Académique Documents
Professionnel Documents
Culture Documents
CP CS24 NuclearPhysics
Transféré par
Siti Ipah Latipah0 évaluation0% ont trouvé ce document utile (0 vote)
26 vues1 pageStrong (nuclear) force is associated with the strong bonds between quarks and other subatomic particles. Weak nuclear force is weaker than the "strong nuclear force" - only 10-13 times as strong! Sometimes overcome by other forces causing the atom to cleave or give off radiation. Neutron: A positively charged subatomic particle forming part of the nucleus of an atom.
Description originale:
Titre original
CP_CS24_NuclearPhysics
Copyright
© Attribution Non-Commercial (BY-NC)
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentStrong (nuclear) force is associated with the strong bonds between quarks and other subatomic particles. Weak nuclear force is weaker than the "strong nuclear force" - only 10-13 times as strong! Sometimes overcome by other forces causing the atom to cleave or give off radiation. Neutron: A positively charged subatomic particle forming part of the nucleus of an atom.
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
26 vues1 pageCP CS24 NuclearPhysics
Transféré par
Siti Ipah LatipahStrong (nuclear) force is associated with the strong bonds between quarks and other subatomic particles. Weak nuclear force is weaker than the "strong nuclear force" - only 10-13 times as strong! Sometimes overcome by other forces causing the atom to cleave or give off radiation. Neutron: A positively charged subatomic particle forming part of the nucleus of an atom.
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 1
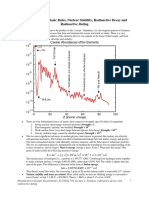
Physics Core Concept Cheat Sheet
24: Nuclear Physics
- The weak nuclear force is weaker than the “strong nuclear
Key Nuclear Physics Terms force” – only 10-13 times as strong! Sometimes is overcome
Proton: A positively charged subatomic particle forming part by other forces causing the atom to cleave or give off
of the nucleus of an atom and determining the atomic radiation.
number of an element. - Binding energy can be calculated using E=mc2.
Neutron: A subatomic particle forming part of the nucleus of - Binding energy is the energy needed to hold the nucleus and
an atom and having no charge. its particles together.
Nucleon: A proton or a neutron. - Binding energy comes from the actual mass of the particles
Atomic Number: The number, equal to the number of in the nucleus!
protons in an atom that determines its chemical properties. - On this scale, it is convenient to use the electron-volt as a
Symbol: Z unit of energy.
Atomic Mass: The mass of an atom expressed in atomic - Conversion: 1 eV= 1.602 x 10-19 J
mass units.
Strong (nuclear) Force: A fundamental force that is The Nucleus and Nuclear Physics
associated with the strong bonds between quarks and other Atomic Nucleus
subatomic particles.
Weak (nuclear) Force: One of the four fundamental forces
that is associated with nuclear decay. Particles Radiation Applications
Binding Energy: The energy needed to separate the
constituent parts of an atom or nucleus
Mass Defect: The difference between the mass of an atom Protons Neutrons Alpha Beta Gamma
and the sum of the masses of its individual components.
Electron-Volt: A unit of energy Half Life Atomic Nuclear
Mass-Energy Equivalence: All mass represents an Bombs Power
equivalent amount of energy. 1 amu = 931 MeV. Medical
Radioactivity: Emission of radiation as a consequence of a
nuclear reaction, or directly from the breakdown of an Atomic Atomic
unstable nucleus. Number Mass
Half Life: The time required for half of the nuclei in a
sample of a specific isotope to undergo radioactive decay.
Alpha Particle: A positively charged helium nucleus Isotopes
(consisting of two protons and two neutrons).
Beta Particle: An energetic electron produced as the result
Calculating Binding Energy
of a nuclear reaction or nuclear decay. 1. Determine the masses of each of the 4 particles
Gamma Particle/Ray: Very high frequency electromagnetic individually.
radiation emitted as a consequence of radioactivity. 2. Determine the mass of a He nucleus.
Fission: The process whereby one item splits to become two. 3. The difference between the two provides “m”.
Tomography: Imaging by sections or sectioning. 4. Use m in the equation E=mc2 to calculate E.
Nuclear Structure Key Facts Radioactive Decay
- The atom is thought of as electrons in a cloud moving Radioactive decay usually takes on one of three specific
around a compact nucleus. forms, known as alpha, beta and gamma decay.
- The nucleus is made of protons and neutrons, also known It is useful to know the amount of time needed for half of
as nucleons. a sample to decay – this is called the “half-life”.
- The nucleus is held together by extremely short-ranged Alpha decay results in the emission of a helium nucleus
forces called the “strong force” and the “weak force” Relatively large
- Conventional symbols are used to quickly communicate the Relatively slow
make-up of a nucleus. Relatively low energy
- The nucleus is made of two types of particles. Atoms may Stopped more easily than other decay particles
have up to about 100 protons and about 150 neutrons! Beta decay results in a different nucleus of the same
- Protons are positively charged particles that have a mass of atomic mass but atomic number 1 greater.
about 1 atomic mass unit (amu). Larger, faster and higher energy than alpha particle
- Neutrons are particles with NO charge and a mass of about Greater ability to penetrate than alpha particle
1 atomic mass unit (amu). Gamma rays are usually highly energetic
- Isotope (ī´sƏ-tōp´) – One of two or more atoms having the No new element is formed; rather the remaining nucleus
same atomic number but different mass numbers. is less energetic
- The strong nuclear force holds nuclear particles together. Massless
- The strong nuclear force keeps protons and neutrons from Move at the speed of light – high energy
breaking apart into yet smaller particles. Penetrate most matter
- The strong nuclear force acts over a very small distance Half-Life – Amount of TIME taken for one half of the
(10-15 m). original nuclei to decay.
- The weak nuclear force is the force that acts to hold nucleus
Nuclear Applications
together.
- The weak nuclear force acts over a very small distance Nuclear power plants have provided energy for half a
(distances no larger than a nucleus). century.
Atomic bombs are based on fission and among the most
How to Use This Cheat Sheet: These are the keys related this destructive weapons ever created.
topic. Try to read through it carefully twice then recite it out Medical applications of radioactivity are commonplace in our
on a blank sheet of paper. Review it again before the exams. society, and are seen in cancer therapy, tracers, tomography
(PET scans), NMRs and MRIs.
RapidLearningCenter.com ©2006 - 2007 All Right Reserved
Vous aimerez peut-être aussi
- Terminologies: 1. Alpha ParticlesDocument7 pagesTerminologies: 1. Alpha ParticlesRalph MazinoPas encore d'évaluation
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3D'EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3Pas encore d'évaluation
- Atomic Theory & Structure: Oakland Schools Chemistry Resource UnitDocument52 pagesAtomic Theory & Structure: Oakland Schools Chemistry Resource UnithendraprimaPas encore d'évaluation
- Your Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2D'EverandYour Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2Évaluation : 5 sur 5 étoiles5/5 (1)
- Unit I Nuclear Structure & RadioactivityDocument66 pagesUnit I Nuclear Structure & RadioactivityAnupriya AnuPas encore d'évaluation
- Nuclei Chapter 12 Class12 PhysicsDocument30 pagesNuclei Chapter 12 Class12 Physicsharikeshsingh45497Pas encore d'évaluation
- RevisionDocument13 pagesRevisionrajayush67780Pas encore d'évaluation
- 12 Physics Notes Ch13 NucleiDocument6 pages12 Physics Notes Ch13 NucleiKOMAL NAVARIYAPas encore d'évaluation
- Nuclei IDocument20 pagesNuclei IShoaibPas encore d'évaluation
- 12 Physics Notes ch13 NucleiDocument6 pages12 Physics Notes ch13 NucleiRonaldo NEXTPas encore d'évaluation
- Modern Physics: Structure of Atoms - Structure of An AtomDocument4 pagesModern Physics: Structure of Atoms - Structure of An AtomAbhay VishwakarmaPas encore d'évaluation
- 12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive DatingDocument8 pages12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Datingshawnah jamesPas encore d'évaluation
- Nuclear ModelDocument11 pagesNuclear Modelzeamayf.biasPas encore d'évaluation
- Final CBRNDocument28 pagesFinal CBRNRavindra JoshiPas encore d'évaluation
- Radioactivity HSCDocument11 pagesRadioactivity HSCRushita LingiahPas encore d'évaluation
- NEUTRON - The Neutron Is ADocument10 pagesNEUTRON - The Neutron Is AsushreenandaPas encore d'évaluation
- Lesson 12 Atomic and Nuclear PhenomenaDocument10 pagesLesson 12 Atomic and Nuclear PhenomenaAjzPas encore d'évaluation
- Nuclearchemistrybsci 170708171032Document42 pagesNuclearchemistrybsci 170708171032Victor OkosunPas encore d'évaluation
- Unit 3 Nuclear Power Plant: StructureDocument34 pagesUnit 3 Nuclear Power Plant: StructureAnand KalPas encore d'évaluation
- 2nd Year Chapter 9Document20 pages2nd Year Chapter 9joy bakshiPas encore d'évaluation
- Lecture Notes in Nuclear Medicine - Edited Version)Document65 pagesLecture Notes in Nuclear Medicine - Edited Version)Jestia Lyn EngracialPas encore d'évaluation
- Atomic Nucleus and Neutrons-SiliconDocument6 pagesAtomic Nucleus and Neutrons-SiliconbobbyjonesnickPas encore d'évaluation
- Mithun's Physics Revision Notes (Unit 1)Document6 pagesMithun's Physics Revision Notes (Unit 1)Mithun Dev SabaratnamPas encore d'évaluation
- Modern Physics-1Document22 pagesModern Physics-1Minato NamikazePas encore d'évaluation
- PC Chapter 46Document93 pagesPC Chapter 46ultimuPas encore d'évaluation
- Chapter 3 ing - - نسخةDocument11 pagesChapter 3 ing - - نسخةThë Nãüght RayenëPas encore d'évaluation
- IB CHAPTER 7 and 12 - Atomic, Nuclear and Particle PhysicsDocument18 pagesIB CHAPTER 7 and 12 - Atomic, Nuclear and Particle PhysicsAziz KarimPas encore d'évaluation
- Nuclear Binding EnergyDocument17 pagesNuclear Binding EnergyTrajce StojanovPas encore d'évaluation
- Nuclear Chemistry With Pen 1106Document63 pagesNuclear Chemistry With Pen 1106JOHN DAVE MOISES BALDRIASPas encore d'évaluation
- Radioactivity: Instructor: MR Toba Al-Kheir Islamic Girls' SeminaryDocument86 pagesRadioactivity: Instructor: MR Toba Al-Kheir Islamic Girls' SeminarySalehe TobaPas encore d'évaluation
- General ChemistryDocument20 pagesGeneral ChemistryEd Ryan RualesPas encore d'évaluation
- Atomic Number and Synthesis of New Elements, Nuclear ReactionDocument58 pagesAtomic Number and Synthesis of New Elements, Nuclear ReactionJesiah PascualPas encore d'évaluation
- Rad PhysicsDocument5 pagesRad PhysicsSoleil SierraPas encore d'évaluation
- C18-Radioactivity and Nuclear ReactionsDocument106 pagesC18-Radioactivity and Nuclear ReactionsAbhishek UpadhyayPas encore d'évaluation
- 13 NucleiDocument13 pages13 Nucleivallabh.moapPas encore d'évaluation
- Atomic NucleusDocument10 pagesAtomic NucleusKristiyan GeorgievPas encore d'évaluation
- Modern and Nuclear Physics - 2019Document41 pagesModern and Nuclear Physics - 2019Ekwama EwugaPas encore d'évaluation
- Atoms Nucleus and Radiation - To Be Filled inDocument15 pagesAtoms Nucleus and Radiation - To Be Filled inNick RandazzoPas encore d'évaluation
- Nuclear Physics (Lecture 22-24) - Compatibility ModeDocument41 pagesNuclear Physics (Lecture 22-24) - Compatibility ModeBibhu Prasad SahooPas encore d'évaluation
- Module Week 1Document2 pagesModule Week 1Maria Christina ManzanoPas encore d'évaluation
- Lec 2 Basic PhysicsDocument26 pagesLec 2 Basic PhysicsHashir AliPas encore d'évaluation
- Energy Conversion: Nuclear Power Plants: Nuclear Physics BasicsDocument22 pagesEnergy Conversion: Nuclear Power Plants: Nuclear Physics BasicsAhmed SamyPas encore d'évaluation
- ATOMDocument40 pagesATOMveronica lunaPas encore d'évaluation
- Modern Physics Atom and Its StructureDocument13 pagesModern Physics Atom and Its StructureRAHUL CHOUDHARYPas encore d'évaluation
- What Is Radioactivity?Document27 pagesWhat Is Radioactivity?Davies MasumbaPas encore d'évaluation
- GRE FactsDocument25 pagesGRE FactsMohamed ElashriPas encore d'évaluation
- Notes: Background RadiationDocument15 pagesNotes: Background Radiationanwar9602020Pas encore d'évaluation
- Radioactivity: Background RadiationDocument13 pagesRadioactivity: Background RadiationSuresh SenanayakePas encore d'évaluation
- Basic Electricity and Motor ControlsDocument15 pagesBasic Electricity and Motor ControlsAlex RamosPas encore d'évaluation
- Lect No 1 Nuclear Imaging FRCR PhysicsDocument22 pagesLect No 1 Nuclear Imaging FRCR Physicszlatan.srt8Pas encore d'évaluation
- Energy Unit 4Document22 pagesEnergy Unit 4Joseph MartinPas encore d'évaluation
- Nuclear PhysicsDocument40 pagesNuclear PhysicsShakir KhattakPas encore d'évaluation
- VIP Nuclear BasicsDocument166 pagesVIP Nuclear BasicsgetachewPas encore d'évaluation
- Nuclear Forces and Energy LevelsDocument5 pagesNuclear Forces and Energy Levelsbilalkhan3567Pas encore d'évaluation
- Chemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 1Document6 pagesChemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 1Kristine AlcantaraPas encore d'évaluation
- Unit 2 Notes - Teacher 2Document13 pagesUnit 2 Notes - Teacher 2noPas encore d'évaluation
- Elementary Particles and Their InteractionsDocument22 pagesElementary Particles and Their InteractionsCecilia PicayoPas encore d'évaluation
- Emags and Circ1Document14 pagesEmags and Circ1Compl3x CSGOPas encore d'évaluation
- Balancing Nuclear EquationsDocument1 pageBalancing Nuclear EquationsninnabananaPas encore d'évaluation
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyD'EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyPas encore d'évaluation
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceD'EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceÉvaluation : 4 sur 5 étoiles4/5 (51)
- A Brief History of Time: From the Big Bang to Black HolesD'EverandA Brief History of Time: From the Big Bang to Black HolesÉvaluation : 4 sur 5 étoiles4/5 (2193)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldD'EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldÉvaluation : 3.5 sur 5 étoiles3.5/5 (64)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseD'EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseÉvaluation : 3.5 sur 5 étoiles3.5/5 (69)
- Bedeviled: A Shadow History of Demons in ScienceD'EverandBedeviled: A Shadow History of Demons in ScienceÉvaluation : 5 sur 5 étoiles5/5 (5)
- Quantum Physics: What Everyone Needs to KnowD'EverandQuantum Physics: What Everyone Needs to KnowÉvaluation : 4.5 sur 5 étoiles4.5/5 (49)
- AP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticeD'EverandAP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticePas encore d'évaluation
- Summary and Interpretation of Reality TransurfingD'EverandSummary and Interpretation of Reality TransurfingÉvaluation : 5 sur 5 étoiles5/5 (5)
- Lost in Math: How Beauty Leads Physics AstrayD'EverandLost in Math: How Beauty Leads Physics AstrayÉvaluation : 4.5 sur 5 étoiles4.5/5 (125)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterD'EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterÉvaluation : 4.5 sur 5 étoiles4.5/5 (410)
- Packing for Mars: The Curious Science of Life in the VoidD'EverandPacking for Mars: The Curious Science of Life in the VoidÉvaluation : 4 sur 5 étoiles4/5 (1396)
- The End of Everything: (Astrophysically Speaking)D'EverandThe End of Everything: (Astrophysically Speaking)Évaluation : 4.5 sur 5 étoiles4.5/5 (157)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeD'EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifePas encore d'évaluation
- The Beginning of Infinity: Explanations That Transform the WorldD'EverandThe Beginning of Infinity: Explanations That Transform the WorldÉvaluation : 5 sur 5 étoiles5/5 (60)
- Vibration and Frequency: How to Get What You Want in LifeD'EverandVibration and Frequency: How to Get What You Want in LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (13)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessD'EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessÉvaluation : 4 sur 5 étoiles4/5 (6)
- What If?: Serious Scientific Answers to Absurd Hypothetical QuestionsD'EverandWhat If?: Serious Scientific Answers to Absurd Hypothetical QuestionsÉvaluation : 5 sur 5 étoiles5/5 (5)