemical equation for the reaction in experiment. [2 marks]
(b) Calculate the total volume of hydrogen gas released in experiment I.
[Molar gas volume at room conditions is 24 dm3] [3 marks]
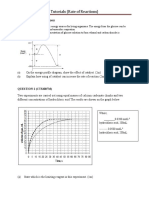
(c) Diagram 1 shows the results of experiments I, II and III.
Diagram 1
Based on the graph,
(i) compare the rate of reaction between experiment I and experiment II.
Explain your answer using the Collision Theory.
[5 marks]
(ii) suggest one way to obtain curve III without changing zink, acid or temperature in experiment II.
Explain your answer using the Collision Theory.
[5 marks]
(iii) explain why the total volume of hydrogen gas released in experiment I and experiment II is doubled that of
experiment I.
[2 marks]
Vous aimerez peut-être aussi
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)D'EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)Pas encore d'évaluation
- The Meaning of The Rate of Reaction Two Factors That Affect The Rate of ReactionDocument2 pagesThe Meaning of The Rate of Reaction Two Factors That Affect The Rate of ReactionKlvin TeePas encore d'évaluation
- O Level Biology Practice Questions And Answers EnzymesD'EverandO Level Biology Practice Questions And Answers EnzymesÉvaluation : 5 sur 5 étoiles5/5 (1)
- Chemistry SPM State Trial Papers-Form5chap1Document17 pagesChemistry SPM State Trial Papers-Form5chap1Law Jin YaoPas encore d'évaluation
- Of Hydroge N Gas (CM) V 2V III II: SPM 2010/paper 2/ Section B/ Question 8: Rate of ReactionDocument1 pageOf Hydroge N Gas (CM) V 2V III II: SPM 2010/paper 2/ Section B/ Question 8: Rate of ReactionSaya RizalPas encore d'évaluation
- Znco HNO Gas Y X H O Heating Crystallisation: Cut It ShortDocument1 pageZnco HNO Gas Y X H O Heating Crystallisation: Cut It ShortNHani IderisPas encore d'évaluation
- 3 Esay Rate of ReactionDocument17 pages3 Esay Rate of ReactionNurul Aini MusaPas encore d'évaluation
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocument29 pagesChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanPas encore d'évaluation
- RTS Chemistry SPM Question Bank Chapter 10Document8 pagesRTS Chemistry SPM Question Bank Chapter 10Scorched ZenPas encore d'évaluation
- Module 7 (Teacher) CHDocument6 pagesModule 7 (Teacher) CHrvinrajPas encore d'évaluation
- f5 Chapter 1 Essay QDocument4 pagesf5 Chapter 1 Essay Qzhen1998Pas encore d'évaluation
- Practicetopics 6 Paper 1.pagesDocument13 pagesPracticetopics 6 Paper 1.pagesnadia sykesPas encore d'évaluation
- SPM Kimia Tingkatan, 5 Rate of Reaction ExerciseDocument7 pagesSPM Kimia Tingkatan, 5 Rate of Reaction Exerciseryder1man6433Pas encore d'évaluation
- F5 Chemistry CHP 1Document12 pagesF5 Chemistry CHP 1muraliMuPas encore d'évaluation
- Chapter 1Document11 pagesChapter 1kenenathPas encore d'évaluation
- RTS Chemistry SPM Question Bank Chapter 10Document8 pagesRTS Chemistry SPM Question Bank Chapter 10dobbybibiPas encore d'évaluation
- Chemistry F5C1Document9 pagesChemistry F5C1Mohammad Nur SyafiqPas encore d'évaluation
- A Student Carried Out Three Experiments To Investigate The Factors Affecting The Rate of ReactionDocument3 pagesA Student Carried Out Three Experiments To Investigate The Factors Affecting The Rate of ReactionazrulakmarPas encore d'évaluation
- SPM Chemistry QuestionDocument6 pagesSPM Chemistry QuestionSaya MenangPas encore d'évaluation
- Marking Scheme AssayDocument5 pagesMarking Scheme AssaySmoi TaiPas encore d'évaluation
- Chem SL QBDocument24 pagesChem SL QBJad GhaouiPas encore d'évaluation
- Ujian Setara 1 2017 KimiaDocument9 pagesUjian Setara 1 2017 KimiaZulkifli Bin PariPas encore d'évaluation
- Chapter 1 QuestionsDocument7 pagesChapter 1 QuestionsfazdirPas encore d'évaluation
- WS Grade 10 IG Chemistry 23-24 - Rate of ReactionDocument6 pagesWS Grade 10 IG Chemistry 23-24 - Rate of ReactionSiyaPas encore d'évaluation
- 2015 - Kem Pecutan Kimia SBP - T5Document24 pages2015 - Kem Pecutan Kimia SBP - T5Mohd HaidilPas encore d'évaluation
- C5 C3 Termokimia 3842Document5 pagesC5 C3 Termokimia 3842Azrel BatistaPas encore d'évaluation
- Kinetics SLDocument16 pagesKinetics SLAmiraliPas encore d'évaluation
- Chemistry Paper 4 November 2009Document13 pagesChemistry Paper 4 November 2009EvansPas encore d'évaluation
- Chemistry Blueprint PDFDocument33 pagesChemistry Blueprint PDFbhagya shree VPas encore d'évaluation
- Factor Affecting Rate of Reaction ExerciseDocument3 pagesFactor Affecting Rate of Reaction ExerciseRafiq IrdhinaPas encore d'évaluation
- Youis - Ushami.IE? - : Instructions: 1. The Question Paper Has Four Parts. All Parts Are CompulsoryDocument4 pagesYouis - Ushami.IE? - : Instructions: 1. The Question Paper Has Four Parts. All Parts Are CompulsoryBazil 9393Pas encore d'évaluation
- 08a. Chemical Kinetics SheetDocument33 pages08a. Chemical Kinetics SheetVIKRANTH KUMAR JAKKOJUPas encore d'évaluation
- Rate of Reaction Factors Affecting Rate of Reaction: Orientation in Space and TimeDocument6 pagesRate of Reaction Factors Affecting Rate of Reaction: Orientation in Space and Timemayana agarwalPas encore d'évaluation
- C3 Exercise 3Document8 pagesC3 Exercise 3Noor Liyana Ahmad FuadPas encore d'évaluation
- Soalan ObjektifDocument9 pagesSoalan ObjektifHairul Nizam OmarPas encore d'évaluation
- Solaf Chemistry SPM 2014: Chapter 1 Form 5: Rate of ReactionDocument18 pagesSolaf Chemistry SPM 2014: Chapter 1 Form 5: Rate of ReactionNik Diana Hartika Nik Husain100% (1)
- Form 5 Chapter 3Document18 pagesForm 5 Chapter 3Kalaiarasu SelvarajanPas encore d'évaluation
- Kadar Tindak Balas.K 2 & K3Document16 pagesKadar Tindak Balas.K 2 & K3Narah NasPas encore d'évaluation
- Tutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Document7 pagesTutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Subesh ShanmugamPas encore d'évaluation
- How FastDocument54 pagesHow FastKaushal Silva RanpatabendigePas encore d'évaluation
- Chemistry Answers PDFDocument126 pagesChemistry Answers PDFNurafiqah FarhaniPas encore d'évaluation
- Physical Chemistry QuestionsDocument22 pagesPhysical Chemistry QuestionshanaPas encore d'évaluation
- SS 2 Chemistry Theory (2nd Term, 2024)Document2 pagesSS 2 Chemistry Theory (2nd Term, 2024)qasimyoosufPas encore d'évaluation
- HKDSE Chemistry: (Paper 2) Mock Examination 4Document6 pagesHKDSE Chemistry: (Paper 2) Mock Examination 4Vinaigrette HePas encore d'évaluation
- Microsoft Word - C18 PS1aDocument6 pagesMicrosoft Word - C18 PS1aabcdelololPas encore d'évaluation
- Bahagian B. StrukturDocument4 pagesBahagian B. StrukturWan Zaharah Wan ZainuddinPas encore d'évaluation
- AP Chemistry Unit 2 Packet 3Document3 pagesAP Chemistry Unit 2 Packet 3Brandon BaxterPas encore d'évaluation
- Level 2 (Some Things Missing)Document4 pagesLevel 2 (Some Things Missing)Viviana PlacentinoPas encore d'évaluation
- Paper - 1 (Theory) : ChemistryDocument7 pagesPaper - 1 (Theory) : Chemistrykaithabjeet singhPas encore d'évaluation
- Chem Test 1 2019 Section BDocument8 pagesChem Test 1 2019 Section BAmirah Noor AffandiPas encore d'évaluation
- Zimbabwe School Examinations Council Chemistry 6031/3Document12 pagesZimbabwe School Examinations Council Chemistry 6031/3takundavs100% (2)
- A Level Chemistry Paper 2 Exam 28Document3 pagesA Level Chemistry Paper 2 Exam 28Anthony AndyPas encore d'évaluation
- K2 KTBDocument9 pagesK2 KTBnur mazrahPas encore d'évaluation
- Heat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Document8 pagesHeat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Mark Riley81% (16)
- Mid Term Examination November 2014 II Puc ChemistryDocument2 pagesMid Term Examination November 2014 II Puc ChemistryRahul PPas encore d'évaluation
- General Chemistry I Final Exam Sem 1 2009Document4 pagesGeneral Chemistry I Final Exam Sem 1 2009John BrownPas encore d'évaluation
- Chemistry Form 4 PP2Document12 pagesChemistry Form 4 PP2jmwalimu81Pas encore d'évaluation
- TrialDocument6 pagesTrialBenjamin TeePas encore d'évaluation
- Reversible Reactions and Equilibria Paper1Document6 pagesReversible Reactions and Equilibria Paper1saipkPas encore d'évaluation
- Assessment 1 Form 5Document12 pagesAssessment 1 Form 5Masitah Abu BakarPas encore d'évaluation
- Redox WrkshtsDocument2 pagesRedox WrkshtsMary Ann DimacaliPas encore d'évaluation
- Hydrogenation of Maltose in Catalytic Membrane ReaDocument4 pagesHydrogenation of Maltose in Catalytic Membrane Reakhaled kaidPas encore d'évaluation
- Functional Group InterconversionDocument7 pagesFunctional Group InterconversionSUBHASISH DASHPas encore d'évaluation
- 4 Chemical KineticsDocument96 pages4 Chemical Kineticsforojaypee2002Pas encore d'évaluation
- Alkylation of Enolate IonsDocument13 pagesAlkylation of Enolate IonsGabriel PekárekPas encore d'évaluation
- Notes and Questions: Aqa GcseDocument16 pagesNotes and Questions: Aqa Gcseapi-422428700Pas encore d'évaluation
- Thermochemistry QuizDocument2 pagesThermochemistry QuizMohit sadhPas encore d'évaluation
- The Collision TheoryDocument5 pagesThe Collision TheoryRhea PardiñasPas encore d'évaluation
- Carboxylic Acids Carboxylic Acids Carboxylic Acids Carboxylic AcidsDocument25 pagesCarboxylic Acids Carboxylic Acids Carboxylic Acids Carboxylic AcidsOhmark VeloriaPas encore d'évaluation
- Senior/principal Scientist/engineerDocument6 pagesSenior/principal Scientist/engineerapi-121342364Pas encore d'évaluation
- Homogeneous & Heterogeneous Reactions-1Document17 pagesHomogeneous & Heterogeneous Reactions-1Muhammad Saim100% (1)
- Accepted Manuscript: Thermochimica ActaDocument22 pagesAccepted Manuscript: Thermochimica ActaAngelica Taipe ZevallosPas encore d'évaluation
- Alkyl Halides and Nucleophilic SubstitutionDocument53 pagesAlkyl Halides and Nucleophilic SubstitutionRaja DanishPas encore d'évaluation
- Vs. E1 vs. E2 - Factors Affecting The Type of Reaction An Alkyl Halide Undergoes IncludeDocument9 pagesVs. E1 vs. E2 - Factors Affecting The Type of Reaction An Alkyl Halide Undergoes Includenikhil jalanPas encore d'évaluation
- Chapter 22: Carbonyl Alpha-Substitution ReactionsDocument4 pagesChapter 22: Carbonyl Alpha-Substitution Reactionsmikec86Pas encore d'évaluation
- Organic Chemistry 7Th Edition Bruice Test Bank Full Chapter PDFDocument64 pagesOrganic Chemistry 7Th Edition Bruice Test Bank Full Chapter PDFjunemojarrazqmxj100% (7)
- CKB 20104 - Reaction EngineeringDocument9 pagesCKB 20104 - Reaction EngineeringNoor FatihahPas encore d'évaluation
- C18 Solved Problems 1 PDFDocument6 pagesC18 Solved Problems 1 PDFGerald Labios100% (2)
- Chemistry 18 - Notes PDFDocument63 pagesChemistry 18 - Notes PDFGeorge Miguel Obusán100% (1)
- Learn: Learning Activity Sheet General Chemistry 2 Week 7B: Rate LawsDocument3 pagesLearn: Learning Activity Sheet General Chemistry 2 Week 7B: Rate LawsJbrePas encore d'évaluation
- CHE S402 Chapter9 Deactivation Part2Document9 pagesCHE S402 Chapter9 Deactivation Part2Sonu SinghPas encore d'évaluation
- Simplified Melc-Based Budget of Lessons in General Biology 2Document3 pagesSimplified Melc-Based Budget of Lessons in General Biology 2irene belle dableolesiguesPas encore d'évaluation
- Chapter 14 LectureDocument55 pagesChapter 14 LectureReinette Carl Sagrio100% (1)
- Chemistry Module Ii Physical Chemistry Ii For Iit Jee Main and Advanced Ranveer Singh Mcgraw Hill Education - Ebook PDFDocument68 pagesChemistry Module Ii Physical Chemistry Ii For Iit Jee Main and Advanced Ranveer Singh Mcgraw Hill Education - Ebook PDFrobert.davidson233100% (28)
- Chem 3 MCQ MedDocument20 pagesChem 3 MCQ Medbrighter716Pas encore d'évaluation
- Application of Factors Affecting Rate of Chemical ReactionDocument2 pagesApplication of Factors Affecting Rate of Chemical Reactiongodlike 3605Pas encore d'évaluation
- Chapters 1 & 2.: Questions For ReasoningDocument8 pagesChapters 1 & 2.: Questions For ReasoningAimane hantoutPas encore d'évaluation
- Haldane RelationshipDocument8 pagesHaldane RelationshipmehakPas encore d'évaluation
- 1 Reaction KineticsDocument41 pages1 Reaction KineticsZIAJIAPas encore d'évaluation
- JJDocument4 pagesJJEureca ParraPas encore d'évaluation
- Process Plant Equipment: Operation, Control, and ReliabilityD'EverandProcess Plant Equipment: Operation, Control, and ReliabilityÉvaluation : 5 sur 5 étoiles5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeD'EverandChemistry for Breakfast: The Amazing Science of Everyday LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (14)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincD'EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincÉvaluation : 3.5 sur 5 étoiles3.5/5 (137)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsD'EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsÉvaluation : 5 sur 5 étoiles5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (5)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeD'EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticePas encore d'évaluation
- ICH Quality Guidelines: An Implementation GuideD'EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdalePas encore d'évaluation
- Sodium Bicarbonate: Nature's Unique First Aid RemedyD'EverandSodium Bicarbonate: Nature's Unique First Aid RemedyÉvaluation : 5 sur 5 étoiles5/5 (21)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeD'EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeÉvaluation : 5 sur 5 étoiles5/5 (1)
- It's Elemental: The Hidden Chemistry in EverythingD'EverandIt's Elemental: The Hidden Chemistry in EverythingÉvaluation : 4 sur 5 étoiles4/5 (10)
- Taste: Surprising Stories and Science About Why Food Tastes GoodD'EverandTaste: Surprising Stories and Science About Why Food Tastes GoodÉvaluation : 3 sur 5 étoiles3/5 (20)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeD'EverandChemistry for Breakfast: The Amazing Science of Everyday LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (90)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeD'EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeÉvaluation : 4 sur 5 étoiles4/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsD'EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsPas encore d'évaluation
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersD'EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersPas encore d'évaluation
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (1)
- Guidelines for Defining Process Safety Competency RequirementsD'EverandGuidelines for Defining Process Safety Competency RequirementsÉvaluation : 3 sur 5 étoiles3/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesD'EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesPas encore d'évaluation
- Guidelines for Chemical Process Quantitative Risk AnalysisD'EverandGuidelines for Chemical Process Quantitative Risk AnalysisÉvaluation : 5 sur 5 étoiles5/5 (1)
- An Applied Guide to Water and Effluent Treatment Plant DesignD'EverandAn Applied Guide to Water and Effluent Treatment Plant DesignÉvaluation : 5 sur 5 étoiles5/5 (4)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesD'EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesÉvaluation : 5 sur 5 étoiles5/5 (2)
- Piping Engineering Leadership for Process Plant ProjectsD'EverandPiping Engineering Leadership for Process Plant ProjectsÉvaluation : 5 sur 5 étoiles5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideD'EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuidePas encore d'évaluation