Académique Documents
Professionnel Documents
Culture Documents
Adrenal Medulla: Pheochromocytoma
Transféré par
Taro RahmatiaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Adrenal Medulla: Pheochromocytoma
Transféré par
Taro RahmatiaDroits d'auteur :
Formats disponibles
Adrenal Medulla
The adrenal medulla is developmentally, functionally, and structurally distinct from the adrenal
cortex. It is composed of specialized neural crest (neuroendocrine) cells, termed chromaffin cells,
and their supporting (sustentacular) cells. The adrenal medulla is the major source of
catecholamines (epinephrine, norepinephrine) in the body. Neuroendocrine cells similar to
chromaffin cells are widely dispersed in an extra-adrenal system of clusters and nodules that,
together with the adrenal medulla, make up the paraganglion system. These extra-adrenal
paraganglia are closely associated with the autonomic nervous system and can be divided into
three groups based on their anatomic distribution: (1) branchiomeric, (2) intravagal, and (3)
aorticosympathetic. The branchiomeric and intravagal paraganglia associated with the
parasympathetic system are located close to the major arteries and cranial nerves of the head and
neck and include the carotid bodies ( Chapter 16 ). The intravagal paraganglia, as the term
implies, are distributed along the vagus nerve. The aorticosympathetic chain is found in
association with segmental ganglia of the sympathetic system and therefore is distributed mainly
alongside of the abdominal aorta. The organs of Zuckerkandl, close to the aortic bifurcation,
belong to this group.
The most important diseases of the adrenal medulla are neoplasms, which include neoplasms of
chromaffin cells (pheochromocytomas) and neuronal neoplasms (neuroblastic tumors).
Neuroblastomas and other neuroblastic tumors are further discussed in Chapter 10 .
PHEOCHROMOCYTOMA
Pheochromocytomas are neoplasms composed of chromaffin cells, which synthesize and release
catecholamines and in some instances peptide hormones. It is important to recognize these
tumors because they are a rare cause of surgically correctable hypertension. Traditionally,
pheochromocytomas have been associated with a“rule of 10s”.
• 10% of pheochromocytomas are extra-adrenal, occurring in sites such as the organs of
Zuckerkandl and the carotid body. Pheochromocytomas that develop in extra-adrenal
paraganglia are designated paragangliomas and are discussed in Chapter 16 .
• 10% of sporadic adrenal pheochromocytomas are bilateral; this figure may rise to as high
as 50% in cases that are associated with familial syndromes (see below).
• 10% of adrenal pheochromocytomas are biologically malignant, defined by the presence
of metastatic disease. Notably, malignancy is more common (20% to 40%) in extra-
adrenal paragangliomas, and in tumors arising in the setting of certain germline mutations
(see below).
• 10% of adrenal pheochromocytomas are not associated with hypertension. Of the 90%
that present with hypertension, approximately two thirds have “paroxysmal” episodes
associated with sudden rise in blood pressure and palpitations, which can, on occasion, be
fatal.
• One “traditional” 10% rule that has now been modified pertains to familial cases. It is now
recognized that as many as 25% of individuals with pheochromocytomas and
paragangliomas harbor a germline mutation in one of at least six known genes ( Table
24-11 ).[79] Patients with germline mutations are typically younger at presentation than
those with sporadic tumors and more often harbor bilateral disease. The incidence of
malignancy is higher (∼30%) in tumors that arise on the backdrop of germline SDHB
mutations. The three succinate dehydrogenase complex subunit genes (SDHB, SDHC, and
SDHD) encode proteins involved in mitochondrial electron transport and oxygen sensing.
It is postulated that loss of function in one or more of these subunits leads to stabilization
of the oncogenic transcription factor hypoxia-inducible factor 1α (HIF-1α), promoting
tumorigenesis.[80] Notably, stabilization of HIF-1α is also the most likely mechanism
underlying cancer predisposition in patients with von Hippel-Lindau (VHL) syndrome,
since the VHL protein normally targets HIF-1α for destruction.
TABLE 24-11 -- Familial Syndromes Associated with Pheochromocytoma and Extra-
Adrenal Paragangliomas
Syndrome Gene Associated Lesion Other Features
Multiple endocrine neoplasia, RET Pheochromocytoma Medullary thyroid
type 2A (MEN-2A) carcinoma
Parathyroid hyperplasia
Multiple endocrine neoplasia, RET Pheochromocytoma Medullary thyroid
type 2B (MEN-2B) carcinoma
Marfanoid habitus
Mucocutaneous GNs
Neurofibromatosis, type 1 NF1 Pheochromocytoma Neurofibromatosis
(NF1)
Café-au-lait spots
Optic nerve glioma
Von Hippel-Lindau (VHL) VHL Pheochromocytoma, Renal cell carcinoma
paraganglioma (uncommon)
Hemangioblastoma
Pancreatic endocrine
neoplasm
Familial paraganglioma 1 SDHD Pheochromocytoma,
paraganglioma
Familial paraganglioma 3 SDHC Paraganglioma
Familial paraganglioma 4 SDHB Pheochromocytoma,
paraganglioma
Adapted with permission from Elder EE et al.: Pheochromocytoma and functional
paraganglioma syndrome: no longer the 10% tumor. J Surg Oncol 89:193–201, 2005.
GN, ganglioneuroma; NF1, neurofibromin; SDHB, succinate dehydrogenase complex, subunit B;
SDHC, succinate dehydrogenase complex, subunit C; SDHD, succinate dehydrogenase complex,
subunit D.
Morphology. Pheochromocytomas range from small, circumscribed lesions confined to the
adrenal ( Fig. 24-53 ) to large hemorrhagic masses weighing kilograms. The average weight of a
pheochromocytoma is 100 gm, but weights from just over 1 gm to almost 4000 gm have been
reported. The larger tumors are well demarcated by either connective tissue or compressed
cortical or medullary tissue. Richly vascularized fibrous trabeculae within the tumor produce a
lobular pattern. In many tumors, remnants of the adrenal gland can be seen, stretched over the
surface or attached at one pole. On section, the cut surfaces of smaller pheochromocytomas are
yellowtan. Larger lesions tend to be hemorrhagic, necrotic, and cystic and typically efface the
adrenal gland. Incubation of fresh tissue with a potassium dichromate solution turns the tumor a
dark brown color due to oxidation of stored catecholamines, thus the term chromaffin.
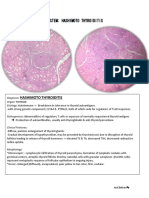
The histologic pattern in pheochromocytoma is quite variable. The tumors are composed of
polygonal to spindle-shaped chromaffin cells or chief cells, clustered with the sustentacular cells
into small nests or alveoli (zellballen) by a rich vascular network ( Fig. 24-54 ). Uncommonly,
the dominant cell type is a spindle or small cell; various patterns can be found in any one tumor.
The cytoplasm has a finely granular appearance, best demonstrated with silver stains, due to the
presence of granules containing catecholamines. The nuclei are usually round to ovoid, with a
stippled “salt and pepper” chromatin that is characteristic of neuroendocrine tumors. Electron
microscopy reveals variable numbers of membrane-bound, electron-dense secretory granules (
Fig. 24-55 ). Immunoreactivity for neuroendocrine markers (chromogranin and synaptophysin)
is seen in the chief cells, while the peripheral sustentacular cells stain with antibodies against S-
100, a calcium-binding protein expressed by a variety of mesenchymal cell types.
Determining malignancy in pheochromocytomas can be vexing. There is no histologic feature
that reliably predicts clinical behavior. Several histologic features, such as numbers of
mitoses, confluent tumor necrosis, and spindle cell morphology, have been associated with an
aggressive behavior and increased risk of metastasis, but these are not entirely reliable. Tumors
with “benign” histologic features may metastasize, while bizarrely pleomorphic tumors may
remain confined to the adrenal gland. In fact, cellular and nuclear pleomorphism, including the
presence of giant cells, and mitotic figures are often seen in benign pheochromocytomas, while
cellular monotony is paradoxically associated with an aggressive behavior. Even capsular and
vascular invasion may be encountered in benign lesions. Therefore, the definitive diagnosis of
malignancy in pheochromocytomas is based exclusively on the presence of metastases.
These may involve regional lymph nodes as well as more distant sites, including liver, lung, and
bone.
FIGURE 24-53 Pheochromocytoma. The tumor is enclosed within an attenuated cortex and demonstrates areas of hemorrhage.
The comma-shaped residual adrenal is seen below. (Courtesy of Dr. Jerrold R. Turner, Department of Pathology, University of
Chicago Hospitals, Chicago, IL.)
FIGURE 24-54 Pheochromocytoma demonstrating characteristic nests of cells (“zellballen”) with abundant cytoplasm. Granules
containing catecholamine are not visible in this preparation. It is not uncommon to find bizarre cells even in pheochromocytomas
that are biologically benign. (Courtesy of Dr. Jerrold R. Turner, Department of Pathology, University of Chicago Hospitals,
Chicago, IL.)
FIGURE 24-55 Electron micrograph of pheochromocytoma. This tumor contains membrane-bound secretory granules in which
catecholamines are stored (30,000×).
Clinical Course.
The dominant clinical manifestation of pheochromocytoma is hypertension, observed in 90% of
patients. Approximately two thirds of patients with hypertension demonstrate paroxysmal
episodes, which are described as an abrupt, precipitous elevation in blood pressure, associated
with tachycardia, palpitations, headache, sweating, tremor, and a sense of apprehension. These
episodes may also be associated with pain in the abdomen or chest, nausea, and vomiting.
Isolated paroxysmal episodes of hypertension occur in fewer than half of patients; more
commonly, patients demonstrate chronic, sustained elevation in blood pressure punctuated by the
aforementioned paroxysms. The paroxysms may be precipitated by emotional stress, exercise,
changes in posture, and palpation in the region of the tumor; patients with urinary bladder
paragangliomas occasionally precipitate a paroxysm during micturition. The elevations of blood
pressure are induced by the sudden release of catecholamines that may acutely precipitate
congestive heart failure, pulmonary edema, myocardial infarction, ventricular fibrillation, and
cerebrovascular accidents. The cardiac complications have been attributed to what has been
called catecholamine cardiomyopathy, or catecholamine-induced myocardial instability and
ventricular arrhythmias. Nonspecific myocardial changes, such as focal necrosis, mononuclear
infiltrates, and interstitial fibrosis, have been attributed either to ischemic damage secondary to
the catecholamine-induced vasomotor constriction of the myocardial circulation or to direct
catecholamine toxicity. In some cases pheochromocytomas secrete other hormones, such as
ACTH and somatostatin, and may therefore be associated with clinical features related to the
secretion of these or other peptide hormones. The laboratory diagnosis of pheochromocytoma is
based on the demonstration of increased urinary excretion of free catecholamines and their
metabolites, such as vanillylmandelic acid and metanephrines.
Isolated benign tumors are treated with surgical excision, after preoperative and intraoperative
medication of patients with adrenergic-blocking agents to prevent a hypertensive crisis.
Multifocal lesions require long-term medical treatment for hypertension.
Vous aimerez peut-être aussi
- Hayashi Reiki ManualDocument14 pagesHayashi Reiki Manualboomerb100% (4)
- Tumours of The Adrenal GlandDocument34 pagesTumours of The Adrenal GlandSonam JoshiPas encore d'évaluation
- Telephone DirectoryDocument4 pagesTelephone DirectoryAnonymous QL0z40Fs9vPas encore d'évaluation
- Clinical Practice Guidelines - 2017Document54 pagesClinical Practice Guidelines - 2017Cem ÜnsalPas encore d'évaluation
- Pheochromocytoma in Genetic Disorders - UpToDateDocument11 pagesPheochromocytoma in Genetic Disorders - UpToDateLaura Marina IlincaPas encore d'évaluation
- SDL 10 BMS16091064Document5 pagesSDL 10 BMS16091064Jonathan YeohPas encore d'évaluation
- Pheochromocytoma - WikipediaDocument1 pagePheochromocytoma - WikipediaIulian GherasimPas encore d'évaluation
- The Endocrine System Path - Week 3Document11 pagesThe Endocrine System Path - Week 3joatasouzaPas encore d'évaluation
- Renal, Urinary Systems - ElectrolytesDocument692 pagesRenal, Urinary Systems - ElectrolytesRoshan MevadaPas encore d'évaluation
- Epidemiology, Natural History, Pathology and Management of Medulloblastoma in ChildrenDocument48 pagesEpidemiology, Natural History, Pathology and Management of Medulloblastoma in ChildrenSam OlukaPas encore d'évaluation
- 1 s2.0 S0085253815518541 MainDocument6 pages1 s2.0 S0085253815518541 MainaripPas encore d'évaluation
- Pituitary AdenomasDocument47 pagesPituitary AdenomasSabrina whtPas encore d'évaluation
- CA AdrenalDocument8 pagesCA AdrenalCleysser Antonio Custodio PolarPas encore d'évaluation
- Ent Mcqs For Part I Exam: Prepared By: Dr. Fouad ShamsanDocument86 pagesEnt Mcqs For Part I Exam: Prepared By: Dr. Fouad ShamsanAli QuwarahPas encore d'évaluation
- Pathology Aspect Pituitary Gland: By. Meike Rachmawati, DR Pathology Anatomy Department Medical Faculty-UnisbaDocument35 pagesPathology Aspect Pituitary Gland: By. Meike Rachmawati, DR Pathology Anatomy Department Medical Faculty-UnisbadeasyahPas encore d'évaluation
- Renal Cell Carcinoma in Young Patients: A Review of Recent LiteratureDocument6 pagesRenal Cell Carcinoma in Young Patients: A Review of Recent LiteratureSAhand HamzaPas encore d'évaluation
- Endocrine System PathologyDocument57 pagesEndocrine System PathologyCARSON 539Pas encore d'évaluation
- Ped Solid TumorDocument52 pagesPed Solid TumorIndranil GhoshPas encore d'évaluation
- Seminar: Jacques W M Lenders, Graeme Eisenhofer, Massimo Mannelli, Karel PacakDocument11 pagesSeminar: Jacques W M Lenders, Graeme Eisenhofer, Massimo Mannelli, Karel PacakAlexandru CozmaPas encore d'évaluation
- Pediatric Brain TumorDocument49 pagesPediatric Brain TumorJessica Victoria SudanawidjajaPas encore d'évaluation
- HHS Public Access: Pituitary TumorsDocument12 pagesHHS Public Access: Pituitary TumorsFauzi NoviaPas encore d'évaluation
- Neuropathologic Features of Central Nervous System HemangioblastomaDocument11 pagesNeuropathologic Features of Central Nervous System HemangioblastomaDavid Camilo GomezPas encore d'évaluation
- Rodgers Et Al-2012-British Journal of HaematologyDocument10 pagesRodgers Et Al-2012-British Journal of HaematologySalwiyadiPas encore d'évaluation
- Pathology of The Endocrine System: Causes A. Pituitary Usually Anterior LobeDocument19 pagesPathology of The Endocrine System: Causes A. Pituitary Usually Anterior LobecystanarisaPas encore d'évaluation
- 4.1c - MEN Syndromes - Nov.10 - Dr. GalangDocument2 pages4.1c - MEN Syndromes - Nov.10 - Dr. GalangMiel Raphael AranillaPas encore d'évaluation
- Path Lab Name: Onyedika Egbujo No: #671 Topic: PheochromocytomaDocument4 pagesPath Lab Name: Onyedika Egbujo No: #671 Topic: PheochromocytomaOnyedika EgbujoPas encore d'évaluation
- Tumors of The Nervous SystemDocument45 pagesTumors of The Nervous SystemIsaac MwangiPas encore d'évaluation
- Parathyroid N AdrenalDocument3 pagesParathyroid N Adrenalvishalyadav5656Pas encore d'évaluation
- Small Round Cell TumorsDocument131 pagesSmall Round Cell TumorschinnnababuPas encore d'évaluation
- Sdxxxxy MRPDocument10 pagesSdxxxxy MRPnon_zensePas encore d'évaluation
- Thyroid Neoplasms: Muhammad Haris Aslam Janjua Resident, Surgical Unit I SIMS/Services Hospital, LahoreDocument116 pagesThyroid Neoplasms: Muhammad Haris Aslam Janjua Resident, Surgical Unit I SIMS/Services Hospital, LahoreHajra IsrarPas encore d'évaluation
- Thyroid: Cytopathology and Its Histopathological BasesDocument49 pagesThyroid: Cytopathology and Its Histopathological BasesYuli Setio Budi PrabowoPas encore d'évaluation
- PheochromocytomaDocument5 pagesPheochromocytomahussain AltaherPas encore d'évaluation
- Basic Science Notes MRCP AssDocument9 pagesBasic Science Notes MRCP AssUm HamoOdPas encore d'évaluation
- 2 Tumour Biology and Histopathology of Neuroendocrine TumoursDocument17 pages2 Tumour Biology and Histopathology of Neuroendocrine TumoursUvi Cancino RamosPas encore d'évaluation
- Patologi Endokrin 2016Document132 pagesPatologi Endokrin 2016agusPas encore d'évaluation
- Patho Prelim 2nd SemDocument14 pagesPatho Prelim 2nd SemIC BPas encore d'évaluation
- 2-Adrenal Path.Document72 pages2-Adrenal Path.Lea MonzerPas encore d'évaluation
- Actualizaciones Oaragangliomas Sociedad EndocrinoDocument9 pagesActualizaciones Oaragangliomas Sociedad EndocrinoCharly FlowPas encore d'évaluation
- FeocromocitomaDocument15 pagesFeocromocitomaRaul GascueñaPas encore d'évaluation
- Teratoma TranslateDocument4 pagesTeratoma TranslateHr. EmhyPas encore d'évaluation
- 2 The Nature of HNSCC-WayanS, DRSPBDocument29 pages2 The Nature of HNSCC-WayanS, DRSPBLeise Kestia Rosalyn LimpelehPas encore d'évaluation
- Leukemias & Lymphomas - HY USMLEDocument87 pagesLeukemias & Lymphomas - HY USMLEMatt McGlothlinPas encore d'évaluation
- Pheochromocytoma and Paraganglioma: A Review of Diagnosis, Management and Treatment of Rare Causes of HypertensionDocument6 pagesPheochromocytoma and Paraganglioma: A Review of Diagnosis, Management and Treatment of Rare Causes of HypertensionBernaHerediaPas encore d'évaluation
- MRCP Notes 2006Document117 pagesMRCP Notes 2006Walaa Ismail HidirbiPas encore d'évaluation
- Retinal Hemangiomas - American Academy of OphthalmologyDocument10 pagesRetinal Hemangiomas - American Academy of OphthalmologyLydia Angelia YanitaPas encore d'évaluation
- Mitochondrial Encephalomyopathies (MEM)Document66 pagesMitochondrial Encephalomyopathies (MEM)Fatma KaledPas encore d'évaluation
- Head and Neck: Salivary Gland Tumors: An OverviewDocument12 pagesHead and Neck: Salivary Gland Tumors: An OverviewVanessa MordiPas encore d'évaluation
- Malignant Pheochromocytoma - A Diagnostic and Therapeutic DilemmaDocument5 pagesMalignant Pheochromocytoma - A Diagnostic and Therapeutic DilemmaJad DegheiliPas encore d'évaluation
- Neoplasms Thyroid N ParathyroidsDocument88 pagesNeoplasms Thyroid N ParathyroidsBkas GrgPas encore d'évaluation
- Hemostasis - Hematology BlockDocument40 pagesHemostasis - Hematology BlockamandaPas encore d'évaluation
- 15c MedullaryThyroidCancer-ITSBookChapterDocument10 pages15c MedullaryThyroidCancer-ITSBookChapterIulia JulyPas encore d'évaluation
- Management of Paraproteinaemia: ReviewDocument7 pagesManagement of Paraproteinaemia: ReviewInaGargPas encore d'évaluation
- Brain TumoursDocument25 pagesBrain Tumoursmulumbamusonda25Pas encore d'évaluation
- Paraneoplastic SyndromesDocument15 pagesParaneoplastic SyndromesironPas encore d'évaluation
- B17M4L4B PheochromocytomaDocument6 pagesB17M4L4B PheochromocytomaJoaquim RodriguezPas encore d'évaluation
- Duchenne Muscular DystrophyDocument10 pagesDuchenne Muscular DystrophyZeeshan KhanPas encore d'évaluation
- The Dysmyelopoietic DisordersDocument26 pagesThe Dysmyelopoietic DisordersJoseph FronterasPas encore d'évaluation
- Pituitary Apoplexy Pathophysiology Diagnosis and MDocument6 pagesPituitary Apoplexy Pathophysiology Diagnosis and MlathifatulPas encore d'évaluation
- Path Prep - GeoffDocument98 pagesPath Prep - GeoffVerlyn YtPas encore d'évaluation
- Fast Facts: Myelofibrosis: Reviewed by Professor Ruben A. MesaD'EverandFast Facts: Myelofibrosis: Reviewed by Professor Ruben A. MesaPas encore d'évaluation
- Neuroendocrine Tumors: Surgical Evaluation and ManagementD'EverandNeuroendocrine Tumors: Surgical Evaluation and ManagementJordan M. CloydPas encore d'évaluation
- Pheochromocytomas, Paragangliomas and Disorders of the Sympathoadrenal System: Clinical Features, Diagnosis and ManagementD'EverandPheochromocytomas, Paragangliomas and Disorders of the Sympathoadrenal System: Clinical Features, Diagnosis and ManagementLewis LandsbergPas encore d'évaluation
- Corneal Degeneration: By-Shweta Santosh Maurya 2 Year B. Optometry Institute For Technology and ManagementDocument43 pagesCorneal Degeneration: By-Shweta Santosh Maurya 2 Year B. Optometry Institute For Technology and ManagementTaro RahmatiaPas encore d'évaluation
- Jve 2 27Document5 pagesJve 2 27Taro RahmatiaPas encore d'évaluation
- Potensi Cabai Sebagai Anti-Aterosklerosis: Fakultas Kedokteran Universitas LampungDocument6 pagesPotensi Cabai Sebagai Anti-Aterosklerosis: Fakultas Kedokteran Universitas LampungTaro RahmatiaPas encore d'évaluation
- Journal Reading DR Arief DADocument26 pagesJournal Reading DR Arief DATaro RahmatiaPas encore d'évaluation
- Basic Musculoskeletal Radiologic InterpretationDocument2 pagesBasic Musculoskeletal Radiologic InterpretationTaro RahmatiaPas encore d'évaluation
- Arthropod BitesDocument60 pagesArthropod BitesTaro RahmatiaPas encore d'évaluation
- Treatment Using Ethanolic Extract of Cocoa Beans)Document5 pagesTreatment Using Ethanolic Extract of Cocoa Beans)Hidayah HarahapPas encore d'évaluation
- Ukuran BurnazinDocument2 pagesUkuran BurnazinTaro RahmatiaPas encore d'évaluation
- Namdaemun Market Location:: Exit From Line 4 - Hoehyeon StationDocument3 pagesNamdaemun Market Location:: Exit From Line 4 - Hoehyeon StationTaro RahmatiaPas encore d'évaluation
- Abdul RohimDocument1 pageAbdul RohimTaro RahmatiaPas encore d'évaluation
- Jadwal Minggu I Blok EmergencyDocument1 pageJadwal Minggu I Blok EmergencyTaro RahmatiaPas encore d'évaluation
- Penilaian Konsumsi Food Recall NikenDocument3 pagesPenilaian Konsumsi Food Recall NikenTaro RahmatiaPas encore d'évaluation
- Qwertyuiop LatihannnnDocument2 pagesQwertyuiop LatihannnnTaro RahmatiaPas encore d'évaluation
- Niken ByssinosisDocument23 pagesNiken ByssinosisTaro RahmatiaPas encore d'évaluation
- Pesticide Illness: Recognition, Diagnosis, ManagementDocument31 pagesPesticide Illness: Recognition, Diagnosis, Managementكسلان اكتب اسميPas encore d'évaluation
- Viral Skin InfectionDocument23 pagesViral Skin InfectionTaro RahmatiaPas encore d'évaluation
- Review Article: The Use of Stem Cells in Burn Wound Healing: A ReviewDocument9 pagesReview Article: The Use of Stem Cells in Burn Wound Healing: A ReviewTaro RahmatiaPas encore d'évaluation
- Pesticide PoisoningDocument34 pagesPesticide PoisoningappealingashishPas encore d'évaluation
- Facial Fractures: Sylvia Aparicio Harvard Medical School Year IVDocument0 pageFacial Fractures: Sylvia Aparicio Harvard Medical School Year IVRumah Kost Kontrakan AntapaniPas encore d'évaluation
- 22 Varicella Pink BookDocument24 pages22 Varicella Pink BookMohamad Syaikhul IslamPas encore d'évaluation
- Chronic Bronchitis in West Sweden A Matter of Smoking and Social ClassDocument10 pagesChronic Bronchitis in West Sweden A Matter of Smoking and Social ClassTaro RahmatiaPas encore d'évaluation
- Facial Fractures: Sylvia Aparicio Harvard Medical School Year IVDocument0 pageFacial Fractures: Sylvia Aparicio Harvard Medical School Year IVRumah Kost Kontrakan AntapaniPas encore d'évaluation
- Herbicide and Fungicide Toxicology 2014Document34 pagesHerbicide and Fungicide Toxicology 2014Taro RahmatiaPas encore d'évaluation
- 22 Varicella Pink BookDocument24 pages22 Varicella Pink BookMohamad Syaikhul IslamPas encore d'évaluation
- 2016 Article 111Document8 pages2016 Article 111Taro RahmatiaPas encore d'évaluation
- Facial Fractures: Sylvia Aparicio Harvard Medical School Year IVDocument0 pageFacial Fractures: Sylvia Aparicio Harvard Medical School Year IVRumah Kost Kontrakan AntapaniPas encore d'évaluation
- Family Eco Map Bapak ZulkiDocument1 pageFamily Eco Map Bapak ZulkiTaro RahmatiaPas encore d'évaluation
- Learning Objectives: Niken Rahmatia 1418011152Document17 pagesLearning Objectives: Niken Rahmatia 1418011152Taro RahmatiaPas encore d'évaluation
- Cushing SyndromeDocument17 pagesCushing SyndromeTaro RahmatiaPas encore d'évaluation
- Clinical Session Adult I-1 3Document34 pagesClinical Session Adult I-1 3Juan LinPas encore d'évaluation
- Respiratory PhysiologyDocument9 pagesRespiratory PhysiologyBrent TorresPas encore d'évaluation
- Modul Normal Delivery 2016Document8 pagesModul Normal Delivery 2016Alvin FarhanPas encore d'évaluation
- Immunization Schedule in India 2017 (Latest !!)Document13 pagesImmunization Schedule in India 2017 (Latest !!)rajPas encore d'évaluation
- HSG PresentationDocument18 pagesHSG Presentationashikin92Pas encore d'évaluation
- TELECARDIOLOGYDocument11 pagesTELECARDIOLOGYNancy Prasad100% (1)
- Basic and Clinical Pharmacology 12th Edition-Bertram Katzung Susan Masters Anthony Trevor-290-296 PDFDocument7 pagesBasic and Clinical Pharmacology 12th Edition-Bertram Katzung Susan Masters Anthony Trevor-290-296 PDFalinamatei1000000Pas encore d'évaluation
- Case StudyDocument4 pagesCase StudyTariq shahPas encore d'évaluation
- PALS Skills ChecklistDocument5 pagesPALS Skills ChecklistGiulia MeniconziPas encore d'évaluation
- 1.1.4.B ConcentrationDocument3 pages1.1.4.B ConcentrationAnonymous lFgKXClH0% (1)
- Indian Perspective For Probiotics: A Review: ArticleDocument12 pagesIndian Perspective For Probiotics: A Review: ArticleMurali DathanPas encore d'évaluation
- Dementia - StatPearls - NCBI BookshelfDocument7 pagesDementia - StatPearls - NCBI BookshelfSMA N 1 TOROHPas encore d'évaluation
- Epidemiology, Prevention and Control of Dengue: DR Tahira JaffarDocument36 pagesEpidemiology, Prevention and Control of Dengue: DR Tahira JaffarRaza UzuPas encore d'évaluation
- Case Study 103Document8 pagesCase Study 103Jonah MaasinPas encore d'évaluation
- Norethisterone JournalDocument29 pagesNorethisterone JournalAditya Syah PutraPas encore d'évaluation
- IPT Students - Non-Hospital Based Specialists KUANTANDocument113 pagesIPT Students - Non-Hospital Based Specialists KUANTANFatin nadhirah Kamaludin latifiPas encore d'évaluation
- Pathology MnemonicsDocument27 pagesPathology MnemonicsdyaPas encore d'évaluation
- 4 EL Husseinys Essentials of Cardiovascular System @eduwaves360Document236 pages4 EL Husseinys Essentials of Cardiovascular System @eduwaves360ahmed_abu_alrobPas encore d'évaluation
- EzhilDocument92 pagesEzhilRachitha GuttaPas encore d'évaluation
- Haemostasis: 1. Vascular SpasmDocument5 pagesHaemostasis: 1. Vascular SpasmAnurag YadavPas encore d'évaluation
- Brief Intervention: Mhgap-Ig Base Course - Field Test Version 1.00 - May 2012 1Document16 pagesBrief Intervention: Mhgap-Ig Base Course - Field Test Version 1.00 - May 2012 1TEOFILO PALSIMON JR.Pas encore d'évaluation
- Teleradiologi: Cross Reporting & Smart AssignDocument17 pagesTeleradiologi: Cross Reporting & Smart Assignrafael100% (1)
- 0 - Review of Post Graduate Medical Entrance Examination (PGMEE) (AAA) (PDFDrive - Com) Export PDFDocument39 pages0 - Review of Post Graduate Medical Entrance Examination (PGMEE) (AAA) (PDFDrive - Com) Export PDFAbu ÂwŞmž100% (2)
- All India Institute of Medical Sciences: List of Faculty - Departmentswise As On 10.10.2014 at The A.I.I.M.S., New DelhiDocument23 pagesAll India Institute of Medical Sciences: List of Faculty - Departmentswise As On 10.10.2014 at The A.I.I.M.S., New DelhiSaif AliPas encore d'évaluation
- Microbiological Quality of Non-SterileDocument5 pagesMicrobiological Quality of Non-SterilePaula BelloPas encore d'évaluation
- Chemical Peel Guidelines PDFDocument1 pageChemical Peel Guidelines PDFHasan MurdimanPas encore d'évaluation
- Photodiagnosis and Photodynamic Therapy: Case ReportDocument2 pagesPhotodiagnosis and Photodynamic Therapy: Case ReportMedPas encore d'évaluation