Académique Documents
Professionnel Documents
Culture Documents
Geometría Molecular PDF
Transféré par
GeanellaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Geometría Molecular PDF
Transféré par
GeanellaDroits d'auteur :
Formats disponibles
436
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
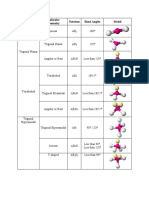
T A B L E 1 0 . 1 Electron and Molecular Geometries
Electron Bonding Lone Electron Molecular Approximate
Groups* Groups Pairs Geometry Geometry Bond Angles Example
2 2 0 Linear Linear 180° O C O
Trigonal F
3 3 0 Trigonal planar 120°
planar
F B F
3 2 1 Trigonal planar Bent <120° O S O
4 4 0 Tetrahedral Tetrahedral 109.5° H C H
H
Trigonal H N H
4 3 1 Tetrahedral <109.5°
pyramidal
H
4 2 2 Tetrahedral Bent <109.5° H O H
Cl
Cl
Trigonal Trigonal 120° (equatorial)
5 5 0
bipyramidal bipyramidal 90° (axial) P Cl
Cl
Cl
F
Trigonal <120° (equatorial)
5 4 1
bipyramidal
Seesaw
<90° (axial)
F S F
Trigonal F Br F
5 3 2 T-shaped <90°
bipyramidal
F
Trigonal
5 2 3 Linear 180° F Xe F
bipyramidal
F
F F
6 6 0 Octahedral Octahedral 90° S
F F
F
F
Square
6 5 1 Octahedral <90° F Br F
pyramidal
F F
F
Square
6 4 2 Octahedral
planar
90° F Xe F
*Count only electron groups around the central atom. Each of the following is considered one electron group: a lone pair, a single bond, a double bond, a triple bond,
or a single electron.
M10_TRO5187_04_SE_C10_426-483v4.0.2.indd 436 2015/11/20 7:15 PM
Vous aimerez peut-être aussi
- Mobil 1 Engine Oil GuideDocument2 pagesMobil 1 Engine Oil GuideTudor RatiuPas encore d'évaluation
- CPAA Cable Installation Manual-GJ + GL MAZDA 6Document20 pagesCPAA Cable Installation Manual-GJ + GL MAZDA 6Viktor XPas encore d'évaluation
- Adult Therapy Guide-9020-7705 PDFDocument62 pagesAdult Therapy Guide-9020-7705 PDFbogdan.tomosPas encore d'évaluation
- Configuratii ElectroniceDocument1 pageConfiguratii ElectroniceVasile FlaviusPas encore d'évaluation
- Vsepr ChartDocument2 pagesVsepr Chartapi-239855791Pas encore d'évaluation
- 4.3-VSEPR - Shapes of MoleculesDocument1 page4.3-VSEPR - Shapes of MoleculesStephan MinhPas encore d'évaluation
- 3 AB Trigonal Planar Trigonal Planar 120 Between All BondsDocument5 pages3 AB Trigonal Planar Trigonal Planar 120 Between All BondsVedantPas encore d'évaluation
- VSEPR Theory Bond Angle TableDocument1 pageVSEPR Theory Bond Angle TableAudrey HizonPas encore d'évaluation
- Electron Bonding Molecular Geometry GuideDocument2 pagesElectron Bonding Molecular Geometry GuideRichamille Ann RicafortePas encore d'évaluation
- Lewis Structures Molecular Geometry and Polarity 1A KEYDocument3 pagesLewis Structures Molecular Geometry and Polarity 1A KEYrsleoPas encore d'évaluation
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaPas encore d'évaluation
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles BubblesPas encore d'évaluation
- 2..chemical Bonding Theory-12-12Document1 page2..chemical Bonding Theory-12-12Ashish SharmaPas encore d'évaluation
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkePas encore d'évaluation
- Nota VSEPR PDFDocument1 pageNota VSEPR PDFMarlene GazconPas encore d'évaluation
- KYOCERA CatalogueDocument95 pagesKYOCERA CatalogueMANIT KUMAR BHOIPas encore d'évaluation
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoPas encore d'évaluation
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjPas encore d'évaluation
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahPas encore d'évaluation
- Iso DJ Milling InsertsDocument21 pagesIso DJ Milling InsertsalphatoolsPas encore d'évaluation
- CLASSIFICATION AND PROPERTIES OF TRIANGLESDocument8 pagesCLASSIFICATION AND PROPERTIES OF TRIANGLEScharmaine padorPas encore d'évaluation
- K 5Document44 pagesK 5Ricardo Rincon Vega100% (1)
- Sa&Vol FormulaeDocument1 pageSa&Vol FormulaeComputer ioPas encore d'évaluation
- Precision mitre saws with rotating tables and self-centering lockingDocument4 pagesPrecision mitre saws with rotating tables and self-centering lockingTerra MachinesPas encore d'évaluation
- Sample - Thickness Test Measurement For Steam DrumDocument1 pageSample - Thickness Test Measurement For Steam DrumAzim AsriPas encore d'évaluation
- Iso Turning InsertsDocument37 pagesIso Turning InsertsalphatoolsPas encore d'évaluation
- 110022-1 Extraction Fan For VapourDocument1 page110022-1 Extraction Fan For VapourpaulPas encore d'évaluation
- Cast Iron Epoxy Hub Spigot 102221Document15 pagesCast Iron Epoxy Hub Spigot 102221Anonymous wt2BA7uPas encore d'évaluation
- LiTech DatasheetDocument1 pageLiTech DatasheetMadhankumarPas encore d'évaluation
- Catalog of Milling Solution 2020Document31 pagesCatalog of Milling Solution 2020vedrenne92Pas encore d'évaluation
- Geometry Exercise - 1 (Redo) (Cie Cmabridge Mathematics Answer Guide)Document11 pagesGeometry Exercise - 1 (Redo) (Cie Cmabridge Mathematics Answer Guide)Jenna HanyPas encore d'évaluation
- Circular MeasureDocument7 pagesCircular MeasureaffendePas encore d'évaluation
- Geometry - HW 31 Angles Formed by Secants and TangentsDocument4 pagesGeometry - HW 31 Angles Formed by Secants and Tangentsroselyn panganibanPas encore d'évaluation
- 8inchdrumd10 lh6Document1 page8inchdrumd10 lh6mohammad khoraminiaPas encore d'évaluation
- Part DrawingDocument1 pagePart DrawingAbhishek PatilPas encore d'évaluation
- LiTech DatasheetDocument1 pageLiTech DatasheetMadhankumarPas encore d'évaluation
- Correlation 1 - Trigonometry NotesDocument7 pagesCorrelation 1 - Trigonometry Notesdoni poPas encore d'évaluation
- Tabelas Roscas TrapezoidaisDocument49 pagesTabelas Roscas TrapezoidaisDesenvolvimento MHPas encore d'évaluation
- CF 10 Vbuurm KRV 22PPDocument1 pageCF 10 Vbuurm KRV 22PPHERNAN MESIASPas encore d'évaluation
- 13C and 1H NMR (RMN 1H y 13C)Document1 page13C and 1H NMR (RMN 1H y 13C)veromendoPas encore d'évaluation
- Cazoom Maths. Lines and Angles. Angles On Parallel Lines (A) - AnswersDocument2 pagesCazoom Maths. Lines and Angles. Angles On Parallel Lines (A) - AnswersChelsea ChikafuPas encore d'évaluation
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolPas encore d'évaluation
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaPas encore d'évaluation
- Trapezgewinde Musterzeichnung PDFDocument1 pageTrapezgewinde Musterzeichnung PDFHMPGPas encore d'évaluation
- EDLW DortmundDocument17 pagesEDLW Dortmunddaniel.namendorfPas encore d'évaluation
- Saegengewinde MusterzeichnungDocument1 pageSaegengewinde MusterzeichnunggeigerPas encore d'évaluation
- Tip VDocument1 pageTip VKenan RamićPas encore d'évaluation
- SumiSmall 2015-16 CATALOG LR PDFDocument249 pagesSumiSmall 2015-16 CATALOG LR PDFAlbertPas encore d'évaluation
- Dimensions: UnitsDocument58 pagesDimensions: UnitsNaman MahawarPas encore d'évaluation
- Square and RectangleDocument5 pagesSquare and RectangleVishnu PrasadPas encore d'évaluation
- Solving Geometry Problems Using Angle and Distance TheoremsDocument6 pagesSolving Geometry Problems Using Angle and Distance Theoremsjv680802Pas encore d'évaluation
- Molecular GeometryDocument1 pageMolecular GeometryDean Joyce Alboroto100% (1)
- Ambiciclo Rampa Debaixo MMCDocument1 pageAmbiciclo Rampa Debaixo MMCAndréAmsPas encore d'évaluation
- U-Section: Values For CalculationDocument3 pagesU-Section: Values For CalculationEng-CalculationsPas encore d'évaluation
- Tme R: Technical Data SheetDocument5 pagesTme R: Technical Data SheetEnrique HortaPas encore d'évaluation
- Insert Designation ChartDocument10 pagesInsert Designation ChartEmba MadrasPas encore d'évaluation
- 05 Geometric Details Hairpin Bends 05 Hairpin BendsDocument1 page05 Geometric Details Hairpin Bends 05 Hairpin BendsBinod Raj GiriPas encore d'évaluation
- Wallmount PDFDocument1 pageWallmount PDFAris RisnandarPas encore d'évaluation
- Cazoom Maths. Lines and Angles. Angles On Parallel Lines (A)Document2 pagesCazoom Maths. Lines and Angles. Angles On Parallel Lines (A)Apex GamingPas encore d'évaluation
- ZYHBDocument30 pagesZYHBGio GPas encore d'évaluation
- Elastic LiDAR, Theory, Practice, and Analysis MethodsDocument572 pagesElastic LiDAR, Theory, Practice, and Analysis MethodsTumis BuduPas encore d'évaluation
- 4501 Homework04solDocument9 pages4501 Homework04solDaudi Erasto MlangiPas encore d'évaluation
- PrinciplesDocument608 pagesPrinciplesspeedkillz83% (6)
- ZeemanDocument6 pagesZeemanMateo BarraganPas encore d'évaluation
- Chemistry Page 4 To 20Document17 pagesChemistry Page 4 To 20Boopathi SarvesanPas encore d'évaluation
- LAB REPORT 3 ChemDocument4 pagesLAB REPORT 3 ChemSofia GarciaPas encore d'évaluation
- TsonopoulosDocument16 pagesTsonopoulosCaique FerreiraPas encore d'évaluation
- CHEMISTRY-04-06 - 11th (PQRS)Document8 pagesCHEMISTRY-04-06 - 11th (PQRS)Raju SinghPas encore d'évaluation
- 2.5.2 Energy Levels PDFDocument3 pages2.5.2 Energy Levels PDFpraveen alwisPas encore d'évaluation
- Soldering BrazingDocument31 pagesSoldering BrazingchchuanPas encore d'évaluation
- AP Chemistry Course OverviewDocument1 pageAP Chemistry Course OverviewhersheymkmPas encore d'évaluation
- ASTM D2386-15e1 PDFDocument5 pagesASTM D2386-15e1 PDFAndres Muñoz AguirrePas encore d'évaluation
- Lesson 2 Puu-0 4400 Operating Principles of ScrubbersDocument12 pagesLesson 2 Puu-0 4400 Operating Principles of Scrubbersssmith2007100% (1)
- Oil Tanker Familiarization Handout Seaskills Maritime AcademyDocument63 pagesOil Tanker Familiarization Handout Seaskills Maritime AcademyruchirrathorePas encore d'évaluation
- Kompedium FrosioDocument126 pagesKompedium Frosioprotein100% (5)
- Organic Chemistry Principles and TechniquesDocument27 pagesOrganic Chemistry Principles and TechniquesAwan DubeyPas encore d'évaluation
- Directional Anisotropy of The Vibrational Modes in 2D-Layered PerovskitesDocument9 pagesDirectional Anisotropy of The Vibrational Modes in 2D-Layered PerovskitesDibyajyoti GhoshPas encore d'évaluation
- Sae Technical Paper SeriesDocument7 pagesSae Technical Paper SeriesManuel LentiPas encore d'évaluation
- Flares ImDocument25 pagesFlares ImBaba JohnehPas encore d'évaluation
- Et - Test PPFDocument6 pagesEt - Test PPFclvsh1985Pas encore d'évaluation
- PDF PDFDocument25 pagesPDF PDFelbronPas encore d'évaluation
- Conformaations Chairs Energy ProfilingDocument24 pagesConformaations Chairs Energy ProfilingMaxi MaPas encore d'évaluation
- Introduction To NanomaterialsDocument73 pagesIntroduction To Nanomaterialsabhay yelmulePas encore d'évaluation
- مكامن ١٢Document11 pagesمكامن ١٢MohammedalwaelyPas encore d'évaluation
- Application of Mossbauer Spectroscopy in Fe and SNDocument17 pagesApplication of Mossbauer Spectroscopy in Fe and SNMeghnath100% (2)
- 1 Organic Chemistry of Aliphatic CompoundsDocument12 pages1 Organic Chemistry of Aliphatic CompoundsSholpanPas encore d'évaluation
- Cbse Class XI Chemistry Sample Paper - 3 Solution Section ADocument13 pagesCbse Class XI Chemistry Sample Paper - 3 Solution Section ABhabaniPas encore d'évaluation
- 1 s2.0 S2772801322000185 Main 2Document17 pages1 s2.0 S2772801322000185 Main 2Mani VrsPas encore d'évaluation
- Appendix B: International Standard AtmosphereDocument2 pagesAppendix B: International Standard AtmosphereredhielPas encore d'évaluation