Académique Documents
Professionnel Documents
Culture Documents
Buffer Solution: Abstract: The Following Report Presents
Transféré par
Andrés Camilo Moreno Barón0 évaluation0% ont trouvé ce document utile (0 vote)
13 vues2 pagesThis document discusses buffer solutions, which use a mixture of a weak acid and its conjugate base to stabilize pH when small amounts of acid or base are added. Typical buffer systems include ammonia-ammonium, acetic acid-acetate, and carbonic acid-bicarbonate. The document presents a lab experiment to create a buffer solution with a pH of 4 using acetic acid and sodium acetate. Calculations using the Henderson-Hasselbach equation accurately predicted the pH. Buffer solutions are important in biology, chemistry, and industry to maintain stable pH levels.
Description originale:
quimica basica 2, soluciones buffer.
Titre original
soluciones buffer
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentThis document discusses buffer solutions, which use a mixture of a weak acid and its conjugate base to stabilize pH when small amounts of acid or base are added. Typical buffer systems include ammonia-ammonium, acetic acid-acetate, and carbonic acid-bicarbonate. The document presents a lab experiment to create a buffer solution with a pH of 4 using acetic acid and sodium acetate. Calculations using the Henderson-Hasselbach equation accurately predicted the pH. Buffer solutions are important in biology, chemistry, and industry to maintain stable pH levels.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
13 vues2 pagesBuffer Solution: Abstract: The Following Report Presents
Transféré par
Andrés Camilo Moreno BarónThis document discusses buffer solutions, which use a mixture of a weak acid and its conjugate base to stabilize pH when small amounts of acid or base are added. Typical buffer systems include ammonia-ammonium, acetic acid-acetate, and carbonic acid-bicarbonate. The document presents a lab experiment to create a buffer solution with a pH of 4 using acetic acid and sodium acetate. Calculations using the Henderson-Hasselbach equation accurately predicted the pH. Buffer solutions are important in biology, chemistry, and industry to maintain stable pH levels.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 2
BUFFER SOLUTION Each buffer system has its own effective
Andrés Camilo moreno Barón pH range, which will depend on the
equilibrium constant of the acid or base
Universidad Distrital Francisco José De used. They are important in the
Caldas laboratory and in industry, and also in the
chemistry of life. Typical buffers are the
ammonia-ammonium cation pair, acetic
Abstract: The following report presents acid-anion acetate, carbonate-anion
the results obtained by using a bicarbonate anion, citric acid-anion
mathematical equation to prepare a citrate or one of the pairs in the
buffer solution. dissociation of phosphoric acid.
The ions of these salts combine with
acids and alkalis. These hydrolytically
active salts are the products that result
Keyworks: pH, Solution, Base, Acid.
from the reaction between weak acids
and strong alkalis such as calcium
carbonate (from carbonic acid and
calcium hydroxide) or between strong
acids and weak alkalies such as
ammonium chloride (afrom hydrochloric
acid and ammonium hydroxide).
A buffer acid reacts when a weak acid or
weak base is combined with its
corresponding hydrolytic salt in a water
solution, forming a buffer system called
buffer.
Not always a buffer system is
Introduction
appropriate, because the ions of some
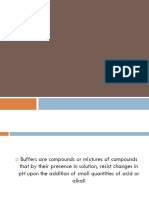
A buffer, buffer, buffer or buffer is a
hydrolytic salts can, for example,
mixture in relatively high concentrations
damage the organisms that come into
of an acid and its conjugate base,
contact with it.
hydrolytically active salts. They have the
On the other hand, each buffer system
property of keeping the pH of a solution
has its own effective pH range, some of
stable against the addition of relatively
which are not suitable for aquariums.
small amounts of strong acids or bases.
This fact is of vital importance in diverse
contexts where it is necessary to Materials
maintain the pH in a narrow threshold, For the practice, two beakers of 100 and
for example, with a slight change in the 250ml were used, as well as a glass
concentration of hydrogen ion in the cell clock, a spatula, a washing bottle,
a stoppage in the activity of the enzymes sodium acetate, 0.8N acetic acid.
can take place.
This property can be understood as a Method
consequence of the common ion effect The necessary calculations are made to
and the prepare a solution of 15 ml of acetic acid
different constants of acidity or basicity: and sodium acetate with a pH equal to 4.
a small amount of acid or base slightly
displaces the weak acid-base balance,
which has a minor consequence on pH.1
Results
An exact solution of a pH equal to 4 was
obtained, this is a buffer since it requires
a higher level than other solutions so that
its pH varied.
Conclusions
The Henderson-Hasselbach equation is
an effective theoretical way to calculate
the pH of a base acid pair
Bibliography
ET SAPIENCE, M. (2019). Buffer
solution - Monografias.com. Retrieved
from
https://www.monografias.com/trabajos8
2/solucion-tampon/solucion-
tampon.shtml-
Vous aimerez peut-être aussi
- Buffers Notes1Document3 pagesBuffers Notes1Lara MonevaPas encore d'évaluation
- Buffer Solution (1.2)Document5 pagesBuffer Solution (1.2)Kuldipsinh ZalaPas encore d'évaluation
- Buffer SolutionsDocument19 pagesBuffer SolutionsMuskaan BindalPas encore d'évaluation
- Chem 2066 Biochemistry Experiment 1 Preparation of Buffers 2020-07007!Document5 pagesChem 2066 Biochemistry Experiment 1 Preparation of Buffers 2020-07007!Mohamidin MamalapatPas encore d'évaluation
- Acids Bases BuffersDocument10 pagesAcids Bases BuffersLouie G NavaltaPas encore d'évaluation
- Biochemistry LN03Document16 pagesBiochemistry LN03Rahaf Al-muhtasebPas encore d'évaluation
- Chem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesDocument13 pagesChem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesFaith VillahermosaPas encore d'évaluation
- Buffer Solutions FinalDocument62 pagesBuffer Solutions Finalshripathyd1100% (1)
- Buffers Solution & Its Applications: Abuzar KhalidDocument8 pagesBuffers Solution & Its Applications: Abuzar Khalidabuzar khalidPas encore d'évaluation
- Buffer Solution - Acidic and Basic Buffers, Preparations, Examples PDFDocument5 pagesBuffer Solution - Acidic and Basic Buffers, Preparations, Examples PDFSakshi DevalekarPas encore d'évaluation
- Buffe by F S SHAH (Autosaved) (Autosaved)Document28 pagesBuffe by F S SHAH (Autosaved) (Autosaved)farooq shah shabbirPas encore d'évaluation
- Buffer PreparationDocument6 pagesBuffer PreparationPraveen KumarPas encore d'évaluation
- Acid-Base Equilibria in Aqueous SolutionsDocument48 pagesAcid-Base Equilibria in Aqueous SolutionsAdrian ChombaPas encore d'évaluation
- Buffers: Analytical TechniquesDocument17 pagesBuffers: Analytical TechniquesAbdul FarooqPas encore d'évaluation
- Lecture 1Document24 pagesLecture 1mvps9248Pas encore d'évaluation
- Buffer Solutions: September 2020Document22 pagesBuffer Solutions: September 2020Nitin DapkePas encore d'évaluation
- Biochemistry LN02Document9 pagesBiochemistry LN02Rahaf Al-muhtasebPas encore d'évaluation
- BuffersDocument20 pagesBuffersgopimpharmPas encore d'évaluation
- Buffer SolutionsDocument10 pagesBuffer SolutionsELYANPas encore d'évaluation
- Buffer ChemistryDocument9 pagesBuffer ChemistrySidra chaudharyPas encore d'évaluation
- Pharmaceutical Chemistry NotesDocument8 pagesPharmaceutical Chemistry NotesZaheer Uddin100% (1)
- Chem 40.1 - Midterms Reviewer Discussion: Buffers: - (Aq) 2 4 - (Aq) 4 2 - (Aq) 2Document5 pagesChem 40.1 - Midterms Reviewer Discussion: Buffers: - (Aq) 2 4 - (Aq) 4 2 - (Aq) 2Steffi GatdulaPas encore d'évaluation
- Buffers CompleteDocument46 pagesBuffers CompleteSunshine_Bacla_4275100% (2)
- PH and Buffer System - NotesDocument29 pagesPH and Buffer System - Noteskatherine morenoPas encore d'évaluation
- Buffer - Adrean HensleyDocument2 pagesBuffer - Adrean HensleyJamaica SalvadorPas encore d'évaluation
- Analytical Techniques: Topic: BuffersDocument18 pagesAnalytical Techniques: Topic: BuffersAbdul FarooqPas encore d'évaluation
- My Notes: - Bonds That Share Electrons UnequallyDocument5 pagesMy Notes: - Bonds That Share Electrons UnequallyPatricia Bianca BunagPas encore d'évaluation
- The Solution For All of Your Buffer Needs: BuffersDocument10 pagesThe Solution For All of Your Buffer Needs: BuffersFaycel FaidiPas encore d'évaluation
- Acid-Base Homeostasis: Acids and BasesDocument14 pagesAcid-Base Homeostasis: Acids and BasessakuraleeshaoranPas encore d'évaluation
- Lec 7 Analytical (Buffer Solution)Document13 pagesLec 7 Analytical (Buffer Solution)alhyaly857Pas encore d'évaluation
- Lecture 3 Buffer Solutions (Буферные Растворы)Document27 pagesLecture 3 Buffer Solutions (Буферные Растворы)Adbhut YadavPas encore d'évaluation
- PH AND BUFFER SOLUTIONS - Google DriveDocument22 pagesPH AND BUFFER SOLUTIONS - Google DrivewilhelminaanimPas encore d'évaluation
- Buffer Solutions.: Ass. Prof. I. R. BekusDocument27 pagesBuffer Solutions.: Ass. Prof. I. R. BekusNanda ThyarezaPas encore d'évaluation
- Equilibrium Constants: Needed in ChemistryDocument28 pagesEquilibrium Constants: Needed in ChemistryGea EcoyPas encore d'évaluation
- AssignmentDocument3 pagesAssignmentahsansalman4004Pas encore d'évaluation
- Separation of Salicylic Acid Impurities With DiffeDocument4 pagesSeparation of Salicylic Acid Impurities With Diffemic92833292Pas encore d'évaluation
- Scientific Method: Jayson Nota Maria Angelica BalicocoDocument21 pagesScientific Method: Jayson Nota Maria Angelica BalicocoGrace Ann ArimadoPas encore d'évaluation
- Acids Bases SaltsDocument13 pagesAcids Bases SaltsChito JarciaPas encore d'évaluation
- Buffers For Biological SystemsDocument28 pagesBuffers For Biological SystemsMuhammed Shafi TkPas encore d'évaluation
- Artículo PH para EstudiantesDocument5 pagesArtículo PH para EstudiantesAndrés CardonaPas encore d'évaluation
- Unit 4 Chemical Equilibrium PDFDocument27 pagesUnit 4 Chemical Equilibrium PDFmisganamarcos10Pas encore d'évaluation
- Biological Buffer SystemDocument4 pagesBiological Buffer SystemVern NuquiPas encore d'évaluation
- Properties of WaterDocument4 pagesProperties of WaterMikhael Jay IglesiasPas encore d'évaluation
- BC34.1 E1 Amino Acids As AmpholytesDocument4 pagesBC34.1 E1 Amino Acids As AmpholytesGlenn Vincent Tumimbang100% (1)
- Mechanism of Buffering SystemDocument8 pagesMechanism of Buffering SystemRezaul Karim TutulPas encore d'évaluation
- Non Aqeuous TitrationDocument7 pagesNon Aqeuous Titrationsurabhi tadePas encore d'évaluation
- Unit-V - PH, Buffer, Buffer Equation, Isotonicity-1Document27 pagesUnit-V - PH, Buffer, Buffer Equation, Isotonicity-1IKHLASH MOHDPas encore d'évaluation
- Buffer Solution PDFDocument5 pagesBuffer Solution PDFNarasimha MurthyPas encore d'évaluation
- CHEM 102 Instructional Objectives: - Additional Aqueous EquilibriaDocument29 pagesCHEM 102 Instructional Objectives: - Additional Aqueous EquilibriarajPas encore d'évaluation
- Solutions Constant of A Weak Acid or BaseDocument8 pagesSolutions Constant of A Weak Acid or BaseJeromePas encore d'évaluation
- Buffers Experiment 1: Rüveyda AKÇİN, Gebze Technical University, TurkeyDocument6 pagesBuffers Experiment 1: Rüveyda AKÇİN, Gebze Technical University, TurkeyRüveyda Akçin100% (1)
- BioChem34: Exp1Document2 pagesBioChem34: Exp1Nonee Quesada CornebyPas encore d'évaluation
- The Amino AcidsDocument4 pagesThe Amino AcidsNatasha MahatantilaPas encore d'évaluation
- Buffers: Prepared By: La Vera U. Sombito Natural Sciences Department, CASDocument17 pagesBuffers: Prepared By: La Vera U. Sombito Natural Sciences Department, CASJacqueline Rose Alipo-onPas encore d'évaluation
- BCH211 Water and PH 2021Document24 pagesBCH211 Water and PH 2021Sefrinmi AyodejiPas encore d'évaluation
- BufferDocument26 pagesBufferAlia RizanPas encore d'évaluation
- Fundamentals of General Organic and Biological Chemistry 8th Edition Mcmurry Solutions ManualDocument8 pagesFundamentals of General Organic and Biological Chemistry 8th Edition Mcmurry Solutions Manualglendalaeliaqm5lr9100% (30)
- Buffer SolutionDocument7 pagesBuffer SolutionMahmoud AbdAllahPas encore d'évaluation
- Biochemistry : A Practical ManualD'EverandBiochemistry : A Practical ManualÉvaluation : 5 sur 5 étoiles5/5 (1)
- Author's Accepted Manuscript: Nano EnergyDocument52 pagesAuthor's Accepted Manuscript: Nano EnergySteven KmiecPas encore d'évaluation
- SEM 1 OVERALL (Summary)Document16 pagesSEM 1 OVERALL (Summary)Peh ZeroxPas encore d'évaluation
- PolymerSelector OnlineDocument5 pagesPolymerSelector OnlineAntonio C olmosPas encore d'évaluation
- Properties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryDocument13 pagesProperties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryFOUTOUPas encore d'évaluation
- United States Patent: (10) Patent No.: US 6,420,470 B1Document6 pagesUnited States Patent: (10) Patent No.: US 6,420,470 B1SiddharthBhasneyPas encore d'évaluation
- US20130344550A1Document18 pagesUS20130344550A1Lourence Albert Moreno MoleñoPas encore d'évaluation
- Bacterial MetabolismDocument13 pagesBacterial MetabolismololadePas encore d'évaluation
- New Fent SynthesisDocument2 pagesNew Fent SynthesisGireesh Chowdary GarikapatiPas encore d'évaluation
- Chalk ResearchDocument10 pagesChalk ResearchRosemenjelPas encore d'évaluation
- Sterilization of Ot EquipmentsDocument20 pagesSterilization of Ot EquipmentsZiaudin ZiaPas encore d'évaluation
- Experiment 7: Redox Reactions and The Metal Activity Series OutcomesDocument4 pagesExperiment 7: Redox Reactions and The Metal Activity Series OutcomesSafwan m.tPas encore d'évaluation
- Bab Iv Formulasi Dan Perhitungan 4.1 Formulasi: Iso IsoDocument4 pagesBab Iv Formulasi Dan Perhitungan 4.1 Formulasi: Iso IsoIstiqomah Sa'adahPas encore d'évaluation
- Intertek Testing Services LTD., Shanghai Ningbo BranchDocument4 pagesIntertek Testing Services LTD., Shanghai Ningbo BranchKalpesh PatelPas encore d'évaluation
- U FactorDocument22 pagesU Factorandry_setiawanall4jcPas encore d'évaluation
- Tutorial 3 Electrochemistry - AnswersDocument10 pagesTutorial 3 Electrochemistry - AnswerssgarrabPas encore d'évaluation
- Enargite Leaching Under Ammoniacal MediaDocument10 pagesEnargite Leaching Under Ammoniacal MediaEnrique QuezadaPas encore d'évaluation
- ISOTOPESDocument4 pagesISOTOPESfatzyPas encore d'évaluation
- H2 - Prelim 2009 Paper1Document16 pagesH2 - Prelim 2009 Paper1Augustine NgPas encore d'évaluation
- 1152 Lab CarbohydratesDocument8 pages1152 Lab Carbohydratesanyss_afiezaPas encore d'évaluation
- R-2A Agar: Intended Use: CompositionDocument3 pagesR-2A Agar: Intended Use: CompositionABDO ELJAPas encore d'évaluation
- Mendes Et Al. 2019. Provenance of Upper PDFDocument17 pagesMendes Et Al. 2019. Provenance of Upper PDFANDERSON MENDESPas encore d'évaluation
- Lecture 4: Adsorption: The Islamic University of Gaza-Environmental Engineering Department Water Treatment (EENV - 4331)Document45 pagesLecture 4: Adsorption: The Islamic University of Gaza-Environmental Engineering Department Water Treatment (EENV - 4331)Anuja Padole100% (1)
- Nitoprime PrimerDocument2 pagesNitoprime PrimerBalasubramanian AnanthPas encore d'évaluation
- Chemistry Pre-U Chemistry Sem 1 Chap 5 PDFDocument85 pagesChemistry Pre-U Chemistry Sem 1 Chap 5 PDFJIANHUI0160% (1)
- Worksheet On General ChemistryDocument2 pagesWorksheet On General ChemistryMay Conde Aguilar50% (2)
- Presented By: Jenelyn P. Cadion BS Biology 4: Leyte Normal University Tacloban CityDocument31 pagesPresented By: Jenelyn P. Cadion BS Biology 4: Leyte Normal University Tacloban CityJen Perez CadionPas encore d'évaluation
- FDA Det Flame ArrestorsDocument2 pagesFDA Det Flame Arrestorsali kararPas encore d'évaluation
- Components of Coal AshDocument4 pagesComponents of Coal AshDulguun BayPas encore d'évaluation
- Cleaning Procedures DOW Filmtec Membranes LDocument7 pagesCleaning Procedures DOW Filmtec Membranes LAjay PatelPas encore d'évaluation
- 2020 H2 MCT Section C Ans (2023 Edition) 2Document4 pages2020 H2 MCT Section C Ans (2023 Edition) 2Shereen LimPas encore d'évaluation