Académique Documents
Professionnel Documents
Culture Documents
PP
Transféré par
Tian Nopita SariTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
PP
Transféré par
Tian Nopita SariDroits d'auteur :
Formats disponibles
Letters
RESEARCH LETTER Table. Baseline Patient Characteristics at Time of Diagnosis of Brain
Metastases in Patients Who Did or Did Not Receive Immunotherapy
Immunotherapy and Symptomatic Radiation
No. (%)
Necrosis in Patients With Brain Metastases No
Treated With Stereotactic Radiation Immuno- Immuno-
therapy therapy
Immunotherapeutic checkpoint inhibitors are commonly used Characteristic (n = 365) (n = 115) P Valuea
in patients with melanoma, non–small cell lung cancer Age, mean (SD), y 62 (11) 61 (11) .17
(NSCLC), and renal cell carcinoma (RCC), all cancers that fre- Sex .13
quently metastasize to the Female 166 (45) 43 (37)
brain. Radiation therapy is Male 199 (55) 72 (63)
Invited Commentary page 1116 frequently used for brain Race .04
metastases bec ause few White 313 (86) 109 (95)
Related article page 1112 systemic agents effectively African American 17 (5) 0
penetrate the blood-brain Hispanic 6 (2) 1 (1)
barrier. The most deleterious consequence of brain-directed, Asian 15 (4) 1 (1)
Other 1 (<1) 0
stereotactic radiation is radiation necrosis—inflammation
Unknown 13 (4) 4 (3)
and/or injury to the brain abutting the treated tumor.1 Pub-
Primary cancer <.001
lished literature has suggested that brain-directed stereotac-
Melanoma 73 (20) 72 (63)
tic radiation in patients also receiving immunotherapy may
NSCLC 256 (70) 38 (33)
yield beneficial, synergistic effects; however, few studies have
Renal cell carcinoma 36 (10) 5 (4)
examined radiation necrosis. We investigated the association
KPS, median (IQR) 90 (80-90) 90 (80-90) .16
between immunotherapy and symptomatic radiation necro-
Size of largest metastasis, 14 (8-22) 12 (6-22) .20
sis in patients with melanoma, NSCLC, or RCC and newly di- median (IQR), mm
agnosed brain metastases treated with stereotactic radiation. Neurologic symptoms 170 (47) 64 (57) .07
Seizures 6 (2) 2 (2) .99
Methods | The institutional review board of Dana-Farber Can- Primary cancer controlled 172 (47) 62 (54) .31
cer Institute approved the project and informed consent was Extracranial disease 290 (79) 102 (89) .03
waived. We identified 480 patients with newly diagnosed Extracranial disease sites
Lung 169 (46) 72 (63) .003
brain metastases secondary to NSCLC (n = 294), melanoma
Liver 35 (10) 24 (21) .003
(n = 145), and RCC (n = 41) treated with stereotactic radiation
Bone 69 (19) 35 (30) .01
at Brigham and Women’s Hospital/Dana-Farber Cancer Insti-
Lymph nodes 110 (30) 47 (41) .04
tute between 2001 and 2015; of these, 115 patients received
Soft tissue 47 (13) 34 (30) <.001
immunotherapeutic checkpoint inhibitors (ipilimumab,
Adrenal 37 (10) 9 (8) .59
pembrolizumab, or nivolumab) whereas 365 did not. Target
Type of initial radiation .24
tumors that were 0 to 2 cm, 2 to 3 cm, and larger than 3 cm
SRS 291 (80) 88 (77)
in maximal diameter generally received 18 to 20 Gy in 1 frac- SRT 55 (15) 16 (14)
tion, 18 Gy in 1 fraction, and 25 to 30 Gy in 5 fractions, SRS and SRT 19 (5) 11 (10)
respectively. Patients received immunotherapy for a median Systemic therapy prior to SRS/SRT 106 (29) 41 (36) .20
of 14.3 (IQR, 8.0-31.0) weeks. Symptomatic radiation necro- No. of prior systemic therapy 0 (0-1) 0 (0-1) .29
sis was defined as an enlarging lesion after stereotactic regimens, median (IQR)
Salvage whole brain radiation 95 (26) 33 (29) .63
radiation causing neurologic symptomatology that displayed after initial SRS/SRT
one of the following characteristics: pathology specimen SRS/SRT after initial SRS/SRT 127 (35) 59 (51) .002
showing only necrosis (if surgical resection performed) or
Abbreviations: IQR, interquartile range; KPS, Karnsofsky performance status;
changes consistent with necrosis on dual-phase positron NSCLC, non–small-cell lung cancer; SD, standard deviation; SRS, stereotactic
emission tomography–computed tomography (PET-CT) or radiosurgery; SRT, stereotactic radiotherapy.
serial magnetic resonance imaging (MRI). a
Baseline patient characteristics in patients who received immunotherapy vs
All time-to-event analyses were performed using Kaplan- not were compared using the t test, Wilcoxon rank sum test, and Fisher exact
test, as indicated.
Meier plots and Cox regression in SAS statistical software pack-
age (version 9.4, SAS Institute). The assumption of propor-
tional hazards was verified. The median follow-up of surviving Results | Patient characteristics at diagnosis of brain metasta-
patients who did vs did not receive immunotherapy was 23.1 ses in patients who did and did not receive immunotherapy
(IQR, 15.4-42.1) vs 25.1 (IQR, 15.2-34.3) months, respectively. were generally well balanced (Table). Symptomatic necrosis
jamaoncology.com (Reprinted) JAMA Oncology August 2018 Volume 4, Number 8 1123
© 2018 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 09/23/2019
Letters
Conclusions | We found an association between receipt of im-
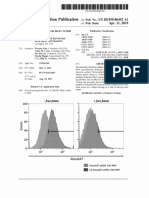
Figure. Kaplan-Meier Curves Displaying Freedom From Symptomatic
Necrosis as Stratified by Receipt of Immunotherapy
munotherapy and symptomatic radiation necrosis in pa-
vs No Receipt of Immunotherapy tients undergoing stereotactic radiation for brain metastases.
Prospective studies are needed to better characterize the risks
1.0
Freedom From Symptomatic Necrosis
and benefits of combining brain-directed stereotactic radia-
No immunotherapy tion with immunotherapy in this population.
0.8
Allison M. Martin, BS
0.6
Immunotherapy
Daniel N. Cagney, MD
Paul J. Catalano, ScD
0.4
Brian M. Alexander, MD, MPH
0.2 Amanda J. Redig, MD, PhD
Jon D. Schoenfeld, MD, MPH
0 Ayal A. Aizer, MD, MHS
0 1 2 3 4 5 6 7 8 9 10
Time, y Author Affiliations: Department of Radiation Oncology, Dana-Farber/Brigham and
No. at risk Women’s Cancer Center, Harvard Medical School, Boston, Massachusetts (Martin,
No immunotherapy 422 203 82 41 20 13 6 3 2 2 1
Immunotherapy 129 67 21 7 4 4 3 2 1 1 0 Cagney,Alexander,Schoenfeld,Aizer); Department of Biostatistics, Harvard T. H. Chan
School of Public Health, Boston, Massachusetts (Catalano); Department of
Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston,
Log rank P<.001.
Massachusetts (Catalano); Department of Medical Oncology, Dana-Farber Cancer
Institute, Harvard Medical School, Boston, Massachusetts (Redig).
Corresponding Author: Ayal Aizer, MD, MHS, Department of Radiation Oncology,
occurred in 23 of 115 and 25 of 365 patients who did vs did Brigham and Women’s Hospital, 75 Francis St, ASB1-L2, Boston, MA 02115
not receive immunotherapy, respectively (Figure). Receipt of (aaaizer@partners.org).
immunotherapy was associated with symptomatic radiation Accepted for Publication: September 16, 2017.
necrosis after adjustment for tumor histology (HR, 2.56; 95% Published Online: January 11, 2018. doi:10.1001/jamaoncol.2017.3993
CI, 1.35-4.86; P = .004); this association was especially
Author Contributions: Drs Martin and Aizer had full access to all of the data in
strong in patients with melanoma (HR, 4.02; 95% CI, 1.17- the study and take responsibility for the integrity of the data and the accuracy
13.82; P = .03). Among patients with melanoma, receipt of of the data analysis.
ipilimumab vs no immunotherapy (HR, 4.70; 95% CI, 1.36- Study concept and design: Martin, Redig, Schoenfeld, Aizer.
Acquisition, analysis, or interpretation of data: Martin, Cagney, Catalano,
16.19; P = .01) and programmed cell death protein 1 (PD-1) Alexander, Schoenfeld, Aizer.
inhibition vs no immunotherapy (HR, 3.57; 95% CI, 0.94- Drafting of the manuscript: Martin, Cagney, Catalano, Redig, Aizer.
13.53; P = .06) were associated with development of sympto- Critical revision of the manuscript for important intellectual content: Martin,
Alexander, Redig, Schoenfeld, Aizer.
matic necrosis, although the association with PD-1 inhibition
Statistical analysis: Martin, Catalano, Aizer.
was not statistically significant. Of the 23 patients who Administrative, technical, or material support: Martin, Cagney, Alexander, Redig,
received immunotherapy and developed symptomatic Schoenfeld, Aizer.
necrosis, 18 (78%) were treated with dexamethasone (me- Study supervision: Alexander, Schoenfeld, Aizer.
dian duration, 1.8 months). Conflict of Interest Disclosures: Dr Redig reports consulting fees from Medtronic,
Boehringer-Ingelheim,andProactiveWorldwideInc.DrSchoenfeldreportsconsulting
andscientificadvisoryboardfeesforBMS,AstraZeneca,Nanobiotix,Tilos,Debiopharm
Discussion | Radiation necrosis significantly impacts quality of aswellasresearchfundingfromMerckandBMS.DrAizerreportsresearchfundingfrom
life; focal neurologic deficits are common, as are headaches, Varian Medical Systems. No other conflicts are reported.
nausea, and seizures. Steroids are often given, potentially mini- 1. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):
mizing the efficacy of immunotherapy.2 Given the strong as- 180-188.
sociation between immunotherapy and symptomatic radia- 2. Chasset F, Pages C, Biard L, et al. Single-center study under a French
Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic
tion necrosis that we observed, utilization of immunotherapy
melanoma: negative impact of baseline corticosteroids. Eur J Dermatol. 2015;25
as monotherapy for treatment of brain metastases has ap- (1):36-44.
peal. However, intracranial response rates to immune- 3. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients
checkpoint monotherapy in patients with brain metastases are with melanoma or non-small-cell lung cancer and untreated brain metastases:
generally low, 3,4 although concurrent ipilimumab plus early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol.
2016;17(7):976-983.
nivolumab in melanoma has promise.5,6
4. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with
melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol.
Limitations. Limitations of our study include its retrospective 2012;13(5):459-465.
nature and the small sample size, which prevented use of a pro- 5. Tawbi HA-H, Forsyth PAJ, Algazi AP, et al. Efficacy and safety of nivolumab
pensity score matching analysis. In addition, there is poten- (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the
tial for error in radiographic delineation of necrosis from tu- brain: results of the phase II study CheckMate 204. J Clin Oncol. 2017;35(suppl
15):9507-9507.
mor progression. However, we found high agreement between
6. Long GV, Atkinson V, Menzies AM, et al. A randomized phase II study of
radiographic-only delineation of necrosis vs progression in pa-
nivolumab or nivolumab combined with ipilimumab in patients (pts) with
tients undergoing surgery (by the senior author, A.A., blinded melanoma brain metastases (mets): The Anti-PD1 Brain Collaboration (ABC).
to the surgical results) and the surgical pathology (κ = .76). J Clin Oncol. 2017;35(suppl 15):9508-9508.
1124 JAMA Oncology August 2018 Volume 4, Number 8 (Reprinted) jamaoncology.com
© 2018 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 09/23/2019
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- First Page PDFDocument1 pageFirst Page PDFTian Nopita SariPas encore d'évaluation
- P E N Elitian I LM I Ah: Effect of Early Mobilization Post Partum Mother To Decrease Uterine Fundus HeightDocument7 pagesP E N Elitian I LM I Ah: Effect of Early Mobilization Post Partum Mother To Decrease Uterine Fundus HeightTian Nopita SariPas encore d'évaluation
- Warta Pengabdian AndalasDocument8 pagesWarta Pengabdian AndalasTian Nopita SariPas encore d'évaluation
- J Cogdev 2006 06 007Document21 pagesJ Cogdev 2006 06 007Tian Nopita SariPas encore d'évaluation
- Colorectal Liver Metastases - An Update On Multidisciplinary Approach - WJHDocument24 pagesColorectal Liver Metastases - An Update On Multidisciplinary Approach - WJHTrí Cương NguyễnPas encore d'évaluation
- Composite Hemangioendothelioma An Unusual Presentation of A Rare Vascular TumorDocument5 pagesComposite Hemangioendothelioma An Unusual Presentation of A Rare Vascular TumorTian Nopita SariPas encore d'évaluation
- Level 1 Japanese: Course IntroductionDocument3 pagesLevel 1 Japanese: Course IntroductionAl-mansorr ManibpalPas encore d'évaluation
- Hubungan Tingkat Pengetahuan Kesehatan Reproduksi Remaja Dengan Sikap Seksual Pranikah Di SMK Taman Siswa Nanggulan Tahun 2017Document4 pagesHubungan Tingkat Pengetahuan Kesehatan Reproduksi Remaja Dengan Sikap Seksual Pranikah Di SMK Taman Siswa Nanggulan Tahun 2017Tian Nopita SariPas encore d'évaluation
- 7758 22294 1 PBDocument7 pages7758 22294 1 PBTian Nopita SariPas encore d'évaluation
- Modpathol 201783Document14 pagesModpathol 201783Tian Nopita SariPas encore d'évaluation
- 03 GualandiDocument10 pages03 GualandiTian Nopita SariPas encore d'évaluation
- Jamaoncology Sonbol 2019 Oi 190087Document7 pagesJamaoncology Sonbol 2019 Oi 190087Tian Nopita SariPas encore d'évaluation
- Theories and Etiologies of Sexual Orientation: January 2014Document43 pagesTheories and Etiologies of Sexual Orientation: January 2014Tian Nopita SariPas encore d'évaluation
- 2529 10019 1 PBDocument9 pages2529 10019 1 PBSii KecilPas encore d'évaluation
- ADLDocument1 pageADLTian Nopita SariPas encore d'évaluation
- Assumption of The Myths of Romantic Love: Its Relationship With Sex, Type of Sex-Affective Relationship, and Sexual OrientationDocument14 pagesAssumption of The Myths of Romantic Love: Its Relationship With Sex, Type of Sex-Affective Relationship, and Sexual OrientationTian Nopita SariPas encore d'évaluation
- Adolescent Sexual Orientation: P S (AH 2008-03)Document5 pagesAdolescent Sexual Orientation: P S (AH 2008-03)Tian Nopita SariPas encore d'évaluation
- Back Massage Therapy To Reduce Low Back Pain in PRDocument4 pagesBack Massage Therapy To Reduce Low Back Pain in PRTian Nopita SariPas encore d'évaluation
- J Cogdev 2006 06 007Document21 pagesJ Cogdev 2006 06 007Tian Nopita SariPas encore d'évaluation
- Iya ItuDocument34 pagesIya ItuTian Nopita SariPas encore d'évaluation
- Pengkajian ICUDocument19 pagesPengkajian ICUWulan Rahmadani FauziPas encore d'évaluation
- Massage Therapy Reduces Pain in Pregnant Women, Alleviates Prenatal Depression in Both Parents and Improves Their RelationshipsDocument5 pagesMassage Therapy Reduces Pain in Pregnant Women, Alleviates Prenatal Depression in Both Parents and Improves Their RelationshipsTian Nopita SariPas encore d'évaluation
- 7758 22294 1 PBDocument7 pages7758 22294 1 PBTian Nopita SariPas encore d'évaluation
- JurnalDocument6 pagesJurnalTian Nopita SariPas encore d'évaluation
- Jurnal CholelitiasisDocument8 pagesJurnal Cholelitiasisdr.deyshiePas encore d'évaluation
- 2 PB PDFDocument9 pages2 PB PDFYuliet SusantoPas encore d'évaluation
- Cognitive Behavioral Therapy in Movement Disorders: A ReviewDocument9 pagesCognitive Behavioral Therapy in Movement Disorders: A ReviewTian Nopita SariPas encore d'évaluation
- Cognitive Behavioral Therapy in Movement Disorders: A ReviewDocument9 pagesCognitive Behavioral Therapy in Movement Disorders: A ReviewTian Nopita SariPas encore d'évaluation
- 43 1 126 1 10 20170613Document7 pages43 1 126 1 10 20170613Mega Ayu NuragaPas encore d'évaluation
- MedicalDocument12 pagesMedicalKristin AndrewsPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Dopamine What It Is, Function & SymptomsDocument7 pagesDopamine What It Is, Function & SymptomsRaj KumarPas encore d'évaluation
- 8 Powerful Methods People Use To Bounce Back From FailureDocument7 pages8 Powerful Methods People Use To Bounce Back From FailureGrego CentillasPas encore d'évaluation
- Resume of Masterchef Contestant, Melissa GutierrezDocument3 pagesResume of Masterchef Contestant, Melissa GutierrezMikhail GalatinovPas encore d'évaluation
- Spouses Aggabao V. Parulan, Jr. and Parulan G.R. No. 165803, (September 1, 2010) Doctrine (S)Document9 pagesSpouses Aggabao V. Parulan, Jr. and Parulan G.R. No. 165803, (September 1, 2010) Doctrine (S)RJPas encore d'évaluation
- A100K10873 VSP-12-Way Technical ManualDocument20 pagesA100K10873 VSP-12-Way Technical Manualchufta50% (2)
- Tamil Ilakkanam Books For TNPSCDocument113 pagesTamil Ilakkanam Books For TNPSCkk_kamalakkannan100% (1)
- Ross, D. (2013) - Field Guide To Jumping Spiders of Southeast Idaho.Document4 pagesRoss, D. (2013) - Field Guide To Jumping Spiders of Southeast Idaho.Dave RossPas encore d'évaluation
- Elementary SurveyingDocument19 pagesElementary SurveyingJefferson EscobidoPas encore d'évaluation
- A Global StudyDocument57 pagesA Global StudyRoynal PasaribuPas encore d'évaluation
- (Durt, - Christoph - Fuchs, - Thomas - Tewes, - Christian) Embodiment, Enaction, and Culture PDFDocument451 pages(Durt, - Christoph - Fuchs, - Thomas - Tewes, - Christian) Embodiment, Enaction, and Culture PDFnlf2205100% (3)
- Introduction To E-Business SystemsDocument19 pagesIntroduction To E-Business SystemsArtur97% (79)
- Adolescent Violence Towards Parents Myths and RealitiesDocument25 pagesAdolescent Violence Towards Parents Myths and RealitiesJoão D C MendonçaPas encore d'évaluation
- VestibuleDocument17 pagesVestibuleDeepti MangalPas encore d'évaluation
- Module6 (Margie Ybanez)Document3 pagesModule6 (Margie Ybanez)Margie Ybañez82% (17)
- Determining Rounding Common CoreDocument2 pagesDetermining Rounding Common Coreapi-3662903730% (1)
- Life in The Past - Year 6 WorksheetsDocument11 pagesLife in The Past - Year 6 WorksheetstinaPas encore d'évaluation
- Reflection Paper-The Elephant Man PDFDocument1 pageReflection Paper-The Elephant Man PDFCarlosJohn02Pas encore d'évaluation
- Asian Journal of ForestryDocument3 pagesAsian Journal of ForestryTeguh MuslimPas encore d'évaluation
- Debus Medical RenaissanceDocument3 pagesDebus Medical RenaissanceMarijaPas encore d'évaluation
- Fallopian Tube BlockageDocument11 pagesFallopian Tube Blockagesimran kaurPas encore d'évaluation
- Articles 62 & 63: Presented By: Muhammad Saad Umar FROM: BS (ACF) - B 2K20Document10 pagesArticles 62 & 63: Presented By: Muhammad Saad Umar FROM: BS (ACF) - B 2K20Muhammad Saad UmarPas encore d'évaluation
- Drug Distribution MethodsDocument40 pagesDrug Distribution MethodsMuhammad Masoom Akhtar100% (1)
- Carbon Facial Copies of SlidesDocument33 pagesCarbon Facial Copies of Slides77yr72cdh6Pas encore d'évaluation
- "Shiksha Se Hi Suraksha": Literacy Campaign WeekDocument4 pages"Shiksha Se Hi Suraksha": Literacy Campaign WeekVaishali100% (1)
- Sickle Cell AnemiaDocument13 pagesSickle Cell Anemiamayra100% (1)
- Rectification or Correction of Sale DeedDocument4 pagesRectification or Correction of Sale Deedsumanth_0678Pas encore d'évaluation
- J of Cosmetic Dermatology - 2019 - Zhang - A Cream of Herbal Mixture To Improve MelasmaDocument8 pagesJ of Cosmetic Dermatology - 2019 - Zhang - A Cream of Herbal Mixture To Improve Melasmaemily emiPas encore d'évaluation
- INTRODUCTIONDocument1 pageINTRODUCTIONNabila Gaming09Pas encore d'évaluation
- Practice Makes Perfect Basic Spanish Premium Third Edition Dorothy Richmond All ChapterDocument67 pagesPractice Makes Perfect Basic Spanish Premium Third Edition Dorothy Richmond All Chaptereric.temple792100% (3)
- Hard Soft Acid Base TheoryDocument41 pagesHard Soft Acid Base TheorythinhbuPas encore d'évaluation