Académique Documents
Professionnel Documents
Culture Documents
New Microsoft Word Document
Transféré par
Ahmad Abd0 évaluation0% ont trouvé ce document utile (0 vote)
14 vues5 pagesصثي
Copyright
© © All Rights Reserved
Formats disponibles
DOCX, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentصثي
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
14 vues5 pagesNew Microsoft Word Document
Transféré par
Ahmad Abdصثي
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 5
Under these conditions, water exists in the liquid phase, and it is called
a compressed liquid, or a subcooled liquid, meaning that it is not about
to vaporize. Heat is now transferred to the water until its temperature
rises to, say, 40°C. As the temperature rises, the liquid water expands
slightly, and so its specific volume increases. To accommodate this
expansion, the piston moves up slightly. The pressure in the cylinder
remains constant at 1 atm during this process since it depends on the
outside barometric pressure and the weight of the piston, both of which
are constant. Water is still a compressed liquid at this state since it has
not started to vaporize. As more heat is transferred, the temperature
keeps rising until it reaches 100°C (state 2). At this point water is still a
liquid, but any heat addition will cause some of the liquid to vaporize.
That is, a phase-change process from liquid to vapor is about to take
place. A liquid that is about to vaporize is called a saturated liquid.
Therefore, state 2 is a saturated liquid state. Once boiling starts, the
temperature stops rising until the liquid is completely vaporized. That
is, the temperature will remain constant during the entire phase-
change process if the pressure is held constant. This can easily be
verified by placing a thermometer into boiling pure water on top of a
stove. At sea level (P = 1 atm), the thermometer will always read
100°C if the pan is uncovered or covered with a light lid. During a
boiling process, the only change we will observe is a large increase in
the volume and a steady decline in the liquid level as a result of more
liquid turning to vapor. Midway about the vaporization line (state 3), the
cylinder contains equal amounts of liquid and vapor. As we continue
transferring heat, the vaporization process continues until the last drop
of liquid is vaporized (state 4). At this point, the entire cylinder is filled
with vapor that is on the borderline of the liquid phase. Any heat loss
from this vapor will cause some of the vapor to condense (phase
change from vapor to liquid). A vapor that is about to condense is
called a saturated vapor. Therefore, state 4 is a saturated vapor state.
A substance at states between 2 and 4 is referred to as a saturated
liquid-vapor mixture since the liquid and vapor phases coexist in
equilibrium at these states.
Once the phase-change process is completed, we are back to a
single-phase region again (this time vapor), and further transfer of
heat results in an increase in both the temperature and the specific
volume. At state 5, the temperature of the vapor is, let us say,
300°C; and if we transfer some heat from the vapor, the
temperature may drop somewhat but no condensation will take
place as long as the temperature remains above 100°C (for P = 1
atm). A vapor that is not about to condense (i.e., not a saturated
vapor) is called a superheated vapor. Therefore, water at state 5 is
a superheated vapor. This constant-pressure phase-change
process is illustrated on a T-v diagram in Fig.1. If the entire process
described here is reversed by cooling the water while maintaining
the pressure at the same value, the water will go back to state 1,
retracing the same path, and in so doing, the amount of heat
released will exactly match the amount of heat added during the
heating process. In our daily life, water implies liquid water and
steam implies water vapor. In thermodynamics, however, both
water and steam usually mean only one thing: H2O.
Saturation Temperature and Saturation Pressure: It probably came
as no surprise to you that water started to boil at 100°C. Strictly
speaking, the statement “water boils at 100°C” is incorrect. The
correct statement is “water boils at 100°C at 1 atm pressure.” the
only reason water started boiling at 100°C was because we held the
pressure constant at 1 atm (101.325 kPa). If the pressure inside the
cylinder were raised to 500 kPa by adding weights on top of the
piston, water would start boiling at 151.8°C. That is, the
temperature at which water starts boiling depends on the pressure;
therefore, if the pressure is fixed, so is the boiling temperature. At a
given pressure, the temperature at which a pure substance
changes phase is called the saturation temperature Tsat . Likewise,
at a given temperature, the pressure at which a pure substance
changes phase is called the saturation pressure Psat. At a pressure
of 101.325 kPa, Tsat is 99.97°C. Conversely, at a temperature of
99.97°C, Psat is 101.325 kPa. (At 100.00°C, Psat is 101.42 kPa )
Saturation tables that list the saturation pressure against the
temperature (or the saturation temperature against the pressure)
are available for practically all substances.
Effect of pressure on evaporation temperature: It is well known that
water boils and evaporates at 100°C under atmospheric pressure.
By higher pressure, water evaporates at higher temperature - e.g. a
pressure of 10 bar equals an evaporation temperature of 184°C.
The pressure and the corresponding temperature when a phase
change occurs are called the saturation temperature and saturation
pressure. During the evaporation process, pressure and
temperature are constant, but if the vaporization occurs in a closed
vessel, the expansion that occurs due to the phase change of water
into steam causes the pressure to rise and thus the boiling
temperature rises.
PROPERTY DIAGRAMS FOR PHASE-CHANGE PROCESSES
The variations of properties during phase-change processes are
best studied and understood with the help of property diagrams.
Next, we develop and discuss the T-v, P-v, and P-T diagrams for
pure substances.
Critical point: it is defined as the point at which the saturated liquid
and saturated vapor stales are identical.
The temperature, pressure, and specific volume of a substance at
the critical point are called, respectively, the critical temperature Tcr
critical pressure Pcr and critical specific volume ver. The critical-
point properties of water are Pcr = 22.06 MPa, Tcr = 373.95°C, and
vcr= 0.003106 m3/kg. For helium, they are 0.23 MPa, 267.85°C,
and 0.01444 m3/kg. At pressures above the critical pressure, there
is not a distinct phase change process (Fig. 9). Instead, the specific
volume of the substance continually increases, and at all times
there is only one phase present. Eventually, it resembles a vapor,
but we can never tell when the change has occurred. Above the
critical state, there is no line that separates the compressed liquid
region and the superheated vapor region. However, it is customary
to refer to the substance as superheated vapor at temperatures
above the critical temperature and as compressed liquid at
temperatures below the critical temperature.
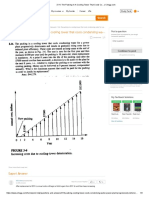
From the diagram (Figure 3) we can see that when we exceed a
certain pressure, 22.12 Mpa (the corresponding temperature is
374°C), the line stops. The reason is that the border between gas
phase and liquid phase is blurred out at that pressure. That point,
where the different phases cease to exist, is called the critical point
of water.
The P-T Diagram: Figure 4 shows the P-T diagram of a pure
substance. This diagram is often called the phase diagram since all
three phases are separated from each other by three lines. The
sublimation line separates the solid and vapor regions, the
vaporization line separates the liquid and vapor regions, and the
melting (or fusion) line separates the solid and liquid regions. These
three lines meet at the triple point, where all three phases coexist in
equilibrium. The vaporization line ends at the critical point because
no distinction can be made between liquid and Vapor phases -
above the critical point Substances that expand and contract on
freezing differ only in the melting line on the P-T diagram.
H.W. The following data were obtained in a test on-a combined
separating and throttling calorimeter: Pressure of steam sample =
15 bar, pressure of steam at exit = 1 bar, temperature of steam at
the exit = 150°C, discharge from separating calorimeter = 0.5
kg/min, discharge from throttling calorimeter = 10 kg/min. Determine
the dryness fraction of the sample steam.
Example 2. The following observations were taken with a
separating and a throttling calorimeter arranged in series: Water
separated = 2 kg, steam discharged from the throttling calorimeter =
20.5 kg, temperature of steam after throttling = 110°C, initial
pressure = 12 bar, barometer = 760 mm of Hg, final pressure = 5
mm of Hg. Estimate the quality of steam supplied.
Vous aimerez peut-être aussi
- Thermo Chapter 6 ExamplesDocument1 pageThermo Chapter 6 ExamplesAhmad AbdPas encore d'évaluation
- Speak Now Student Book 3Document132 pagesSpeak Now Student Book 3Ahmad AbdPas encore d'évaluation
- 650 English Phrases For Everyday Speaking - Facebook Com LinguaLIB PDFDocument50 pages650 English Phrases For Everyday Speaking - Facebook Com LinguaLIB PDFMahmoud Abd El HadiPas encore d'évaluation
- Speak Now Student Book 3Document132 pagesSpeak Now Student Book 3Ahmad AbdPas encore d'évaluation
- Speak Now 3Document26 pagesSpeak Now 3ileana stoica47% (15)
- Speak Now 3 WorkbookDocument20 pagesSpeak Now 3 WorkbookAnan33% (3)
- Thermo Chapter 6 ExamplesDocument5 pagesThermo Chapter 6 ExamplesAhmad AbdPas encore d'évaluation
- Around Town: Conversation Cheat SheetDocument2 pagesAround Town: Conversation Cheat SheetherrerafaridPas encore d'évaluation
- Checking in A Hotel: Conversation Cheat SheetDocument2 pagesChecking in A Hotel: Conversation Cheat SheetDivino Henrique SantanaPas encore d'évaluation
- EnglishDocument2 pagesEnglishSuarez O.Pas encore d'évaluation
- Business English: Business Conversation Cheat SheetDocument2 pagesBusiness English: Business Conversation Cheat Sheetoracle plsqlPas encore d'évaluation
- Travel To Usa PDFDocument2 pagesTravel To Usa PDFHenrik SzingerPas encore d'évaluation
- Geography in English: Conversation Cheat SheetDocument2 pagesGeography in English: Conversation Cheat SheetJuan Jose SotoPas encore d'évaluation
- How'S The Weather?: Conversation Cheat SheetDocument2 pagesHow'S The Weather?: Conversation Cheat SheetLuis PalmerPas encore d'évaluation
- A Smaller Power Plant Produces Steam at 3 MPa, 600...Document3 pagesA Smaller Power Plant Produces Steam at 3 MPa, 600...Ahmad AbdPas encore d'évaluation
- Planning Your Time: Conversation Cheat SheetDocument2 pagesPlanning Your Time: Conversation Cheat SheetDany HuamanPas encore d'évaluation
- Measurements: InstructorDocument38 pagesMeasurements: InstructorAhmad AbdPas encore d'évaluation
- Measurements: InstructorDocument42 pagesMeasurements: InstructorAhmad AbdPas encore d'évaluation
- A 20 Year Mortgage Set Up For Uniform Monthly Paym...Document3 pagesA 20 Year Mortgage Set Up For Uniform Monthly Paym...Ahmad AbdPas encore d'évaluation
- ACFrOgAlSbXHol7X5DCh7AxhfktcUDPYrfN3azOCdL8n w0uIfRwG5eExpx5o5M39eBr6flbL4jKbJQr5lHThcZBkyXMxp-ToA0tTBR4-0kpI 0Pvs52jZmo JZy6vTDinXL3OiGxJSHKeOq WwoDocument290 pagesACFrOgAlSbXHol7X5DCh7AxhfktcUDPYrfN3azOCdL8n w0uIfRwG5eExpx5o5M39eBr6flbL4jKbJQr5lHThcZBkyXMxp-ToA0tTBR4-0kpI 0Pvs52jZmo JZy6vTDinXL3OiGxJSHKeOq WwoAhmad AbdPas encore d'évaluation
- A Mortgage That Was Originally $20,000 Is Being Pa...Document4 pagesA Mortgage That Was Originally $20,000 Is Being Pa...Ahmad AbdPas encore d'évaluation
- 4.9. The Pumping Capacity of A Refrigerating Compr...Document2 pages4.9. The Pumping Capacity of A Refrigerating Compr...Ahmad AbdPas encore d'évaluation
- Measurements: InstructorDocument27 pagesMeasurements: InstructorAhmad AbdPas encore d'évaluation
- Measurements: Instructor: InstructorDocument34 pagesMeasurements: Instructor: InstructorAhmad AbdPas encore d'évaluation
- 3.14. The Packing in A Cooling Tower That Cools Co...Document3 pages3.14. The Packing in A Cooling Tower That Cools Co...Ahmad Abd0% (1)
- A Sum of Su Cient Magnitude Is To Be Invested Now So That..Document2 pagesA Sum of Su Cient Magnitude Is To Be Invested Now So That..Ahmad AbdPas encore d'évaluation
- Measurements: InstructorDocument33 pagesMeasurements: InstructorAhmad AbdPas encore d'évaluation
- Acfrogbej Pmyue Cm6fwgbiguwrxwyogxonm0cvk4jf8v Axtgttyu83kcrva-Dbplruvz2duwxummsjlgfnmseypatv5uzuejh510htsgtij9e5yxdlpm4hfqlmwkl40oxt Lhofnidpiq6ufaDocument15 pagesAcfrogbej Pmyue Cm6fwgbiguwrxwyogxonm0cvk4jf8v Axtgttyu83kcrva-Dbplruvz2duwxummsjlgfnmseypatv5uzuejh510htsgtij9e5yxdlpm4hfqlmwkl40oxt Lhofnidpiq6ufaAhmad AbdPas encore d'évaluation
- 4-6 Using The Data From Table 4-3 For VG at 40, ...Document2 pages4-6 Using The Data From Table 4-3 For VG at 40, ...Ahmad AbdPas encore d'évaluation
- Annual Investments Are Being Made So That $20,000 ...Document1 pageAnnual Investments Are Being Made So That $20,000 ...Ahmad AbdPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Adnan AlamDocument54 pagesAdnan AlamarshadscmePas encore d'évaluation
- Vacuum PumpDocument2 pagesVacuum PumpGonzhalo Ramireç ChaveçPas encore d'évaluation
- Adipic AcidDocument7 pagesAdipic AcidsadiaPas encore d'évaluation
- ASTM A537 Class 1: General Product DescriptionDocument2 pagesASTM A537 Class 1: General Product Descriptionfelipercaliariyahoo.com.brPas encore d'évaluation
- Coupling Agents and Composites: Group 2Document5 pagesCoupling Agents and Composites: Group 2Nicole Anne BorromeoPas encore d'évaluation
- Staad Length ParametersDocument2 pagesStaad Length ParametersAkhil VNPas encore d'évaluation
- Bamboo Reinforcement Concrete - Ghavami - 2004Document13 pagesBamboo Reinforcement Concrete - Ghavami - 2004immortalsky100% (2)
- 2013-013-00 Rev ZD JOULE Operator Manual LR (2) Sterilization RCADocument3 pages2013-013-00 Rev ZD JOULE Operator Manual LR (2) Sterilization RCAEsteban PradaPas encore d'évaluation
- FiberTite Membrane Waterproofing SystemDocument10 pagesFiberTite Membrane Waterproofing SystemSylvester PadacaPas encore d'évaluation
- DHM Series Horizontal Multistage Pumps: WarrantyDocument4 pagesDHM Series Horizontal Multistage Pumps: WarrantysepasepasepaPas encore d'évaluation
- Sensors and ActuatorsDocument109 pagesSensors and ActuatorsDr-Amit Kumar Singh100% (1)
- Magnetic Hysteresis and Ferromagnetic MaterialsDocument67 pagesMagnetic Hysteresis and Ferromagnetic MaterialsafwdPas encore d'évaluation
- Bial T-2 Forecourt DBRDocument18 pagesBial T-2 Forecourt DBRShaileshRastogiPas encore d'évaluation
- Fundamentals of RheologyDocument76 pagesFundamentals of Rheologytonysanchez67Pas encore d'évaluation
- Sales Materials Insulign Guy Strain Insulator - FiberglassDocument2 pagesSales Materials Insulign Guy Strain Insulator - Fiberglassmed ali guiaPas encore d'évaluation
- 1 s2.0 S2666912923000144 Main - CompressedDocument30 pages1 s2.0 S2666912923000144 Main - CompressedFAUZHAN RAMADHAN UBPPas encore d'évaluation
- Pivot-Table-Exercise PracticeDocument151 pagesPivot-Table-Exercise PracticePriyanshu KumarPas encore d'évaluation
- Supplementary Note335Document21 pagesSupplementary Note335Syafiq ArtPas encore d'évaluation
- 1975 Dafalias Y.F. Popov E.P. Acta MechDocument20 pages1975 Dafalias Y.F. Popov E.P. Acta MechAna-Maria PopPas encore d'évaluation
- Properties of Alkyd ResinsDocument2 pagesProperties of Alkyd ResinsDhivya PrabanjanPas encore d'évaluation
- Group1 - Precast and Cast-In-placeDocument16 pagesGroup1 - Precast and Cast-In-placeJumeirahlyn TutaanPas encore d'évaluation
- Interaction DiagramDocument27 pagesInteraction DiagramAlfie Angelo ReyesPas encore d'évaluation
- Irc 022-1986Document36 pagesIrc 022-1986kruttika_apPas encore d'évaluation
- مول - فرمول تجربی - فرمول مولکولی - عدد آووگادروDocument38 pagesمول - فرمول تجربی - فرمول مولکولی - عدد آووگادروapi-3706290Pas encore d'évaluation
- Batch PurificationDocument4 pagesBatch PurificationthirdPas encore d'évaluation
- Unconventional MatterialsDocument4 pagesUnconventional MatterialsAlex GunăPas encore d'évaluation
- Electrochemistry Theory EDocument30 pagesElectrochemistry Theory Ethinkiit100% (2)
- Science 5 - Quarter 4 - Week 1Document36 pagesScience 5 - Quarter 4 - Week 1clyde domingoPas encore d'évaluation
- Introduction To EOR ProcessesDocument23 pagesIntroduction To EOR Processesحسين رامي كريم A 12Pas encore d'évaluation
- 111 WeldDocument13 pages111 Weldpavlosmakridakis2525Pas encore d'évaluation