Académique Documents
Professionnel Documents
Culture Documents
Precipitation Reactions Notes
Transféré par
sprijayaDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Precipitation Reactions Notes
Transféré par
sprijayaDroits d'auteur :
Formats disponibles
Solutions
Precipitation Reaction Teaching Notes
OVERVIEW This main objectives of this lab are to have students use solubility tables to predict precipitation reactions and then test their predictions by mixing solutions of ionic compounds. The lab also provides the students with practice in understanding and writing net ionic equations. Students often like the immediate results they see with precipitation reactions, and the results correlate well with the class material on precipitation reactions. Except for the initial preparation of the solutions this is a relatively quick and easy lab for the students to perform. The solutions may be stored in dropper bottles and can be used numerous times if contamination does not occur. This lab could be done as two separate labs. In the first lab students could prepare the standard solutions . The second lab would be used to conduct the precipitation tests . OPTIONS It is not necessary to use the full sets of solutions. However students should perform tests in which no reaction occurs in addition to reactions that produce a precipitate.
Set A: Source of Cations
Molar Mass (g mol-1 ) NaNO3 KNO3 AgNO 3 NH4 NO3 Pb(NO3 )2 Ca(NO3 )2 Mg(NO 3 )2 Ba(NO3 )2 Cu(NO 3 )2 Fe(NO3 )3 85.0 101.1 169.9 80.0 331.2 164.1 148.3 261.3 187.6 241.9 Mass (g)
0.85 1.01 1.70 0.80 3.31 1.64 1.48 2.61 1.88 2.42
Alternate Sources of Cations
NaC2H3 O2 Zn(C2 H3 O2)2 82.0 183.5 0.82 0.18
Set B: Source of Anions
Molar Mass (g mol-1 ) Mass (g)
EQUIPMENT AND MATERIALS
If all solutions listed are used, 18 dropper bottles per group of students will be needed. A blank test grid is included this may be photocopied on overheads for the students to use for their test grid.
NaCl NaOH NaBr Na 2 S NaI
58.5 40.0 102.9 78.0 149.9 163.9 142.0 106.0
0.59 0.40 1.03 0.78 1.50 1.64 1.42 1.06
SOLUTION PREPARATION
To prepare 100 mL of 0.1M solutions of the following compounds, dissolve the mass indicated in the table in a small amount of distilled water. Add this to a 100-mL volumetric flask or 100-mL graduated cylinder. Fill to the mark with distilled water. For the purposes of the precipitation reactions, measurements do not need to be precise. However if students are preparing the solutions this is a good opportunity for them to practice good techniques for preparing standard solutions. Precipitation Reactions Teaching Notes
Na 3 PO4 Na 2 SO4 Na 2 CO3
Alternate Sources of Anions
K2 CO3 K3 PO4 (NH4 )2 SO4 138.2 212.3 132.1 1.38 2.12 1.32
1
PREDICTIONS
Reactants NaCl + NaNO 3 NaCl + KNO 3 NaCl + AgNO3 NaCl + NH4 NO3 NaCl + Pb(NO3 )2 NaCl + Ca(NO3 )2 NaCl + Mg(NO3 )2 NaCl + Ba(NO3 )2 NaCl + Cu(NO3 )2 NaCl + Fe(NO3 )3
Predicted Precipitate NaBr + NaNO3 NaBr + KNO3 AgCl NaBr + AgNO3 NaBr + NH4NO3 Pb(NO3 )2 NaBr + Pb(NO3 )2 NaBr + Ca(NO3 )2 NaBr + Mg(NO3 )2 NaBr + Ba(NO3 )2 NaBr + Cu(NO 3 )2 NaBr + Fe(NO 3 )3
Predicted Precipitate
AgBr
PbBr 2
NaOH + NaNO3 NaOH + KNO3 NaOH + AgNO3 NaOH + NH4 NO3 NaOH + Pb(NO3 )2 NaOH + Ca(NO3)2 NaOH + Mg(NO3)2 NaOH + Ba(NO3)2 NaOH + Cu(NO3)2 NaOH + Fe(NO3 )3 Cu(OH)2 Fe(OH)3 Pb(OH)2 Ca(OH)2 Mg(OH)2 AgOH
Na 2 S + NaNO 3 Na 2 S + KNO3 Na 2 S + AgNO 3 Na 2 S + NH4NO3 Na 2 S + Pb(NO 3 )2 Na 2 S + Ca(NO3 )2 Na 2 S + Mg(NO3 )2 Na 2 S + Ba(NO3 )2 Na 2 S + Cu(NO3 )2 Na 2 S + Fe(NO3 )3 CuS Fe2 S3 PbS Ag 2S
Precipitation Reactions Teaching Notes
Reactants NaI + NaNO3 NaI + KNO3 NaI + AgNO3 NaI + NH4 NO3 NaI + Pb(NO3 )2 NaI + Ca(NO3 )2 NaI + Mg(NO 3 )2 NaI + Ba(NO3 )2 NaI + Cu(NO 3 )2 NaI + Fe(NO 3 )3
Predicted Precipitate Na 2 SO4 + NaNO3 Na 2 SO4 + KNO3 AgI Na 2 SO4 + AgNO3 Na 2 SO4 + NH4 NO3 PbI2 Na 2 SO4 + Pb(NO3)2 Na 2 SO4 + Ca(NO3)2 Na 2 SO4 + Mg(NO3 )2 Na 2 SO4 + Ba(NO3)2 Na 2 SO4 + Cu(NO3)2 Na 2 SO4 + Fe(NO3)3
Predicted Precipitate
Ag 2 SO4
PbSO4 CaSO4
BaSO4
Na 3 PO4 + NaNO3 Na 3 PO4 + KNO3 Na 3 PO4 + AgNO3 Na 3 PO4 + NH4 NO3 Na 3 PO4 + Pb(NO3 )2 Na 3 PO4 + Ca(NO3 )2 Na 3 PO4 + Mg(NO3 )2 Na 3 PO4 + Ba(NO3 )2 Na 3 PO4 + Cu(NO3 )2 Na 3 PO4 + Fe(NO3 )3 Pb 3(PO4 )2 Ca 3(PO4 )2 Mg 3(PO4 )2 Ba 3(PO4 )2 Cu 3(PO4 )2 FePO4 Ag3 PO4
Na 2 CO3 + NaNO3 Na 2 CO3 + KNO3 Na 2 CO3 + AgNO3 Na 2 CO3 + NH4 NO3 Na 2 CO3 + Pb(NO3)2 Na 2 CO3 + Ca(NO3)2 Na 2 CO3 + Mg(NO3 )2 Na 2 CO3 + Ba(NO3)2 Na 2 CO3 + Cu(NO3)2 Na 2 CO3 + Fe(NO3)3 PbCO3 CaCO3 MgCO3 Ba CO3 CuCO3 Fe2 (CO3)3 Ag 2 CO3
Precipitation Reactions Teaching Notes
CONCLUSIONS AND QUESTIONS
1. For every reaction in which a precipitate occurred, write both the full reaction equation and also the net ionic equation. In both equations be sure to identify the precipitate as a solid, by (s) after the formula. Be sure to balance all equations.
Full Equation NaCl(aq) + AgNO3(aq) ? AgCl ( s ) + NaNO3(aq) NaOH(aq) + AgNO3(aq) ? AgOH( s ) + NaNO3(aq) NaBr(aq) + AgNO3(aq) ? AgBr ( s ) + NaNO3(aq) Na 2 S(aq) + 2 AgNO3(aq) ? Ag2 S( s ) + 2 NaNO3(aq) NaI(aq) + AgNO3(aq) ? AgI(s) + NaNO3(aq) Na 3 PO4(aq) + 3 AgNO3(aq) ? Ag 3 PO4(s) + 3 NaNO 3(aq) Na 2 SO4(aq) + 2 AgNO3(aq) ? Ag 2 SO4(s) + 2 NaNO 3(aq) Na 2 CO3(aq) + 2 AgNO3(aq) ? Ag2 CO3(s) + 2 NaNO3(aq)
Net Ionic Equation Ag +(aq) + Cl-(aq) AgCl ( s ) Ag +(aq) + OH-(aq) AgOH( s ) Ag +(aq) + Br-(aq) AgBr (s) 2 Ag+(aq) + S2-(aq) Ag2 S(s) Ag +(aq) + I-(aq) AgI(s) 3 Ag+(aq) + PO4 3-(aq) Ag3 PO4(s) 2 Ag+(aq) + SO4 2-(aq) Ag2 SO4(s) 2 Ag+(aq) + CO3 2- (aq) Ag2 CO3(s)
2 NaCl(aq) + Pb(NO3 )2(aq) ? PbCl2(s) + 2 NaNO3(aq) 2 NaOH(aq) + Pb(NO3 )2(aq) ? Pb(OH)2( s ) + 2 NaNO3(aq) 2 NaBr(aq) + Pb(NO3 )2(aq) ? PbBr2(s) + 2 NaNO3(aq) Na 2 S(aq) + Pb(NO3 )2(aq) ? PbS( s ) + 2 NaNO3(aq) 2 NaI(aq) + Pb(NO3 )2(aq) ? PbI2(s) + 2 NaNO3(aq) 2 Na3 PO4(aq) + 3 Pb(NO3 )2(aq) ? Pb3 (PO4)2(s) + 6 NaNO 3(aq) Na 2 SO4(aq) + Pb(NO3 )2(aq) ? PbSO4( s ) + 2 NaNO3(aq) Na 2 CO3(aq) + Pb(NO3 )2(aq) ? PbCO3( s ) + 2 NaNO3(aq)
Pb 2+(aq) + 2 Cl-(aq) PbCl2( s ) Pb 2+ (aq) + 2 OH-(aq) Pb(OH)2(s) Pb 2+ (aq) + 2 Br-(aq) PbBr2(s) Pb 2+ (aq) + S2-(aq) PbS( s ) Pb 2+ (aq) + 2 I-(aq) PbI2(s) 3 Pb 2+ (aq) + 2 PO4 3-(aq) Pb 3(PO4 )2(s) Pb 2+ (aq) + SO4 2-(aq) PbSO4( s ) Pb 2+ (aq) + CO3 2- (aq) PbCO3(s)
2 NaOH(aq) + Ca(NO3 )2(aq) Ca(OH)2( s ) + 2 NaNO3(aq) 2 Na3 PO4(aq) + 3Ca(NO3 )2(aq) Ca3 (PO4)2(s) + 6 NaNO 3(aq) Na 2 SO4(aq) + Ca(NO3 )2(aq) CaSO4(s) + 2 NaNO 3(aq) Na 2 CO3(aq) + Ca(NO3 )2(aq) CaCO3(s) + 2 NaNO 3(aq)
Ca 2+(aq) + 2 OH-(aq) Ca(OH) 2( s ) 3Ca 2+(aq) + 2 PO4 3-(aq) Ca 3 (PO4 )2(s) Ca 2+(aq) + SO4 2-(aq) CaSO4(s) Ca 2+(aq) + CO3 2- (aq) CaCO3( s )
Precipitation Reactions Teaching Notes
Full Equation 2 NaOH(aq) + Mg(NO3 )2(aq) Mg(OH)2(s) + 2 NaNO3(aq) 2 Na3 PO4(aq) + 3Mg(NO3 )2(aq) Mg 3 (PO4 )2(s) + 6 NaNO3(aq) Na 2 CO3(aq) + Mg(NO3 )2(aq) MgCO 3( s ) + 2 NaNO3(aq)
Net Ionic Equation Mg 2+(aq) + 2 OH-(aq) Mg(OH)2(s) 3Mg2+(aq) + 2 PO4 3-(aq) Mg3 (PO4)2(s) Mg 2+(aq) + CO3 2- (aq) MgCO 3(s)
2 Na3 PO4(aq) + 3Ba(NO3 )2(aq) Ba3 (PO4)2(s) + 6 NaNO 3(aq) Na 2 SO4(aq) + Ba(NO3 )2(aq) BaSO4(s) + 2 NaNO 3(aq) Na 2 CO3(aq) + Ba(NO3 )2(aq) BaCO3(s) + 2 NaNO 3(aq)
3Ba 2+(aq) + 2 PO4 3-(aq) Ba 3(PO4 )2(s) Ba 2+(aq) + SO4 2-(aq) BaSO4(s) Ba 2+(aq) + CO3 2- (aq) BaCO3( s )
2 NaOH(aq) + Cu(NO3 )2(aq) Cu(OH)2(s) + 2 NaNO3(aq) Na 2 S(aq) + Cu(NO3 )2(aq) CuS( s ) + 2 NaNO3(aq) 2 Na3 PO4(aq) + 3Cu(NO3 )2(aq) Cu3 (PO4)2(s) + 6 NaNO3(aq) Na 2 CO3(aq) + Cu(NO3 )2(aq) CuCO3( s ) + 2 NaNO3(aq)
Cu 2+(aq) + 2 OH-(aq) Cu(OH)2(s) Cu 2+ (aq) + S2-(aq) CuS( s ) 3Cu 2+(aq) + 2 PO4 3-(aq) Cu 3(PO4 )2(s) Cu 2+(aq) + CO3 2- (aq) CuCO3(s)
3 NaOH(aq) + Fe(NO3 )3(aq) Fe(OH)3( s ) + 3 NaNO3(aq) 3 Na2 S(aq) + 2 Fe(NO3 )3(aq) Fe2 S3(s) + 6 NaNO3(aq) Na 3 PO4(aq) + 3Fe(NO3 )3(aq) FePO4( s ) + 3 NaNO3(aq) 3 Na2 CO3(aq) + 2 Fe(NO3 )3(aq) Fe2 (CO3)3( s ) + 6 NaNO3(aq)
Fe3+(aq) + 3 OH-(aq) Fe(OH) 3( s ) 2 Fe3+ (aq) + 3 S2-(aq) Fe2 S3(s) Fe3+(aq) + PO4 3-(aq) FePO4(s) 2 Fe3+(aq) + 3 CO3 2- (aq) Fe2 (CO3)3(s)
Precipitation Reactions Teaching Notes
SET A Na+ K+ Ag+ NO3NH4+ NO3Pb2+ NO3-
SOLUTIONS Ca2+ NO3Mg2+ NO3Ba2+ NO3Cu2+ NO3Fe3+ NO3-
NO3- NO3Na+ ClNa+ OHNa+ SET B SOLUTIONS BrNa+ S2Na+ INa + PO43Na+ SO42Na+ CO32-
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
PPT
Precipitation Reactions Teaching Notes
SET A Na+ NO3Na+ ClNa+ OHNa+ SET B SOLUTIONS BrNa+ S2Na+ INa + PO43Na+ SO42Na+ CO32K+ NO3Ag+ NO3NH4+ NO3-
SOLUTIONS Ca2+ NO3Mg2+ NO3Ba2+ NO3Cu2+ NO3Fe3+ NO3-
Pb2+ NO3-
Precipitation Reactions Teaching Notes
SET A
SOLUTIONS
Precipitation Reactions Teaching Notes
SET B SOLUTIONS
Vous aimerez peut-être aussi
- 6 Precipitation ReactionsDocument2 pages6 Precipitation ReactionsJacob DaughertyPas encore d'évaluation
- Reviewer in ChemistryDocument8 pagesReviewer in ChemistryChristineManrique89% (9)
- EXPERIMENT 5 - Double Displacement Reactions: Report FormDocument6 pagesEXPERIMENT 5 - Double Displacement Reactions: Report FormNguyễn Hoàng ĐăngPas encore d'évaluation
- Softcopy of CHEMISTRY-F5 PDFDocument188 pagesSoftcopy of CHEMISTRY-F5 PDFainihasshim79% (282)
- Chemical Reactions in Aqueous SolutionDocument5 pagesChemical Reactions in Aqueous Solutioniam_crazii_4_mhe100% (2)
- Chem 142 Lecture on Precipitation ReactionsDocument10 pagesChem 142 Lecture on Precipitation ReactionsJunel Dave SalapantanPas encore d'évaluation
- Precipitates and Solubility Rules Pre-Lab Discussion:: Solution Set A Solution Set BDocument7 pagesPrecipitates and Solubility Rules Pre-Lab Discussion:: Solution Set A Solution Set Bapi-296518880Pas encore d'évaluation
- NIE - and - Particulate - Drawings - Worksheet - Answers at End - 2017-1Document8 pagesNIE - and - Particulate - Drawings - Worksheet - Answers at End - 2017-1Jane Ivanova100% (1)
- Net Ionic EditedDocument8 pagesNet Ionic EditedMuhammad AbdullahPas encore d'évaluation
- Solution Stoichiometry - Andnetionic.answers 3Document2 pagesSolution Stoichiometry - Andnetionic.answers 3Rahill SafiPas encore d'évaluation
- Persamaan Uji Reaksi A. Niso: (S) + (NH (Aq) (Aq) NicoDocument2 pagesPersamaan Uji Reaksi A. Niso: (S) + (NH (Aq) (Aq) NicoEko SumiyantoPas encore d'évaluation
- Practice Problems On Net Ionic EquationsDocument3 pagesPractice Problems On Net Ionic EquationsMaddison LilyPas encore d'évaluation
- Bab 6Document31 pagesBab 6Timothy HillPas encore d'évaluation
- Net Ionic EquationDocument3 pagesNet Ionic Equationsara bdeirPas encore d'évaluation
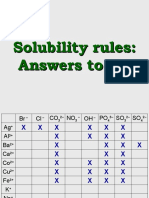
- Solubility Rules: Answers To LabDocument7 pagesSolubility Rules: Answers To LabDeba Jyoti NeogPas encore d'évaluation
- Practice Problems on Net Ionic EquationsDocument3 pagesPractice Problems on Net Ionic EquationsZainabPas encore d'évaluation
- Answer Key To Practice Problems On Net Ionic EquationsDocument4 pagesAnswer Key To Practice Problems On Net Ionic EquationsmerlindikaPas encore d'évaluation
- Net Ionic Equations-ProblemsDocument3 pagesNet Ionic Equations-ProblemsChikuta ShingaliliPas encore d'évaluation
- Ionic Equations wksht2 PDFDocument2 pagesIonic Equations wksht2 PDFBrandeice Barrett0% (1)
- Francisco Vigil - CHDocument5 pagesFrancisco Vigil - CHapi-528208996Pas encore d'évaluation
- Chem 110 Quiz BalancingDocument1 pageChem 110 Quiz BalancinglucasPas encore d'évaluation
- Chapter 5 Answers Practice Examples: ReductionDocument7 pagesChapter 5 Answers Practice Examples: ReductionEmre Enes EdizPas encore d'évaluation
- Sap 5Document22 pagesSap 5reza noviyantiPas encore d'évaluation
- Net Ionic EquationsDocument7 pagesNet Ionic EquationscelinePas encore d'évaluation
- Balancing Equations Worksheet Key: ZN (S) + 2 AgnoDocument1 pageBalancing Equations Worksheet Key: ZN (S) + 2 AgnoIgnacio Jr. PaguyoPas encore d'évaluation
- Precipitation RxnsDocument5 pagesPrecipitation RxnsSavie:D100% (1)
- Soluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DDocument4 pagesSoluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DBEST OF ONE PIECEPas encore d'évaluation
- C4 - StoichiometryDocument36 pagesC4 - StoichiometryAbhay BhingradiaPas encore d'évaluation
- Writing Chemical Equations: Test Yourself 8.1 (Page 130)Document1 pageWriting Chemical Equations: Test Yourself 8.1 (Page 130)Zeeshan MunirPas encore d'évaluation
- Cover Page Chemistry 2 AdobeDocument7 pagesCover Page Chemistry 2 AdobeJWAN RA YA3QOBPas encore d'évaluation
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFMalancha high school HS100% (1)
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFMalancha high school HS0% (1)
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsMalancha high school HS100% (1)
- Identify Colorless SolutionsDocument6 pagesIdentify Colorless SolutionsVasu JayanthiPas encore d'évaluation
- Identify Chemical Reactions and Balance EquationsDocument1 pageIdentify Chemical Reactions and Balance EquationsKarmelo LazaroPas encore d'évaluation
- Chemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryDocument31 pagesChemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryLakshmi SinghPas encore d'évaluation
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiPas encore d'évaluation
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimPas encore d'évaluation
- Elements, Compounds and Molecules ExplainedDocument20 pagesElements, Compounds and Molecules ExplainedPevin De silvaPas encore d'évaluation
- Chem 2Document8 pagesChem 22021302095Pas encore d'évaluation
- Post-lab 4 Qualitative Analysis-solutionsDocument7 pagesPost-lab 4 Qualitative Analysis-solutionsUzo Paul NwabuisiPas encore d'évaluation
- Preparing Soluble and Insoluble SaltsDocument12 pagesPreparing Soluble and Insoluble SaltsAzie Nurul Akhtar100% (1)
- Writing Ionic Equations MSDocument3 pagesWriting Ionic Equations MSSmahs ZabirPas encore d'évaluation
- QUANTITATIVE ANALYSIS OF CATIONSDocument12 pagesQUANTITATIVE ANALYSIS OF CATIONSShafiqahFazyaziqahPas encore d'évaluation
- Lesson Plan 5Document18 pagesLesson Plan 5Peng PengPas encore d'évaluation
- Balancing Equations Worksheet - Answers: © 2004 Cavalcade Publishing, All Rights ReservedDocument2 pagesBalancing Equations Worksheet - Answers: © 2004 Cavalcade Publishing, All Rights ReservedAnshul SoniPas encore d'évaluation
- Answer Key -Exam Review_Dec 2022_ChemistryDocument14 pagesAnswer Key -Exam Review_Dec 2022_Chemistrynicolas.randaxhePas encore d'évaluation
- Assignment F22 1Document15 pagesAssignment F22 1linkeyue330Pas encore d'évaluation
- Nitrogen Family Lecture 2: Preparation, Reactions and Compounds of NitrogenDocument28 pagesNitrogen Family Lecture 2: Preparation, Reactions and Compounds of NitrogenDtyuijPas encore d'évaluation
- (NH4) 2CO3(s) 2 NH3 (G) + CO2 (G) + H2O (G)Document2 pages(NH4) 2CO3(s) 2 NH3 (G) + CO2 (G) + H2O (G)Overlord MomonPas encore d'évaluation
- Reaksi Lap 5Document2 pagesReaksi Lap 5YuliaKamilawatiIIPas encore d'évaluation
- Week06outlinesf11 PDFDocument6 pagesWeek06outlinesf11 PDFaashique hussainPas encore d'évaluation
- Chemical reactions and formulas worksheetDocument2 pagesChemical reactions and formulas worksheetnurulragilPas encore d'évaluation
- 05 Petrucci10e CSMDocument45 pages05 Petrucci10e CSMAlexPas encore d'évaluation
- Ions in SolutionDocument8 pagesIons in SolutionNaufal TsabitadzPas encore d'évaluation
- Pitogo, Chanie Experiment 2Document7 pagesPitogo, Chanie Experiment 2Chanie Baguio PitogoPas encore d'évaluation
- Main Group Elements Practice ProblemsDocument20 pagesMain Group Elements Practice Problemskennethleo69Pas encore d'évaluation
- Precipitation ReactionDocument3 pagesPrecipitation ReactionMostafa NajjarinPas encore d'évaluation
- Practice Makes Perfect in Chemistry: Oxidation-ReductionD'EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionÉvaluation : 5 sur 5 étoiles5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersD'EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersPas encore d'évaluation
- Crystalline and Amorphous SolidsDocument10 pagesCrystalline and Amorphous SolidsAham GtyPas encore d'évaluation
- ThermodynamicsDocument112 pagesThermodynamicsdebaprasad ghoshPas encore d'évaluation
- Team Quastars ReportDocument25 pagesTeam Quastars ReportMedhansh GargPas encore d'évaluation
- Chapter 24 Molecular Absorption Spectrometry Molecular Spectrometry Uv / Vis / Ir Widely Used Identification Inorganic Organic Biochemical SpeciesDocument42 pagesChapter 24 Molecular Absorption Spectrometry Molecular Spectrometry Uv / Vis / Ir Widely Used Identification Inorganic Organic Biochemical Species陳奕廷go.thuPas encore d'évaluation
- Dual Nature of Radiation: in 1 ShotDocument64 pagesDual Nature of Radiation: in 1 ShotDisney DoreamonPas encore d'évaluation
- Chemguard 3% x 6% AR-AFFF for hydrocarbon and polar solvent fuelsDocument2 pagesChemguard 3% x 6% AR-AFFF for hydrocarbon and polar solvent fuelsTri Cahyono YuniantoPas encore d'évaluation
- Alexa Riley - Transpiration LabDocument7 pagesAlexa Riley - Transpiration Labapi-553676905Pas encore d'évaluation
- Int MSDocument24 pagesInt MSCHAVAN VIJAYSINGH MOHANRAOPas encore d'évaluation
- Tabelul Periodic - Google SearchDocument1 pageTabelul Periodic - Google SearchMariaPas encore d'évaluation
- Archimedes Number ExplainedDocument86 pagesArchimedes Number ExplainedRicardo Zevallos CruzPas encore d'évaluation
- Sensorless Control of Electrical DrivesDocument53 pagesSensorless Control of Electrical DrivesSamuel M.Pas encore d'évaluation
- A01. MCAT Uhs Past Paper 2008 - GreenDocument17 pagesA01. MCAT Uhs Past Paper 2008 - GreenAdnan Siddique100% (1)
- 2-Vle Part 2Document22 pages2-Vle Part 2Arfa Zulkifli01Pas encore d'évaluation
- Mucoadhesive Microspheres: A Short Review: Asian Journal of Pharmaceutical and Clinical Research January 2012Document5 pagesMucoadhesive Microspheres: A Short Review: Asian Journal of Pharmaceutical and Clinical Research January 2012Indri AuraliaPas encore d'évaluation
- Toc VWDocument16 pagesToc VWanilkumar995472Pas encore d'évaluation
- Ceramic Pigments Based On Natural Minerals: ArticleDocument5 pagesCeramic Pigments Based On Natural Minerals: ArticlezePas encore d'évaluation
- Ceramic Tiles and Vitrified TilesDocument15 pagesCeramic Tiles and Vitrified TilesSamyukthaPas encore d'évaluation
- The Golden Book of Chemistry Experiments (Banned in The 60-s)Document114 pagesThe Golden Book of Chemistry Experiments (Banned in The 60-s)mvpratt100% (6)
- Star and GalaxiesDocument32 pagesStar and GalaxiesMazura AhmadPas encore d'évaluation
- Singh B 1960 PHD ThesisDocument285 pagesSingh B 1960 PHD ThesisMarcoTacoPas encore d'évaluation
- Quantum Reality May Be Incomplete, Einstein Argument ShowsDocument4 pagesQuantum Reality May Be Incomplete, Einstein Argument ShowsAndy HoPas encore d'évaluation
- Separation and Concentration Technologies in Food ProcessingDocument84 pagesSeparation and Concentration Technologies in Food Processingjoenni hansPas encore d'évaluation
- Extended AbstractDocument10 pagesExtended AbstractSarang GohPas encore d'évaluation
- 7697A HeadspaceSamplers Site Preparation GuideDocument28 pages7697A HeadspaceSamplers Site Preparation GuidedrivePas encore d'évaluation
- Edexcel AS Physics Experiment Questions Unit 1Document7 pagesEdexcel AS Physics Experiment Questions Unit 1RaShid KhAnPas encore d'évaluation
- Physics I ProblemsDocument1 pagePhysics I ProblemsbosschellenPas encore d'évaluation
- CFDeffectoffluidviscosityDocument7 pagesCFDeffectoffluidviscosityHarsh TekriwalPas encore d'évaluation
- Enzyme Controlled Reaction LabDocument2 pagesEnzyme Controlled Reaction Labapi-291218692Pas encore d'évaluation
- Computational Chemistry Analysis of Hydrodesulfurization Reactions Catalyzed by Molybdenum Disulfide NanoparticlesDocument37 pagesComputational Chemistry Analysis of Hydrodesulfurization Reactions Catalyzed by Molybdenum Disulfide Nanoparticleshameed66Pas encore d'évaluation