Académique Documents
Professionnel Documents
Culture Documents
SDR 1
Transféré par
techkasambaDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
SDR 1
Transféré par
techkasambaDroits d'auteur :
Formats disponibles
LIQUIDSOLID MASS TRANSFER
IN A ROTORSTATOR SPINNING DISC REACTOR
Marco Meeuwse, John van der Schaaf and Jaap C. Schouten*
Laboratory of Chemical Reactor Engineering, Department of Chemical Engineering and Chemistry,
Eindhoven University of Technology, P.O. Box 513, 5600 MB Eindhoven, The Netherlands
Email: j.c.schouten@tue.nl; website: www.chem.tue.nl/scr
Summary
Liquid-solid mass transfer coefficients in a rotor-stator spinning disc reactor are reported that were
determined from measurements of reaction rates of the heterogeneously catalysed oxidation of glucose.
The overall reaction rates are limited by both liquid-solid mass transfer and the intrinsic oxidation
kinetics at the operating conditions used. The liquid-solid mass transfer increases with increasing rota-
tional disc speed. The estimated liquid-solid mass transfer coefficient kLS increases from 1.5 · 10−4 m s−1
at 49 rad s−1 to 3 · 10−4 m s−1 at 181 rad s−1, which is at least a factor 3 higher than in conventional
reactors.
Keywords
Process intensification, Novel reactor technologies, Multiphase reactors, Spinning disc reactor
Introduction
There is an increasing demand in the chemical industry for because of catalyst overoxidation. Low oxygen concentra-
smaller, safer, and more cost efficient versatile reactors. In tions of 5 and 25 vol.% are therefore used here.
multiphase reactions, gas-liquid and/or liquid-solid mass The overall reaction rate coefficient Kov is a
transfer are often rate limiting and determine the size of function of the volumetric liquid-solid mass transfer
the reactor. A higher mass transfer rate can increase the coefficient and the intrinsic kinetics reaction rate
reactor performance and the reactor can be made smaller. coefficient and can be calculated from the decrease in
Meeuwse et al.1 showed that in a rotor-stator spinning disc oxygen concentration:
reactor, the gas-liquid mass transfer rates are up to a factor −1
φ ⎛ CO ⎞ ⎛ 1
in
1 ⎞
10 higher than in conventional gas-liquid reactors. K ov = L ⎜ out2 − 1⎟ = ⎜ + ⎟
The rotor-stator spinning disc reactor consists of VR ⎜ CO ⎟ ⎝ kls als krη Ccat ⎠
⎝ 2 ⎠
a rotating disc between two stator walls, at 1 mm distance. The rotational disc speed in the reactor only influences the
With a catalytically active disc, this new reactor type can liquid-solid mass transfer term. The temperature in the
also be used for heterogeneously catalysed reactions,
making use of the high liquid-solid surface area available
per unit reactor volume. In this study, we have estimated
the rate of liquid-solid mass transfer in the rotor-stator
spinning disc reactor with a heterogeneous catalyst
deposited on the disc.
Experimental
The experimental setup is shown in Figure 1. Operating
conditions and dimensions are indicated in the figure.
Glucose is oxidized to gluconate by using a 20 wt% Pt/C
catalyst coating on the bottom of the rotating disc. Glucose
is present in excess in the reactor (0.5 mol l−1) and is
therefore not rate determining. The kinetics of the reaction
are an intricate function of the oxygen concentration2,
Figure 1. Schematic drawing of the experimental setup of
* To whom all correspondence should be addressed the rotor-stator spinning disc reactor.
reactor has a much larger influence on the kinetic term catalysed alcohol oxidation: Reaction-engineering
than on the liquid-solid mass transfer term. Whether the modelling and experiments. Catalysis Today, 2004, 96,
system is mass transfer limited or kinetically limited can 223-234.
be determined by varying the rotational disc speed and the (3) Wenmakers, P.W.A.M., van der Schaaf, J., Kuster, B.
temperature. F. M. and Schouten, J. C. Enhanced liquid-solid mass
transfer by carbon nanofibers on solid foam as catalyst
support. Chemical Engineering Science, 2009, doi:
Results 10.1016/j.ces.2009.06.005.
Figure 2 shows the overall reaction rate coefficient versus (4) Shah,Y.T. Gas-liquid-solid reactor design, 1979,
the rotational disc speed. The reaction rate increases with a McGraw-Hill Inc., London.
factor 2 from 45 to 50 °C, which indicates that intrinsic
kinetics are rate limiting, because the temperature
influences intrinsic kinetics most. The reaction rate also
increases by a factor 2 by increasing the rotational disc
speed from 50 to 179 rad/s, which shows that the liquid-
solid mass transfer is rate limiting as well. The comparable
effects of rotational disc speed and temperature indicate
that the kinetics and the mass transfer are of equal
magnitude.
The overall reaction rate coefficient Kov can be
used to estimate the liquid-solid mass transfer coefficient,
however, the catalyst gradually deactivates by over-
oxidation3. This can be seen in Figure 2, where the
reaction rate at 45°C and 181 rad s−1 decreases with 20%
in 20 min. Regeneration in an oxygen free environment
fully restored the catalyst activity. The overoxidation was
less at a 5% oxygen concentration, see Figure 3, which led
to higher overall reaction rate coefficients. Overoxidation
was still observed though, given the observed hysteresis
effect.
The volumetric liquid-solid mass transfer coeffi- Figure 2. Overall reaction rate coefficient of glucose
cient is at least equal to the overall reaction rate coeffi- oxidation at 25% O2, measured with increasing rotational
cient, but is likely to be twice as high. With a liquid-solid disc speed.
interfacial area als of 274 m2 m−3, kls is then estimated to be
3 to 6 · 10−4 m s−1, at 181 rad s−1. This is higher than in
conventional reactors, where kls is typically 10−4 m s−1.4
Conclusions
The overall reaction rate coefficient of glucose oxidation
in the rotor-stator spinning disc reactor is limited by both
liquid-solid mass transfer and intrinsic kinetics. The
overall liquid-solid mass transfer coefficient klsals is
estimated to be between 0.08 and 0.16 m3L m−3R s−1 at 181
rad s−1. This value can be increased up to a factor 8 by
coating the whole disc and both stators, leading to a value
of klsals which is higher than in conventional reactors, and
is of equal order of magnitude as the overall gas-liquid
mass transfer coefficient in the spinning disc reactor1.
References
(1) Meeuwse, M., van der Schaaf, J., Kuster, B. F. M. and
Schouten, J. C. Gas-liquid mass transfer in a rotor-stator Figure 3. Overall reaction rate coefficient at 50 °C and 5%
spinning disc reactor. Chemical Engineering Science, O2, showing deactivation of the catalyst. The measure-
2009, doi:10.1016/j.ces.2009.06.006. ments start at 97 rad s−1, the rotational speed is increased
(2) Gangwal, V.R., van der Schaaf, J., Kuster, B. F. M. every 3 minutes; after reaching 181 rad s−1, the rotational
and Schouten, J. C. Catalyst performance for noble metal speed is decreased.
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- 1977 Puch Maxi Moped Wiring DiagramDocument9 pages1977 Puch Maxi Moped Wiring DiagramMopedgal100% (1)
- Maxx Cold Mxx-23f ServiceDocument314 pagesMaxx Cold Mxx-23f Servicedan theman100% (2)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Pakyaw RatesDocument36 pagesPakyaw RatesKesMercado100% (5)
- Fibre Reinforced Concrete PDFDocument6 pagesFibre Reinforced Concrete PDFAref AbadelPas encore d'évaluation
- DIY Hot Air Soldering Iron 1 PDFDocument11 pagesDIY Hot Air Soldering Iron 1 PDFztmp1Pas encore d'évaluation
- En71 SGSDocument46 pagesEn71 SGSMax Arias100% (1)
- Hydrometallurgical Process: Analysis of Free, Total & WAD Cyanide in Gold Leach Slurry & WastewaterDocument1 pageHydrometallurgical Process: Analysis of Free, Total & WAD Cyanide in Gold Leach Slurry & WastewaterJUNIORPas encore d'évaluation
- ITP SampleDocument2 pagesITP Sampleeiman_zerep50% (2)
- Benzoylation of AcetophenoneDocument3 pagesBenzoylation of AcetophenonetechkasambaPas encore d'évaluation
- Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2 Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2Document9 pagesSheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2 Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2techkasambaPas encore d'évaluation
- Triazoles ChemistryDocument4 pagesTriazoles ChemistrytechkasambaPas encore d'évaluation
- Save Water, Save Life PDFDocument30 pagesSave Water, Save Life PDFtechkasambaPas encore d'évaluation
- The Decomposition of Aqueous Sodium Bromite: Department of Chemistry, University of Toronto, Toronto OntarioDocument6 pagesThe Decomposition of Aqueous Sodium Bromite: Department of Chemistry, University of Toronto, Toronto OntariotechkasambaPas encore d'évaluation
- S.No Company Name Price On 7 April 201 Buy Amount Quantity Current Priceprofit/LossDocument3 pagesS.No Company Name Price On 7 April 201 Buy Amount Quantity Current Priceprofit/LosstechkasambaPas encore d'évaluation
- IPTFADocument9 pagesIPTFAtechkasambaPas encore d'évaluation
- Assessing The Profitability of Intraday Opening Range Breakout StrategiesDocument11 pagesAssessing The Profitability of Intraday Opening Range Breakout StrategiestechkasambaPas encore d'évaluation
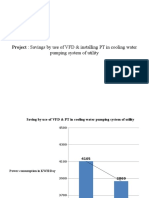
- Project: Savings by Use of VFD & Installing PT in Cooling WaterDocument6 pagesProject: Savings by Use of VFD & Installing PT in Cooling WatertechkasambaPas encore d'évaluation
- Synthesis of 2-Picoline From Acetone Over Modified ZSM-5 CatalystsDocument8 pagesSynthesis of 2-Picoline From Acetone Over Modified ZSM-5 CatalyststechkasambaPas encore d'évaluation
- Isoamyl SalysilateDocument1 pageIsoamyl SalysilatetechkasambaPas encore d'évaluation
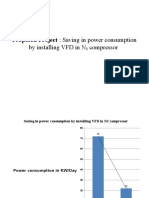
- Proposed Project: Saving in Power Consumption: by Installing VFD in N CompressorDocument3 pagesProposed Project: Saving in Power Consumption: by Installing VFD in N CompressortechkasambaPas encore d'évaluation
- Vishranti Greens BrochureDocument14 pagesVishranti Greens BrochuretechkasambaPas encore d'évaluation
- Stop Worrying About Making Right DecisionsDocument2 pagesStop Worrying About Making Right DecisionstechkasambaPas encore d'évaluation
- 5 Quiz 5 WifeDocument2 pages5 Quiz 5 WifetechkasambaPas encore d'évaluation
- Leather and Leather Products: Food IndustriesDocument4 pagesLeather and Leather Products: Food IndustriestechkasambaPas encore d'évaluation
- Lagnas CharacterDocument14 pagesLagnas CharactertechkasambaPas encore d'évaluation
- BETE TF MetricDocument2 pagesBETE TF Metricajo2402Pas encore d'évaluation
- Manual Instrucciones TyphoonDocument46 pagesManual Instrucciones TyphoonGabriel TanasePas encore d'évaluation
- Industrial Instrumentation NotesDocument13 pagesIndustrial Instrumentation NotesArun RajeshPas encore d'évaluation
- Strength and Microscale Properties of Bamboo FiberDocument14 pagesStrength and Microscale Properties of Bamboo FiberDm EerzaPas encore d'évaluation
- CH 02Document7 pagesCH 02hedayatullahPas encore d'évaluation
- Pittcote 404 PDFDocument2 pagesPittcote 404 PDFJuan Manuel DiazPas encore d'évaluation
- Broad X User Manual 0814 Revised PDFDocument64 pagesBroad X User Manual 0814 Revised PDFs7ukPas encore d'évaluation
- Pre Insulated PipesDocument32 pagesPre Insulated Pipespal_stephenPas encore d'évaluation
- Waste Management Plan: Project Name To Be Written HereDocument16 pagesWaste Management Plan: Project Name To Be Written HerePrashanth JeerPas encore d'évaluation
- Chapter 1 Two-Phase Flow and Boiling Heat TransferDocument44 pagesChapter 1 Two-Phase Flow and Boiling Heat TransferjackleesjPas encore d'évaluation
- (Ensc 13) Probset 1 - Stresses (2ND - Sem - Ay2016-2017)Document4 pages(Ensc 13) Probset 1 - Stresses (2ND - Sem - Ay2016-2017)Bianca AsisPas encore d'évaluation
- Application of Superomniphobic Electrospun Membrane For Treatment of Real Produced Water Through Membrane Distillation - ScienceDirectDocument15 pagesApplication of Superomniphobic Electrospun Membrane For Treatment of Real Produced Water Through Membrane Distillation - ScienceDirectMOH AMANPas encore d'évaluation
- IMCO Catalog 2007Document106 pagesIMCO Catalog 2007shaheenkhan2510Pas encore d'évaluation
- How Can I Help To Protect The Ozone Layer?Document2 pagesHow Can I Help To Protect The Ozone Layer?Muhammad AhmedPas encore d'évaluation
- IMM BR IR enDocument13 pagesIMM BR IR enwatnaPas encore d'évaluation
- MGO Chiller 120kW Cooling Spec. - PDFDocument7 pagesMGO Chiller 120kW Cooling Spec. - PDFAlexandros KritsotakisPas encore d'évaluation
- BMP - Powder MetallurgyDocument71 pagesBMP - Powder Metallurgymantra2010Pas encore d'évaluation
- 02 Generic - Types - Grade1Document34 pages02 Generic - Types - Grade1Aravind BabuPas encore d'évaluation
- Din 571Document3 pagesDin 571mugiwara ruffyPas encore d'évaluation
- Acid Bases and Salts 2022-23Document8 pagesAcid Bases and Salts 2022-23Yasha RizviPas encore d'évaluation
- 2014 1275 SCANFiningLARTCDocument19 pages2014 1275 SCANFiningLARTCdonald55555Pas encore d'évaluation
- Electric Conductivity (EC) MeterDocument3 pagesElectric Conductivity (EC) MeterThanh NguyênPas encore d'évaluation