Académique Documents
Professionnel Documents
Culture Documents
Hospilecass 1
Transféré par
Mylz MendozaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Hospilecass 1
Transféré par
Mylz MendozaDroits d'auteur :
Formats disponibles
1. REPUBLIC ACT No.
3720
Title: This Act shall be known as the "Food, Drug, and Cosmetic Act."
Objective: AN ACT TO ENSURE THE SAFETY AND PURITY OF FOODS, DRUGS, AND
COSMETICS BEING MADE AVAILABLE TO THE PUBLIC BY CREATING THE FOOD AND
DRUG ADMINISTRATION WHICH SHALL ADMINISTER AND ENFORCE THE LAWS
PERTAINING THERETO.
2. Republic Act No. 6675
Title – This Act shall be known as the "Generics Act of 1988."
Objective: AN ACT TO PROMOTE, REQUIRE AND ENSURE THE PRODUCTION OF AN
ADEQUATE SUPPLY, DISTRIBUTION, USE AND ACCEPTANCE OF DRUGS AND MEDICINES
IDENTIFIED BY THEIR GENERIC NAMES
Be it enacted by the Senate and House of Representatives of the Philippines in Congress
assembled::
3. REPUBLIC ACT No. 5921 –
Title : (The Old Pharmacy Law)
Objective:AN ACT REGULATING THE PRACTICE OF PHARMACY AND SETTING STANDARDS
OF PHARMACEUTICAL EDUCATION IN THE PHILIPPINES AND FOR OTHER PURPOSES.
Objectives. This Act provides for and shall govern (a) the standardization and regulation of
pharmaceutical education; (b) the examination for registration of graduates of schools of pharmacy
and (c) the supervision, control and regulation of the practice of pharmacy in the Philippines.
4. REPUBLIC ACT NO. 8203
Title. – This Act shall be known as the “Special Law on Counterfeit Drugs.”
Objective: AN ACT PROHIBITING COUNTERFEIT DRUGS, PROVIDING PENALTIES
FOR VIOLATIONS AND APPROPRIATING FUNDS THEREFOR
5. Republic Act No. 9502
Short Title. - This Act shall be known as the "Universally Accessible Cheaper and Quality
Medicines Act of 2008".
Objective: AN ACT PROVIDING FOR CHEAPER AND QUALITY MEDICINES, AMENDING FOR
THE PURPOSE REPUBLIC ACT NO. 8293 OR THE INTELLECTUAL PROPERTY CODE,
REPUBLIC ACT NO. 6675 OR THE GENERICS ACT OF 1988, AND REPUBLIC ACT NO. 5921
OR THE PHARMACY LAW, AND FOR OTHER PURPOSES
Be it enacted by the Senate and House of Representatives of the Philippines in Congress
assembled::
6. Republic Act No. 9711
Title."Food and Drug Administration (FDA) Act of 2009".
Objective: AN ACT STRENGTHENING AND RATIONALIZING THE REGULATORY CAPACITY
OF THE BUREAU OF FOOD AND DRUGS (BFAD) BY ESTABLISHING ADEQUATE TESTING
LABORATORIES AND FIELD OFFICES, UPGRADING ITS EQUIPMENT, AUGMENTING ITS
HUMAN RESOURCE COMPLEMENT, GIVING AUTHORITY TO RETAIN ITS INCOME,
RENAMING IT THE FOOD AND DRUG ADMINISTRATION (FDA), AMENDING CERTAIN
SECTIONS OF REPUBLIC ACT NO. 3720, AS AMENDED, AND APPROPRIATING FUNDS
THEREOF
Be it enacted by the Senate and House of Representatives of the Philippines in Congress
assembled::
Section 1. The Bureau of Food and Drugs (BFAD) is hereby renamed the Food and Drug
Administration (FDA).
Section 2. This Act shall be known as the "Food and Drug Administration (FDA) Act of 2009".
7. different color and sizes AND USES for IV INJECTION
IV FLUIDS
IV FLUIDS
TYPES of FLUID
1.ISOTONIC Fluid – no movement of fluid.
2.HYPOTONIC Fluid – fluid will enter the cell, the cell will shrink.
3.HYPERTONIC Fluid – fluid will go out from the cell, the cell will shrink
What is dextrose 5% in water?
Dextrose is a form of glucose (sugar). Dextrose 5% in water is injected into a vein
through an IV to replace lost fluids and provide carbohydrates to the body.
Dextrose 5% in water is used to treat low blood sugar (hypoglycemia), insulin shock, or
dehydration (fluid loss). Dextrose 5% in water is also given for nutritional support to
patients who are unable to eat because of illness, injury, or other medical condition.
Dextrose 5% in water is sometimes used as a diluent (liquid) for preparing injectable
medication in an IV bag. A diluent provides a large amount of fluid in which to dilute a
small amount of medicine. The diluent helps carry the medicine into your bloodstream
through the IV. This helps your caregivers inject the medicine slowly and more safely
into your body.
Vous aimerez peut-être aussi
- Republic Act MnemonicsDocument3 pagesRepublic Act MnemonicsJill P100% (1)
- Leo Pharma Research 8Document65 pagesLeo Pharma Research 8Alvin John BalagbagPas encore d'évaluation
- PUBLIC HEALTH LAWS WORD RainDocument8 pagesPUBLIC HEALTH LAWS WORD RainAech EuiePas encore d'évaluation
- Republic ActDocument5 pagesRepublic ActcaptainzackPas encore d'évaluation
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDocument23 pagesBe It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledJanesel Plariza PanerioPas encore d'évaluation
- Republic Act No. 9502Document12 pagesRepublic Act No. 9502Roel AbricaPas encore d'évaluation
- REPUBLIC ACT No 9502Document16 pagesREPUBLIC ACT No 9502Ysiis CCPas encore d'évaluation
- CHN - Phillipine Nursing Laws Final - 010728Document8 pagesCHN - Phillipine Nursing Laws Final - 010728Mi RivarezPas encore d'évaluation
- Republic ActDocument6 pagesRepublic ActLouisaRedoblePas encore d'évaluation
- Legal Aspect in NursingDocument11 pagesLegal Aspect in Nursingdpenaflor100% (7)
- Ethics CHNDocument16 pagesEthics CHNDerick Keith YawanPas encore d'évaluation
- Tumulak, Jamaica Marie M. 45 2B-PHDocument4 pagesTumulak, Jamaica Marie M. 45 2B-PHJaica Mangurali TumulakPas encore d'évaluation
- Pharma Drugs LawDocument6 pagesPharma Drugs LawgreinabelPas encore d'évaluation
- PolicyDocument3 pagesPolicyAlbert Mayo100% (1)
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDocument5 pagesBe It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledRonil ArbisPas encore d'évaluation
- Republic Act 9502Document20 pagesRepublic Act 9502fantasticnerdPas encore d'évaluation
- RA 9502-Amending RA 8293, RA 6675 & RA 5921-Cheaper Medicines Act (2008)Document19 pagesRA 9502-Amending RA 8293, RA 6675 & RA 5921-Cheaper Medicines Act (2008)Duko Alcala EnjambrePas encore d'évaluation
- R.A. 9502Document12 pagesR.A. 9502Daren Mar BumanlagPas encore d'évaluation
- Ethical Considerations in Community Health NursingDocument26 pagesEthical Considerations in Community Health NursingMarlon Glorioso II100% (2)
- Begun and Held Metro Manila, On Monday, The Twerity Third of July, T W o Thousand SevenDocument33 pagesBegun and Held Metro Manila, On Monday, The Twerity Third of July, T W o Thousand SevenSusielyn Anne Tumamao AngobungPas encore d'évaluation
- Public Food LawDocument13 pagesPublic Food LawMickaela MalinaoPas encore d'évaluation
- Reaction PaperDocument2 pagesReaction PaperSherylou Kumo SurioPas encore d'évaluation
- 5 Republic Acts of Food Products Labeling: Devices, and Cosmetics Act" - This Act Governs TheDocument3 pages5 Republic Acts of Food Products Labeling: Devices, and Cosmetics Act" - This Act Governs TheDencil Ramos EspejoPas encore d'évaluation
- Public HealthDocument28 pagesPublic HealthDoc Weh Akut-Salutan100% (1)
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDocument3 pagesBe It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDaren Mar BumanlagPas encore d'évaluation
- Mapeh 10-HealthDocument5 pagesMapeh 10-HealthKatty Cedro Dela CruzPas encore d'évaluation
- Public Health Laws: Romeo R. Andaya, MD, MSCPD, PHD, Fpafp Chair, DPMCH Uph-DjgtmuDocument8 pagesPublic Health Laws: Romeo R. Andaya, MD, MSCPD, PHD, Fpafp Chair, DPMCH Uph-DjgtmuKatPas encore d'évaluation
- Bachelor of Science in Nursing: NCMA 217 RLE: Rle Module Rle Unit WeekDocument5 pagesBachelor of Science in Nursing: NCMA 217 RLE: Rle Module Rle Unit WeekKhams TolentinoPas encore d'évaluation
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDocument14 pagesBe It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembleddoraemoanPas encore d'évaluation
- Differentiate A) Non-Prescription/over-The Counter Drug, B) Prescription, C) Regulated and D) Prohibited DrugsDocument8 pagesDifferentiate A) Non-Prescription/over-The Counter Drug, B) Prescription, C) Regulated and D) Prohibited DrugsVince PaelmoPas encore d'évaluation
- The Generics ActDocument19 pagesThe Generics ActCJ Halasan100% (1)
- Executive Orders (E.O)Document2 pagesExecutive Orders (E.O)Harley Justiniani Dela CruzPas encore d'évaluation
- Universally Accessible Cheaper and Quality Medicines Act ofDocument26 pagesUniversally Accessible Cheaper and Quality Medicines Act ofAlvin John BalagbagPas encore d'évaluation
- Module 02 - Pertinent Laws and Regulations On Public Health - Engr - LCAnonuevoDocument26 pagesModule 02 - Pertinent Laws and Regulations On Public Health - Engr - LCAnonuevoKathleen CastilloPas encore d'évaluation
- Ra 9502Document22 pagesRa 9502Are Pee EtcPas encore d'évaluation
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledDocument4 pagesBe It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledEmerson NunezPas encore d'évaluation
- Ra 9502Document18 pagesRa 9502Ryan AspePas encore d'évaluation
- Begun and Held Metro Manila, On Monday, The Twerity Third of July, T W o Thousand SevenDocument33 pagesBegun and Held Metro Manila, On Monday, The Twerity Third of July, T W o Thousand SevenFaye BaborPas encore d'évaluation
- Public Health Laws in The PhilippinesDocument24 pagesPublic Health Laws in The PhilippinesKarylle PetilPas encore d'évaluation
- The Generic ActDocument32 pagesThe Generic ActElinor Faith V. Retita-CoronadoPas encore d'évaluation
- Republic Act 6675 - Generics Act of 1988Document5 pagesRepublic Act 6675 - Generics Act of 1988Katrina Javier BolivarPas encore d'évaluation
- Ra 9502Document19 pagesRa 9502VicoAdventoPas encore d'évaluation
- Important Acts - ProjectDocument17 pagesImportant Acts - ProjectABDUL RAHMANPas encore d'évaluation
- Cheaper Medicine BillDocument30 pagesCheaper Medicine BillRalph OreiroPas encore d'évaluation
- CU Week 14 PUBLIC HEALTH LAWSDocument8 pagesCU Week 14 PUBLIC HEALTH LAWSAech EuiePas encore d'évaluation
- Generic Act 1988Document3 pagesGeneric Act 1988Pamela Marie O. BaldonadoPas encore d'évaluation
- RA9502 Cheaper MedicineDocument19 pagesRA9502 Cheaper MedicineNikki Rosana RatonPas encore d'évaluation
- IRR of RA 9502 PDFDocument67 pagesIRR of RA 9502 PDFJay Ryan Sy BaylonPas encore d'évaluation
- Joint Doh-Dti-Ipo-Bfad Administrative Order No. 2008-01Document66 pagesJoint Doh-Dti-Ipo-Bfad Administrative Order No. 2008-01gianfranco0613Pas encore d'évaluation
- RA 9502 of The PhilippinesDocument15 pagesRA 9502 of The PhilippinesNikki Francine BaldePas encore d'évaluation
- CHN Salient Laws PDFDocument2 pagesCHN Salient Laws PDFheluPas encore d'évaluation
- ReportingMCN PDFX PDFDocument136 pagesReportingMCN PDFX PDFAndrei BorataPas encore d'évaluation
- Republic Act 6675Document5 pagesRepublic Act 6675Vinson PatronPas encore d'évaluation
- Recent Trends On RA 8976Document2 pagesRecent Trends On RA 8976Bro-wdaalfow DiazPas encore d'évaluation
- RA 6675 Generic Drug ActDocument4 pagesRA 6675 Generic Drug ActMermerRectoPas encore d'évaluation
- MIDTERMS Mazon 3 - Other Laws Relating To Medical TechnologyDocument3 pagesMIDTERMS Mazon 3 - Other Laws Relating To Medical TechnologyMazon, Dinah Melisse P.Pas encore d'évaluation
- REPUBLIC ACT NO 9502 Cheaper Medicine ActDocument3 pagesREPUBLIC ACT NO 9502 Cheaper Medicine ActnathfuentesPas encore d'évaluation
- Law Enactment Related To Health and Nursing ProfessionDocument46 pagesLaw Enactment Related To Health and Nursing ProfessionLilly PattersonPas encore d'évaluation
- SB 2137 18th Cong Sen R Hontiveros Counterfeit Drugs 2021-04-19Document17 pagesSB 2137 18th Cong Sen R Hontiveros Counterfeit Drugs 2021-04-19Rave PerezPas encore d'évaluation
- Asthma QFR 9-11Document2 pagesAsthma QFR 9-11Mylz MendozaPas encore d'évaluation
- IRR of RA 8203Document7 pagesIRR of RA 8203Eah Carit GeremiasPas encore d'évaluation
- Experiment 7 Basic Test For AntibioticsDocument1 pageExperiment 7 Basic Test For AntibioticsMylz MendozaPas encore d'évaluation
- Asthma (Case Study 6)Document14 pagesAsthma (Case Study 6)Mylz MendozaPas encore d'évaluation
- Asthma QFR 1-5Document3 pagesAsthma QFR 1-5Mylz MendozaPas encore d'évaluation
- News Constitutions Acts Batas Pambansa Presidential Decrees Commonwealth Acts Republic Acts Rules of Procedure JurisprudenceDocument35 pagesNews Constitutions Acts Batas Pambansa Presidential Decrees Commonwealth Acts Republic Acts Rules of Procedure JurisprudenceMylz MendozaPas encore d'évaluation
- qfrUTI EditDocument9 pagesqfrUTI EditMylz MendozaPas encore d'évaluation
- News Constitutions Acts Batas Pambansa Presidential Decrees Commonwealth Acts Republic Acts Rules of Procedure JurisprudenceDocument35 pagesNews Constitutions Acts Batas Pambansa Presidential Decrees Commonwealth Acts Republic Acts Rules of Procedure JurisprudenceMylz MendozaPas encore d'évaluation
- Laboratory Waste Disposal: The Importance of Having A Safe Disposal PlanDocument3 pagesLaboratory Waste Disposal: The Importance of Having A Safe Disposal PlanMylz MendozaPas encore d'évaluation
- Asthma QFR 9-11Document2 pagesAsthma QFR 9-11Mylz MendozaPas encore d'évaluation
- Lab and Dignostic Tests of Cardiovascular System - Mylene A. MendozaDocument5 pagesLab and Dignostic Tests of Cardiovascular System - Mylene A. MendozaMylz MendozaPas encore d'évaluation
- Environmental ChemistryDocument12 pagesEnvironmental ChemistryMylz MendozaPas encore d'évaluation
- IRR of RA 8203Document7 pagesIRR of RA 8203Eah Carit GeremiasPas encore d'évaluation
- DiseasesDocument5 pagesDiseasesMylz MendozaPas encore d'évaluation
- Oa 4Document6 pagesOa 4Mylz MendozaPas encore d'évaluation
- Laboratory Waste Disposal: The Importance of Having A Safe Disposal PlanDocument3 pagesLaboratory Waste Disposal: The Importance of Having A Safe Disposal PlanMylz MendozaPas encore d'évaluation
- qfrUTI EditDocument9 pagesqfrUTI EditMylz MendozaPas encore d'évaluation
- Hypertension 3Document5 pagesHypertension 3Mylz MendozaPas encore d'évaluation
- Case Study No. 1 The Polydrug Overdose: Medical TermsDocument15 pagesCase Study No. 1 The Polydrug Overdose: Medical TermsMylz MendozaPas encore d'évaluation
- Biodiversity and A Healthy SocietyDocument6 pagesBiodiversity and A Healthy SocietyMasterJam FernandezPas encore d'évaluation
- Pcog Phytochemical ScreeningDocument2 pagesPcog Phytochemical ScreeningMylz MendozaPas encore d'évaluation
- YogartDocument5 pagesYogartMylz MendozaPas encore d'évaluation
- Pharmacy JurisDocument35 pagesPharmacy JurisMylz MendozaPas encore d'évaluation
- Life and Work of RizalDocument3 pagesLife and Work of RizalMylz MendozaPas encore d'évaluation
- HyperglycemiaDocument11 pagesHyperglycemiaMylz MendozaPas encore d'évaluation
- Hypertension 3Document5 pagesHypertension 3Mylz MendozaPas encore d'évaluation
- What Is A drug-WPS OfficeDocument6 pagesWhat Is A drug-WPS OfficeMylz MendozaPas encore d'évaluation
- Waste ManagementDocument3 pagesWaste ManagementMylz MendozaPas encore d'évaluation
- DiabatesDocument10 pagesDiabatesMuhammad touseefPas encore d'évaluation
- HyperglycemiaDocument11 pagesHyperglycemiaMylz MendozaPas encore d'évaluation
- GAD OrientationDocument58 pagesGAD OrientationKevin Dalangin100% (2)
- Most Important Electrical Engineering NTS Based Short QuestionsDocument63 pagesMost Important Electrical Engineering NTS Based Short QuestionsnageenPas encore d'évaluation
- Leader Ship Assessment: Student No 374212036Document4 pagesLeader Ship Assessment: Student No 374212036Emily KimPas encore d'évaluation
- Stanford - Trauma - Guidelines June 2016 Draft Adult and Peds FINALDocument166 pagesStanford - Trauma - Guidelines June 2016 Draft Adult and Peds FINALHaidir Muhammad100% (1)
- Acalculous Cholecystitis CaseDocument35 pagesAcalculous Cholecystitis CaseSaada Enok MedtamakPas encore d'évaluation
- 2nd Comprehensive ExamDocument15 pages2nd Comprehensive ExamLoala SMDPas encore d'évaluation
- Diana ACCOMPLISHMENTDocument8 pagesDiana ACCOMPLISHMENTMarycon MaapoyPas encore d'évaluation
- Dingenen 2017Document14 pagesDingenen 2017pedro.coleffPas encore d'évaluation
- AssaultDocument12 pagesAssaultEd Mundo100% (3)
- CONTROL & SHUTDOWN PHI R.B - Comment PDFDocument14 pagesCONTROL & SHUTDOWN PHI R.B - Comment PDFmzqaqila100% (1)
- English 10-1 Personal Response EssayDocument2 pagesEnglish 10-1 Personal Response Essayapi-467840192Pas encore d'évaluation
- Argumentative EssayDocument5 pagesArgumentative EssayJoshua MontoyaPas encore d'évaluation
- History of Crime and Criminal Justice in America EditedDocument7 pagesHistory of Crime and Criminal Justice in America EditedcarolinePas encore d'évaluation
- EcodesDocument2 pagesEcodesValentin IonutPas encore d'évaluation
- DICGC - For Depositors - A Guide To Deposit InsuranceDocument10 pagesDICGC - For Depositors - A Guide To Deposit InsuranceSachinPas encore d'évaluation
- Lithium Primary Batteries (Jauch)Document72 pagesLithium Primary Batteries (Jauch)MedSparkPas encore d'évaluation
- The Room - SartreDocument14 pagesThe Room - SartreYue Biohazard100% (1)
- Book Summary - I Hear You Summary Michael Sorensen - Read in 7 MinutesDocument10 pagesBook Summary - I Hear You Summary Michael Sorensen - Read in 7 MinutesNogüey JosePas encore d'évaluation
- LaserDocument12 pagesLasercabe79Pas encore d'évaluation
- Exam1 Key JMB s06Document13 pagesExam1 Key JMB s06Steve DangPas encore d'évaluation
- DBM MonetizationDocument2 pagesDBM MonetizationrsdiamzPas encore d'évaluation
- Plant Based Plan White PaperDocument24 pagesPlant Based Plan White PaperSara AdemovicPas encore d'évaluation
- Presentation 2Document70 pagesPresentation 2Vivek LathPas encore d'évaluation
- MSDS Industrial MargarineDocument3 pagesMSDS Industrial MargarineAeropaulo14Pas encore d'évaluation
- World Wide Emission Free E-Motercycle ProjectDocument20 pagesWorld Wide Emission Free E-Motercycle ProjectAkshay SharmaPas encore d'évaluation
- CV Mary Mugo 2017Document4 pagesCV Mary Mugo 2017Mary Wanja MugoPas encore d'évaluation
- Expository Cause and Effect OUTLINEDocument2 pagesExpository Cause and Effect OUTLINEAutoDefencePas encore d'évaluation
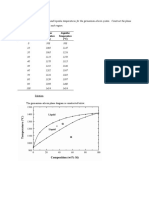
- Composition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Document7 pagesComposition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Muhammad Ibkar YusranPas encore d'évaluation
- MUMMY'S KITCHEN NEW FinalDocument44 pagesMUMMY'S KITCHEN NEW Finalanon_602671575100% (4)
- Manual Servicio Soxte 2050Document46 pagesManual Servicio Soxte 2050Quimica JordanlabPas encore d'évaluation