Académique Documents
Professionnel Documents
Culture Documents
Moles Worksheet
Transféré par
ImranMalikTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Moles Worksheet
Transféré par
ImranMalikDroits d'auteur :
Formats disponibles
Page #1
Worksheet Moles P2 AS
Name:___________________________ Date:________________
1. Ammonium sulfate, (NH4)2SO4, is widely used as a fertiliser.
In order to determine its percentage purity, a sample of ammonium sulfate fertiliser was
analysed by reacting a known amount with an excess of NaOH(aq) and then titrating the
unreacted NaOH with dilute HCl.
(a) Ammonium sulfate reacts with NaOH in a 1 : 2 ratio.
Complete and balance the equation for this reaction.
(NH4)2SO4 + 2NaOH → .......NH3 + ................ + ................
[2]
(b) A 5.00 g sample of a fertiliser containing (NH4)2SO4 was warmed with 50.0 cm3 (an excess)
of 2.00 mol dm–3 NaOH.
When all of the ammonia had been driven off, the solution was cooled.
The remaining NaOH was then titrated with 1.00 mol dm–3 HCl and 31.2 cm3 were required
for neutralisation.
(i) Write a balanced equation for the reaction between NaOH and HCl.
....................................................................................................................................
(ii) Calculate the amount, in moles, of HCl in 31.2 cm3 of 1.00 mol dm–3 HCl.
(iii) Calculate the amount, in moles, of NaOH in 50.0 cm3 of 2.00 mol dm–3 NaOH.
(iv) Use your answers to (i), (ii) and (iii) to calculate the amount, in moles, of NaOH
used up in the reaction with (NH4)2SO4.
Malik Imran Afzal; 0333-4346883
Page #2
(v) Use your answer to (iv) and the equation in (a) to calculate the amount, in moles, of
(NH4)2SO4 that reacted with NaOH.
(vi) Use your answer to (v) to calculate the mass of (NH4)2SO4 that reacted with NaOH.
(vii) Hence, calculate the percentage purity of the ammonium sulfate fertiliser.
[7]
[Total: 9]
Malik Imran Afzal; 0333-4346883
Page #3
2. (a) Chemists recognise that atoms are made of three types of particle.
Complete the following table with their names and properties.
name of particle relative mass relative charge
1/1836
[3]
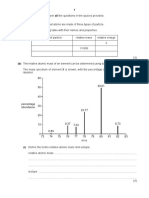
(b) The relative atomic mass of an element can be determined using data from its mass spectrum.
The mass spectrum of element X is shown, with the percentage abundance of each isotope
labelled.
60
49.61
50
40
percentage
abundance 30
23.77
20
10 9.37 7.63 8.73
0.89
0
73 74 75 76 77 78 79 80 81 82 83
m/e
(i) Define the terms relative atomic mass and isotope.
relative atomic mass ...........................................................................................................
.............................................................................................................................................
.............................................................................................................................................
isotope ................................................................................................................................
.............................................................................................................................................
[3]
(ii) Use the data in the mass spectrum to calculate the relative atomic mass, Ar, of X.
Give your answer to two decimal places and suggest the identity of X.
Ar of X ....................................
identity of X ....................................
[2]
Malik Imran Afzal; 0333-4346883
Page #4
(c) The element tellurium, Te, reacts with chlorine to form a single solid product, with a relative
formula mass of 270. The product contains 52.6% chlorine by mass.
(i) Calculate the molecular formula of this chloride.
molecular formula .................................... [3]
3. Some intercontinental jet airliners use kerosene as fuel. The formula of kerosene may be
taken as C14H30.
(a) To which homologous series of compounds does kerosene belong?
.......................................................... [1]
(b) When kerosene burns in an excess of air, carbon dioxide and water form.
Balance the following equation for the complete combustion of kerosene.
......C14H30(l) + ......O2(g) ......CO2(g) + ......H2O(g) [1]
(c) In this section, give your answers to one decimal place.
The flight path from Beijing to Paris is approximately 8195 km.
A typical intercontinental jet airliner burns 10.8 kg of kerosene for each kilometre
covered.
(i) Calculate the mass, in tonnes, of C14H30 burnt on a flight from Beijing to Paris.
[1 tonne = 1 000 kg]
(ii) Use your equation in (b) to calculate the mass, in tonnes, of CO2 produced during
this flight.
[4]
Malik Imran Afzal; 0333-4346883
Page #5
4. A sample of a fertiliser was known to contain ammonium sulfate, (NH4)2SO4, and sand only.
A 2.96 g sample of the solid fertiliser was heated with 40.0 cm3 of NaOH(aq), an excess, and
all of the ammonia produced was boiled away.
After cooling, the remaining NaOH(aq) was exactly neutralised by 29.5 cm3 of 2.00 mol dm–3
HCl.
In a separate experiment, 40.0 cm3 of the original NaOH(aq) was exactly neutralised by
39.2 cm3 of the 2.00 mol dm–3 HCl.
(a) (i) Write balanced equations for the following reactions.
NaOH with HCl
....................................................................................................................................
(NH4)2SO4 with NaOH
....................................................................................................................................
(ii) Calculate the amount, in moles, of NaOH present in the 40.0 cm3 of the original
NaOH(aq) that was neutralised by 39.2 cm3 of 2.00 mol dm–3 HCl.
(iii) Calculate the amount, in moles, of NaOH present in the 40.0 cm3 of NaOH(aq) that
remained after boiling the (NH4)2SO4.
(iv) Use your answers to (ii) and (iii) to calculate the amount, in moles, of NaOH that
reacted with the (NH4)2SO4.
(v) Use your answers to (i) and (iv) to calculate the amount, in moles, of (NH4)2SO4 that
reacted with the NaOH.
Malik Imran Afzal; 0333-4346883
Page #6
(vi) Hence calculate the mass of (NH4)2SO4 that reacted.
(vii) Use your answer to (vi) to calculate the percentage, by mass, of (NH4)2SO4 present
in the fertiliser.
Write your answer to a suitable number of significant figures.
[9]
(b) The uncontrolled use of nitrogenous fertilisers can cause environmental damage to lakes
and streams. This is known as eutrophication.
What are the processes that occur when excessive amounts of nitrogenous fertilisers get
into lakes and streams?
...........................................................................................................................................
...........................................................................................................................................
..................................................................................................................................... [2]
(c) Large quantities of ammonia are manufactured by the Haber process.
Not all of this ammonia is used to make fertilisers.
State one large-scale use for ammonia, other than in the production of nitrogenous
fertilisers.
..................................................................................................................................... [1]
[Total: 12]
Malik Imran Afzal; 0333-4346883
Vous aimerez peut-être aussi
- 2020 Revision For Chapter 3 PDFDocument3 pages2020 Revision For Chapter 3 PDFTimothy Handoko0% (1)
- Reaction Kinetics Question PDFDocument8 pagesReaction Kinetics Question PDFdanielmahsa0% (1)
- Matrix Math: Addition, Subtraction, and PropertiesDocument7 pagesMatrix Math: Addition, Subtraction, and PropertiesMohammad AshfaqPas encore d'évaluation
- Homework Reacting Masses (42 MarksDocument4 pagesHomework Reacting Masses (42 MarksloloPas encore d'évaluation
- Chemistry A: H432/02 Summer 2017 Examination SeriesDocument55 pagesChemistry A: H432/02 Summer 2017 Examination SeriesMohammed AlwajihPas encore d'évaluation
- Partition WallDocument24 pagesPartition WallSHRADDHA GHUGEPas encore d'évaluation
- Free Space Optical Communication PDFDocument233 pagesFree Space Optical Communication PDFIgor Novid100% (1)
- CS5001 CourseworkDocument9 pagesCS5001 CourseworkpeteligijusPas encore d'évaluation
- Thermit Australia Product Catalogue 2012Document20 pagesThermit Australia Product Catalogue 2012Rey Luis TehPas encore d'évaluation
- Stoichiometry 2 QP SolvedDocument10 pagesStoichiometry 2 QP Solveddovoo lolPas encore d'évaluation
- COORDINATE GEOMETRYDocument7 pagesCOORDINATE GEOMETRYMrs R MohammedPas encore d'évaluation
- A2 CHM Sol 05 Acid and Base WSDocument28 pagesA2 CHM Sol 05 Acid and Base WSnsPas encore d'évaluation
- A Level Chemistry: Topic 15 - Transition Metals and Complex IonsDocument15 pagesA Level Chemistry: Topic 15 - Transition Metals and Complex IonsKizzy-Anne BoatswainPas encore d'évaluation
- Chemical Energetics ExplainedDocument16 pagesChemical Energetics ExplainedMuhammad Saif AmirPas encore d'évaluation
- Zimbabwe School Examinations Council Chemistry 6031/3Document12 pagesZimbabwe School Examinations Council Chemistry 6031/3takundavs100% (2)
- Summer Task Mathematics-VIDocument5 pagesSummer Task Mathematics-VIUmair IslamPas encore d'évaluation
- Chemistry Paper 1 HL-Nov2017 PDFDocument17 pagesChemistry Paper 1 HL-Nov2017 PDFIrfan zameerPas encore d'évaluation
- Worksheet On Reactions of Organic Chem - Alkanes PDFDocument2 pagesWorksheet On Reactions of Organic Chem - Alkanes PDFpretzPas encore d'évaluation
- Alkali Metals PPQDocument6 pagesAlkali Metals PPQ张米Pas encore d'évaluation
- AGARWALS INTERNATIONAL 50-MIN TESTDocument7 pagesAGARWALS INTERNATIONAL 50-MIN TESTNyonikaPas encore d'évaluation
- Alkane Alkene QuestionsDocument10 pagesAlkane Alkene QuestionsormattPas encore d'évaluation
- Linear LawDocument4 pagesLinear LawJoann NgPas encore d'évaluation
- Atomic StructureDocument27 pagesAtomic StructureZekZanaPas encore d'évaluation
- Ks3 Chem 9e Eq Q1to10 54marks 4students Metals and Corrosion 11pgsDocument11 pagesKs3 Chem 9e Eq Q1to10 54marks 4students Metals and Corrosion 11pgsmatthewansell6977Pas encore d'évaluation
- A Level Chemistry Acids, Bases and Buffers HomeworkDocument16 pagesA Level Chemistry Acids, Bases and Buffers HomeworkMuhammadPas encore d'évaluation
- Paper 2 Nov 2006Document6 pagesPaper 2 Nov 2006MSHPas encore d'évaluation
- Chemistry 9 Icse Sample Paper 1Document5 pagesChemistry 9 Icse Sample Paper 1Technical VanshPas encore d'évaluation
- 9701 s09 QP 32Document12 pages9701 s09 QP 32Hubbak KhanPas encore d'évaluation
- Answers - H2 Topical Chemistry 2014Document99 pagesAnswers - H2 Topical Chemistry 2014Ruel Arila Jr.Pas encore d'évaluation
- OCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelDocument10 pagesOCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelSigourney MarshPas encore d'évaluation
- Mass Spectra and IRDocument7 pagesMass Spectra and IRSyed FahimPas encore d'évaluation
- ASRJC JC2 Chemistry Prelims 2021 ReviewDocument24 pagesASRJC JC2 Chemistry Prelims 2021 ReviewLorraine HoonPas encore d'évaluation
- Ass 4 O Level Pure Maths Paper 1Document16 pagesAss 4 O Level Pure Maths Paper 1peterchiyanjaPas encore d'évaluation
- Test 1 As Chemistry Unit 2 - KineticsDocument10 pagesTest 1 As Chemistry Unit 2 - KineticsKajana Sivarasa Shenthan100% (1)
- Vector PracticeDocument14 pagesVector Practicecookie1414Pas encore d'évaluation
- 2006 JC 1 H2 JCT & Promo - Differential EquationsDocument3 pages2006 JC 1 H2 JCT & Promo - Differential EquationsOccamsRazorPas encore d'évaluation
- 05 Acid Base and Buffer WS 2021Document37 pages05 Acid Base and Buffer WS 2021VayaPas encore d'évaluation
- ACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1Document10 pagesACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1ofji4oPas encore d'évaluation
- Chemistry Perfect Score Module Form 4 2011 No LogoDocument96 pagesChemistry Perfect Score Module Form 4 2011 No Logohome8008100% (2)
- B16 Adaptations Interdependance CompetitionDocument4 pagesB16 Adaptations Interdependance CompetitionSomeonePas encore d'évaluation
- P9 MotionDocument6 pagesP9 MotionJuyeon NamPas encore d'évaluation
- Tkss Prelim 2009 em p2Document10 pagesTkss Prelim 2009 em p2JASON_INGHAMPas encore d'évaluation
- Experimental Techinque Past Papers QuestionsDocument4 pagesExperimental Techinque Past Papers Questionschemking7933% (3)
- Redox Worksheet New 1Document2 pagesRedox Worksheet New 1Azain CardenasPas encore d'évaluation
- 9701 s06 QP 3Document8 pages9701 s06 QP 3Hubbak KhanPas encore d'évaluation
- Chemistry HL Paper 2 TZ1Document36 pagesChemistry HL Paper 2 TZ1Hasnatul Khaira100% (1)
- G.O.C. Assignment-1Document6 pagesG.O.C. Assignment-1Lakshya ChandakPas encore d'évaluation
- Chemical Bonding (AdvancedDocument28 pagesChemical Bonding (AdvancedAnant JainPas encore d'évaluation
- Shapes of Molecules and Intermol Forces TestDocument5 pagesShapes of Molecules and Intermol Forces TestDevangi VyasPas encore d'évaluation
- JEE Main, Advanced 2023 Cis/Trans Isomerism Practice QuestionsDocument26 pagesJEE Main, Advanced 2023 Cis/Trans Isomerism Practice QuestionsVikas MittalPas encore d'évaluation
- Chemistry SpectDocument51 pagesChemistry SpectOnkar SwamiPas encore d'évaluation
- Circuit QuestionsDocument36 pagesCircuit QuestionsMuhammad TauseefPas encore d'évaluation
- Acids, Bases & Salts 1 QP PDFDocument9 pagesAcids, Bases & Salts 1 QP PDFSatria HalimPas encore d'évaluation
- 9701 s02 ErDocument14 pages9701 s02 ErHubbak KhanPas encore d'évaluation
- Hyper ConjugationDocument3 pagesHyper ConjugationKeval MaldePas encore d'évaluation
- Reversible Reactions 1 QPDocument12 pagesReversible Reactions 1 QPAbed AymanPas encore d'évaluation
- Physics Sample - 2 XII 2024Document18 pagesPhysics Sample - 2 XII 2024Kashif SiddiquiPas encore d'évaluation
- XII - Physics - Preboard 1 - Set A 2023Document6 pagesXII - Physics - Preboard 1 - Set A 2023Anuradha MukherjeePas encore d'évaluation
- 1 Energetics WSDocument28 pages1 Energetics WSGanga GowriPas encore d'évaluation
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsD'EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsPas encore d'évaluation
- Y12Document5 pagesY12Kissiedu YirenkyiPas encore d'évaluation
- 2.1 Atomic Structure TheoryDocument7 pages2.1 Atomic Structure TheoryHiba AsifPas encore d'évaluation
- Atoms & ReactionsDocument38 pagesAtoms & Reactionsdank dankPas encore d'évaluation
- Chemical Equilibria NotesDocument7 pagesChemical Equilibria NotesImranMalikPas encore d'évaluation
- Worksheet EquilibriumDocument8 pagesWorksheet EquilibriumRaj BanwaitPas encore d'évaluation
- Bottom-Up and Top-Down Nanoparticle Synthesis MethodsDocument35 pagesBottom-Up and Top-Down Nanoparticle Synthesis MethodsRaghendra DubeyPas encore d'évaluation
- Chemistry 121: Gaseous State Properties & Gas LawsDocument24 pagesChemistry 121: Gaseous State Properties & Gas LawsImranMalikPas encore d'évaluation
- Thesis 1Document141 pagesThesis 1ImranMalikPas encore d'évaluation
- Malik Imran Afzal, Phd. Scholar, Mphil Chemistry. Visiting Faculty Member O/A Level Lgs/Btsc. 0333-4346883Document2 pagesMalik Imran Afzal, Phd. Scholar, Mphil Chemistry. Visiting Faculty Member O/A Level Lgs/Btsc. 0333-4346883ImranMalikPas encore d'évaluation
- MetalsDocument66 pagesMetalsImranMalikPas encore d'évaluation
- Davis ThesisDocument108 pagesDavis ThesisImranMalikPas encore d'évaluation
- Hardness Vs TextureDocument5 pagesHardness Vs TextureShofa R HaqPas encore d'évaluation
- Room Air Conditioner Owner's Manual GuideDocument19 pagesRoom Air Conditioner Owner's Manual GuideMunteanu LucianPas encore d'évaluation
- Improved M16A2 - A3 - A4 Zero TargetDocument6 pagesImproved M16A2 - A3 - A4 Zero Targetbeetho1990Pas encore d'évaluation
- Silo Fluidizer: Keep Dry Bulk Materials MovingDocument2 pagesSilo Fluidizer: Keep Dry Bulk Materials MovingHùng Thanh NguyễnPas encore d'évaluation
- Asphalt Institute PresentationDocument43 pagesAsphalt Institute PresentationMax Cedeño De LeónPas encore d'évaluation
- Final Page Size: A5 (148mm X 210mm)Document20 pagesFinal Page Size: A5 (148mm X 210mm)RJ BevyPas encore d'évaluation
- Hitachi Sumitomo Scx1500 2 Hydraulic Crawler Crane SpecificationsDocument2 pagesHitachi Sumitomo Scx1500 2 Hydraulic Crawler Crane Specificationsmargeret100% (50)
- Pass4sure 200-120 PDF DownloadDocument8 pagesPass4sure 200-120 PDF DownloadEleanor19Pas encore d'évaluation
- Performance-Creative Design Concept For Concrete InfrastructureDocument11 pagesPerformance-Creative Design Concept For Concrete InfrastructureTuan PnPas encore d'évaluation
- Manufacturing of Urea Through Synthetic Ammonia Project ReportDocument5 pagesManufacturing of Urea Through Synthetic Ammonia Project ReportvishnuPas encore d'évaluation
- E 20925Document214 pagesE 20925Ahmed ElshowbkeyPas encore d'évaluation
- Vdo Pressure GuageDocument14 pagesVdo Pressure Guagezuma zaiamPas encore d'évaluation
- Kinematics Horizontal KinematicsDocument5 pagesKinematics Horizontal KinematicsBaiJPPas encore d'évaluation
- Adf Interview Questions and AnsewrsDocument85 pagesAdf Interview Questions and Ansewrsleninbabus100% (2)
- Python Question Paper Mumbai UnivercityDocument5 pagesPython Question Paper Mumbai UnivercityRahul PawarPas encore d'évaluation
- Arahan Penggunaan Lahan Dan Perencanaan Konservasi Tanah Dan Air Di Das Yeh Empas, Tabanan, BaliDocument9 pagesArahan Penggunaan Lahan Dan Perencanaan Konservasi Tanah Dan Air Di Das Yeh Empas, Tabanan, BalialyciaPas encore d'évaluation
- Testing and Maintenance of Steam TrapsDocument8 pagesTesting and Maintenance of Steam TrapsAjaypalsinh GohilPas encore d'évaluation
- Solar Desalination PlantDocument28 pagesSolar Desalination PlantAnonymous TETH310% (1)
- Vapor Pressure Experiment Data AnalysisDocument3 pagesVapor Pressure Experiment Data AnalysisRanaPas encore d'évaluation
- Engineering Data Sheet: 49187073 E 1145842 1 of 1 October 21, 2016 60HzDocument1 pageEngineering Data Sheet: 49187073 E 1145842 1 of 1 October 21, 2016 60HzGustavo VillarrealPas encore d'évaluation
- 2.3 One Way Slab Design-SS, Cant, ContinuousDocument54 pages2.3 One Way Slab Design-SS, Cant, ContinuousAhmed SiddiquePas encore d'évaluation
- DIN EN 10213 - 2008 - Fundidos em AçoDocument29 pagesDIN EN 10213 - 2008 - Fundidos em AçoLeonardo MartinsPas encore d'évaluation
- Thiourea PDFDocument43 pagesThiourea PDFMohamad EshraPas encore d'évaluation
- Slides - OOP With SmalltalkDocument51 pagesSlides - OOP With Smalltalkapi-3728136Pas encore d'évaluation
- Osciloscopio 1006Document74 pagesOsciloscopio 1006ERNESTO BRAVOPas encore d'évaluation
- Williams CatalogoDocument3 pagesWilliams CatalogoMartin AcuñaPas encore d'évaluation