Académique Documents
Professionnel Documents
Culture Documents
B.tech. Biotechnology Notes
Transféré par
Mudit MisraDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
B.tech. Biotechnology Notes
Transféré par
Mudit MisraDroits d'auteur :
Formats disponibles
Chromatography
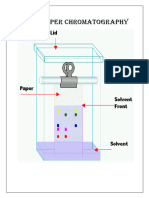
Chromatography is used to separate pure substances from a mixture of substances, such as a cell extract. It is based on different substances having different solubilities in different solvents. A simple and common form of chromatography uses filter paper. 1. Pour some solvent into a chromatography tank and seal it, so the atmosphere is saturated with solvent vapour. Different solvents are suitable for different tasks, but they are usually mixtures of water with organic liquids such as ethanol or propanone. 2. Place a drop of the mixture to be separated onto a sheet of chromatography paper near one end. This is the origin of the chromatogram. The spot should be small but concentrated. Repeat for any other mixtures. Label the spots with pencil, as ink may dissolve. 3. Place the chromatography sheet into the tank so that the origin is just above the level of solvent, and leave for several hours. The solvent will rise up the paper by capillary action carrying the contents of the mixture with it. Any solutes dissolved in the solvent will be partitioned between the organic solvent (the moving phase) and the water, which is held by the paper (the stationary phase). The more soluble a solute is in the solvent the further up the paper it will move. 4. When the solvent has nearly reached the top of the paper, the paper is removed and the position of the solvent front marked. The chromatogram may need to be developed to make the spots visible. For example amino acids stain purple with ninhydrin. 5. The chromatogram can be analysed by measuring the distance travelled by the solvent front, and the distance from the origin to the centre of each spot. This is used to calculate the Rf (relative front) value for each spot:
An Rf value is characteristic of a particular solute in a particular solvent. It can be used to identify components of a mixture by comparing to tables of known Rf values.
Sometimes chromatography with a single solvent is not enough to separate all the constituents of a mixture. In this case the separation can be improved by two-dimensional chromatography, where the chromatography paper is turned through 90 and run a second time in a second solvent. Solutes that didn't
separate in one solvent will separate in another because they have different solubilities.
There are many different types of chromatography.
Paper chromatography is the simplest, but does not always give very clean separation.
Thin layer chromatography (tlc) uses a thin layer of cellulose or silica coated onto a plastic or glass sheet. This is more expensive, but gives much better and more reliable separation.
Column chromatography uses a glass column filled with a cellulose slurry. Large samples can be pumped through the column and the separated fractions can be collected for further experiments, so this is preparative chromatography as opposed to analytical chromatography. High performance liquid chromatography (HPLC) is an improved form of column chromatography that delivers excellent separation very quickly.
Electrophoresis uses an electric current to separate molecules on the basis of charge. It can also be used to separate on the basis of molecular size, and as such is used in DNA sequencing.
Vous aimerez peut-être aussi
- Paper ChromatographyDocument5 pagesPaper ChromatographyMaria Elena PascualPas encore d'évaluation
- Exercise 4 (Chromatography)Document6 pagesExercise 4 (Chromatography)fangirlton0% (1)
- Paper ChromatographyDocument7 pagesPaper Chromatographykiya01Pas encore d'évaluation
- Bioc 211Document6 pagesBioc 211Femina ArgonzaPas encore d'évaluation
- Paper ChromatographyDocument6 pagesPaper ChromatographysamPas encore d'évaluation
- Analytical Techniques Final Note NADocument121 pagesAnalytical Techniques Final Note NAyuvi78312Pas encore d'évaluation
- Types of Chroma To Grap GyDocument75 pagesTypes of Chroma To Grap GyMohammad RehanPas encore d'évaluation
- Chromatography: Organized By: Badahdah, M. S. & Alghazzawi, W. M. 1Document17 pagesChromatography: Organized By: Badahdah, M. S. & Alghazzawi, W. M. 1sivaPas encore d'évaluation
- Experimental Techniques (Chapter 2) : Presented By: Mrs. SaimaDocument16 pagesExperimental Techniques (Chapter 2) : Presented By: Mrs. SaimaMohamed MunirPas encore d'évaluation
- Paper ChromatographyDocument5 pagesPaper ChromatographyDr. P.S.SenguptaPas encore d'évaluation
- ChromatographyDocument12 pagesChromatographyChristian MirandaPas encore d'évaluation
- Paper Chromatography Paper Chromatography Is An Analytical Method Used To Separate Chemicals orDocument4 pagesPaper Chromatography Paper Chromatography Is An Analytical Method Used To Separate Chemicals orMooma fatimaPas encore d'évaluation
- Paper ChromatographyDocument7 pagesPaper ChromatographySEHAR KHAN100% (1)
- Lab Report Exp 6Document5 pagesLab Report Exp 6api-384913960Pas encore d'évaluation
- ChromatographyDocument3 pagesChromatographyAashiPas encore d'évaluation
- Lecture 4 - Microscopy Biochemical TechniquesDocument10 pagesLecture 4 - Microscopy Biochemical TechniqueskkkkllllPas encore d'évaluation
- 083 - Chromatography and Its Uses in BiologyDocument3 pages083 - Chromatography and Its Uses in Biologylastjoe71Pas encore d'évaluation
- Chem Lab Project Paper ChromatographyDocument14 pagesChem Lab Project Paper ChromatographyFarah Kharuddin100% (1)
- Chromatographic Techniques 1200328060603035050Document12 pagesChromatographic Techniques 1200328060603035050KIRAN ALLUPas encore d'évaluation
- Exercise 4 (Chromatography)Document6 pagesExercise 4 (Chromatography)Wendell Kim LlanetaPas encore d'évaluation
- Lab 1 Introduction To SeparationsDocument9 pagesLab 1 Introduction To SeparationsDelicz TanPas encore d'évaluation
- HrmtograhiDocument8 pagesHrmtograhiALJOREY LAZARITOPas encore d'évaluation
- Chromatograp HY: Dr. Andrew.A.Lamare 2 Year PGDocument75 pagesChromatograp HY: Dr. Andrew.A.Lamare 2 Year PGShubhashree SinghPas encore d'évaluation
- Chapter 3.4 Paper Chromatography FinalDocument31 pagesChapter 3.4 Paper Chromatography FinalShalik RazaPas encore d'évaluation
- 37 Analytical Techniques PDFDocument225 pages37 Analytical Techniques PDFKomal EhsanPas encore d'évaluation
- Chapter 18 - ChromatographyDocument16 pagesChapter 18 - ChromatographyJames Miller100% (1)
- Paper ChromatographyDocument4 pagesPaper ChromatographyAsida Maronsing DelionPas encore d'évaluation
- ChromatographyDocument25 pagesChromatographyMani JeePas encore d'évaluation
- Module 3 Part 2 Chromatographic TechniquesDocument11 pagesModule 3 Part 2 Chromatographic TechniquesJyolsna JayarajPas encore d'évaluation
- Jadavpur University: CollegeDocument14 pagesJadavpur University: CollegekuntaljuPas encore d'évaluation
- CHEM 2020 Lab Manual (Introduction, Safety, Exp.1)Document6 pagesCHEM 2020 Lab Manual (Introduction, Safety, Exp.1)Kennedy Avery Morgan Jr.Pas encore d'évaluation
- Paper ChromatographyDocument6 pagesPaper ChromatographyAsida Maronsing DelionPas encore d'évaluation
- Chromatography SLT SpyDocument6 pagesChromatography SLT SpyAbdulnafiu AhmadPas encore d'évaluation
- CHROMATOGRAPHYDocument2 pagesCHROMATOGRAPHYMonica SreePas encore d'évaluation
- Exercise No.2 Paper ChromatographyDocument5 pagesExercise No.2 Paper ChromatographyMary Jane YepesPas encore d'évaluation
- ChromatographyDocument44 pagesChromatographyAravind KanthPas encore d'évaluation
- Chromatography: Is A Technique Used To Separate and Identify The Components of A MixtureDocument72 pagesChromatography: Is A Technique Used To Separate and Identify The Components of A MixtureMustafa KhandgawiPas encore d'évaluation
- Chromatography P1eeaoqbpea91bc5e2b1cc84Document91 pagesChromatography P1eeaoqbpea91bc5e2b1cc84Asif AliPas encore d'évaluation
- Chemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-1 Thin Layer ChromatographyDocument13 pagesChemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-1 Thin Layer ChromatographyHritik LalPas encore d'évaluation
- Paper Chromatography Model and Practical LabDocument4 pagesPaper Chromatography Model and Practical Labdebbie bongPas encore d'évaluation
- Paper ChromatographyDocument13 pagesPaper ChromatographyAlex MagatPas encore d'évaluation
- ChromatographyDocument88 pagesChromatographyMohammad Sabir HussainPas encore d'évaluation
- ChromatographyDocument56 pagesChromatographyapi-26998277100% (14)
- CHEMDocument13 pagesCHEMMohamed MusthapaPas encore d'évaluation
- Air Force School Ambala CanttDocument16 pagesAir Force School Ambala Canttsimran ranaPas encore d'évaluation
- Analytical techniques-TLC, HPLC, GLCDocument27 pagesAnalytical techniques-TLC, HPLC, GLCKudzayi TusaumwePas encore d'évaluation
- Principle of ChromatographyDocument8 pagesPrinciple of ChromatographyMuhammad ZeeshanPas encore d'évaluation
- ChromatographyDocument11 pagesChromatographyAmrit KoiralaPas encore d'évaluation
- AlexDocument2 pagesAlexYasar Arafath SPas encore d'évaluation
- Chroma LangDocument9 pagesChroma LangFaye Dumelod MarianoPas encore d'évaluation
- BPT Chromatography TechniquesDocument48 pagesBPT Chromatography TechniquesMagesh RamasamyPas encore d'évaluation
- ChromatographyDocument18 pagesChromatographyYerram Raju BeharaPas encore d'évaluation
- Separating Mixtures: Inquiry Question: How Do The Properties of Substances Help Us To Classify and Separate Them?Document18 pagesSeparating Mixtures: Inquiry Question: How Do The Properties of Substances Help Us To Classify and Separate Them?Tashna LalPas encore d'évaluation
- A Practical Handbook of Preparative HPLCD'EverandA Practical Handbook of Preparative HPLCÉvaluation : 3 sur 5 étoiles3/5 (3)
- Practical NMR Spectroscopy Laboratory Guide: Using Bruker SpectrometersD'EverandPractical NMR Spectroscopy Laboratory Guide: Using Bruker SpectrometersÉvaluation : 5 sur 5 étoiles5/5 (1)
- Essentials in Modern HPLC SeparationsD'EverandEssentials in Modern HPLC SeparationsÉvaluation : 4 sur 5 étoiles4/5 (4)
- Unit Processes in Pharmacy: Pharmaceutical MonographsD'EverandUnit Processes in Pharmacy: Pharmaceutical MonographsÉvaluation : 4.5 sur 5 étoiles4.5/5 (4)
- Practical Handbook of Pharmaceutical Chemistry for M.PharmD'EverandPractical Handbook of Pharmaceutical Chemistry for M.PharmPas encore d'évaluation
- Voice RecognitionDocument1 pageVoice RecognitionMudit MisraPas encore d'évaluation
- On Study Of: Dr.A.K.RastogiDocument73 pagesOn Study Of: Dr.A.K.RastogiMudit MisraPas encore d'évaluation
- VideoconferencingDocument14 pagesVideoconferencingMudit MisraPas encore d'évaluation
- Imechanisms of Enzymes Reactions: RRRRRRRRRRRRRRRRRRRRRRR RRRRRRRRRRRRRRRRRDocument25 pagesImechanisms of Enzymes Reactions: RRRRRRRRRRRRRRRRRRRRRRR RRRRRRRRRRRRRRRRRMudit MisraPas encore d'évaluation
- VACCINE123Document18 pagesVACCINE123Mudit MisraPas encore d'évaluation
- T Lymphocytes Immunology)Document2 pagesT Lymphocytes Immunology)Mudit MisraPas encore d'évaluation
- T Cell MaturationDocument5 pagesT Cell MaturationMudit Misra100% (1)
- Sequence Alignment and SearchingDocument54 pagesSequence Alignment and SearchingMudit MisraPas encore d'évaluation
- Transpalntation ImmunologyDocument18 pagesTranspalntation ImmunologyMudit MisraPas encore d'évaluation
- T Cell Receptor - Apc - ClonalDocument10 pagesT Cell Receptor - Apc - ClonalMudit MisraPas encore d'évaluation
- Structural BioinfoDocument76 pagesStructural BioinfoMudit MisraPas encore d'évaluation
- Storlllzs On of Blorcsctor: Ead - E Olganbms Ar .Bsent Is Nicoorgan'Sms Dudng The Onti.EDocument10 pagesStorlllzs On of Blorcsctor: Ead - E Olganbms Ar .Bsent Is Nicoorgan'Sms Dudng The Onti.EMudit MisraPas encore d'évaluation
- Statistics and Probability For TicsDocument36 pagesStatistics and Probability For TicsMudit MisraPas encore d'évaluation
- Speech Recognition Technology in A Ubiquitous Computing EnvironmentDocument24 pagesSpeech Recognition Technology in A Ubiquitous Computing EnvironmentMudit MisraPas encore d'évaluation
- Para, Gene DisDocument11 pagesPara, Gene DisMudit MisraPas encore d'évaluation
- Relationship Between Primary Metabolism and Secondary Metabolite AccumulationDocument47 pagesRelationship Between Primary Metabolism and Secondary Metabolite AccumulationMudit Misra100% (2)
- ' "",u " A. Ili,: 4 T (,F/C,/T Ta T) ,!Document30 pages' "",u " A. Ili,: 4 T (,F/C,/T Ta T) ,!Mudit MisraPas encore d'évaluation
- Protein Folds and StructureDocument19 pagesProtein Folds and StructureMudit MisraPas encore d'évaluation
- Production of Monoclonal AntibodiesDocument8 pagesProduction of Monoclonal AntibodiesMudit MisraPas encore d'évaluation
- Protein Structure PredictionDocument52 pagesProtein Structure PredictionMudit MisraPas encore d'évaluation
- R I: P E''tu:+l!") - ' L6t'd - 61 '1: HNR A e (YL,, :+4 - LL ,. - 'Q) L' "EDocument11 pagesR I: P E''tu:+l!") - ' L6t'd - 61 '1: HNR A e (YL,, :+4 - LL ,. - 'Q) L' "EMudit MisraPas encore d'évaluation
- Plant Tissue CultureDocument38 pagesPlant Tissue CultureMudit MisraPas encore d'évaluation
- Production of Enzymes and Phytotoxins by The Plant PathogenDocument40 pagesProduction of Enzymes and Phytotoxins by The Plant PathogenMudit MisraPas encore d'évaluation
- Prospects of Production and Utilization of Medicinal Plants Resources of India An Overview J SingDocument62 pagesProspects of Production and Utilization of Medicinal Plants Resources of India An Overview J SingMudit MisraPas encore d'évaluation
- Allure OrganicDocument2 pagesAllure OrganicDeyanira RubíPas encore d'évaluation
- Phytochemical Characterization of Brown Seaweed Sargassum WightiiDocument5 pagesPhytochemical Characterization of Brown Seaweed Sargassum WightiiMohamed Abou EldahabPas encore d'évaluation
- Existence of Bioactive Flavonoids in Rhizomes and Plant Cell Cultures of Boesenbergia Rotunda (L.) Mansf. KulturpflDocument6 pagesExistence of Bioactive Flavonoids in Rhizomes and Plant Cell Cultures of Boesenbergia Rotunda (L.) Mansf. KulturpflReanaldy Ibrahim Masudi PutraPas encore d'évaluation
- GUID - 2 en-USDocument2 pagesGUID - 2 en-USRima SPas encore d'évaluation
- Comparative Study of New Trends in HPLCDocument6 pagesComparative Study of New Trends in HPLCModPas encore d'évaluation
- Chiral Screening Approach of Atorvastatin Diastereomers by HPLC MethodDocument5 pagesChiral Screening Approach of Atorvastatin Diastereomers by HPLC MethodMediterr J Pharm Pharm SciPas encore d'évaluation
- Chromatography - USP Chapter 621Document7 pagesChromatography - USP Chapter 621jaimin patelPas encore d'évaluation
- Coal Tar Creosote: Concise International Chemical Assessment Document 62Document149 pagesCoal Tar Creosote: Concise International Chemical Assessment Document 62Rohin ChopraPas encore d'évaluation
- SANTE-12682-2019. Analytical Quality ControlDocument52 pagesSANTE-12682-2019. Analytical Quality ControlaaPas encore d'évaluation
- @MedicalBooksStore 2017 Immune Infertility 2nd Edition PDFDocument297 pages@MedicalBooksStore 2017 Immune Infertility 2nd Edition PDFCamilla Fernandez100% (1)
- Nakajima 1993Document12 pagesNakajima 1993Adrián Rojas ÁvilaPas encore d'évaluation
- HPLC User's Guide, 32 Karat 5.0Document148 pagesHPLC User's Guide, 32 Karat 5.0thynameisraymondPas encore d'évaluation
- MAN Lactic Acid Bacteria in Philippine Traditional Fermented FoodsDocument19 pagesMAN Lactic Acid Bacteria in Philippine Traditional Fermented FoodsVincent ReyesPas encore d'évaluation
- Sensors 17 02891 PDFDocument10 pagesSensors 17 02891 PDFWatcharapongWongkaewPas encore d'évaluation
- Lecture HPLC StudentDocument243 pagesLecture HPLC StudentNur Hariani100% (1)
- Poly (Epsilon-Caprolactone) Nanocapsules As Carrier Systems For Herbicides - Physico-Chemical Characterization and Genotoxicity EvaluationDocument9 pagesPoly (Epsilon-Caprolactone) Nanocapsules As Carrier Systems For Herbicides - Physico-Chemical Characterization and Genotoxicity EvaluationSilvio Toledo de LimaPas encore d'évaluation
- Instrument Analysis: Analysis Carbohydrate Contained in Palm Sugar by High Performance Liquid Chromatography (HPLC)Document7 pagesInstrument Analysis: Analysis Carbohydrate Contained in Palm Sugar by High Performance Liquid Chromatography (HPLC)Muhammad FahmiPas encore d'évaluation
- 2019-Simultaneous Estimation of Gabapentin and Methylcobalamin in Bulk and Pharmaceutical Dosage Form by RP-HPLC Simulta PDFDocument5 pages2019-Simultaneous Estimation of Gabapentin and Methylcobalamin in Bulk and Pharmaceutical Dosage Form by RP-HPLC Simulta PDFShweta BhutadaPas encore d'évaluation
- ChromatographyDocument18 pagesChromatographyYerram Raju BeharaPas encore d'évaluation
- Paper Chromatography: Presented By-Dr/ Samir MorshedyDocument36 pagesPaper Chromatography: Presented By-Dr/ Samir MorshedyAhmed AliPas encore d'évaluation
- Invitro Studies On The Effect of Gardenia Gummifera Methanol Extracts in MDA - MB - 231 Cell Lines PDFDocument7 pagesInvitro Studies On The Effect of Gardenia Gummifera Methanol Extracts in MDA - MB - 231 Cell Lines PDFDR. BALASUBRAMANIAN SATHYAMURTHYPas encore d'évaluation
- Paracetamol 9Document6 pagesParacetamol 9danielafarmacie_1617Pas encore d'évaluation
- Trite R Pen OidsDocument51 pagesTrite R Pen Oidspja890223Pas encore d'évaluation
- Aoac 979.08Document1 pageAoac 979.08blink scientificPas encore d'évaluation
- BiotechniquesDocument17 pagesBiotechniquesPoypoy Postrano Rojo-ArongPas encore d'évaluation
- Inglés Nivel 2 Fcen: Reading MaterialDocument31 pagesInglés Nivel 2 Fcen: Reading Materialcindy solPas encore d'évaluation
- High Performance Liquid ChromatographyDocument14 pagesHigh Performance Liquid Chromatographyghaluh parahitaPas encore d'évaluation
- 1 s2.0 S0889157517302788 MainDocument9 pages1 s2.0 S0889157517302788 MainNurma SabilalaPas encore d'évaluation
- User Guide For Organic Acids Analysis Columns: The Power of DiscoveryDocument17 pagesUser Guide For Organic Acids Analysis Columns: The Power of DiscoveryGeetha ThiruvengadamPas encore d'évaluation
- Chemical Composition, Physicochemical and Bioactive Properties of Avocado SeedsDocument13 pagesChemical Composition, Physicochemical and Bioactive Properties of Avocado SeedsHuỳnh Như Đặng ThụyPas encore d'évaluation