Académique Documents
Professionnel Documents
Culture Documents
A Fla Toxins 1
Transféré par
Niamatullah KhanDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
A Fla Toxins 1
Transféré par
Niamatullah KhanDroits d'auteur :
Formats disponibles
FACT SHEET - www.micotoxinas.com.
br
THE AFLATOXINS
Leatherhead Food Research Association, Randalls Road, Leatherhead, Surrey KT22 7RY, England Naturally occurring aflatoxins Aflatoxins consist of a group of approximately 20 related fungal metabolites, although only aflatoxins B1, B2, G1 and G2 are normally found in foods. Aflatoxins B2 and G2 are the dihydro derivatives of the parent compounds. They are produced by at least three species of Aspergillus, A. flavus, A. parasiticus and A. nomius, and can occur in a wide range of important raw food commodities, including cereals, nuts, spices, figs and dried fruit.
Aflatoxin B1
Although the highest concentrations are formed in food crops grown and stored in the warmer areas of the world, the international trading of these important commodities ensures that aflatoxins are not only a problem for the producing nations but are also of concern for importing countries. Aflatoxins M1 and M2 are the hydroxylated metabolites of aflatoxins B1 and B2 and are produced when cows or other ruminants ingest feed contaminated with these mycotoxins. They are then excreted in the milk and may subsequently contaminate other dairy products such as cheese and yoghurt. Chemical and physical properties Some important physical and chemical properties of the aflatoxins are given in the table UV absorption max (e ), nm, methanol Aflatoxin Molecular formula B1 B2 C17H12O6 C17H14O6 Molecular weight 312 314 Melting Point 268-269 286-289 265 12,400 12,100 360-362 21,800 24,000
G1 G2 M1 M2
C17H12O7 C17H14O7 C17H12O7 C17H14O7
328 330 328 330
244-246 237-240 299 293
9,600 8,200 14,150 12,100 (264)
17,700 17,100 21,250 (357) 22,900 (357)
Aflatoxins are crystalline substances, freely soluble in moderately polar solvents such as chloroform, methanol and dimethyl sulfoxide, and dissolve in water to the extent of 10-20 mg/litre. They fluoresce under UV radiation. Crystalline aflatoxins are extremely stable in the absence of light and particularly UV radiation, even at temperatures in excess of 100 C. A solution prepared in chloroform or benzene is stable for years if kept cold and in the dark. The lactone ring makes them susceptible to alkaline hydrolysis, and processes involving ammonia or hypochlorite have been investigated as means for their removal from food commodities, although questions concerning the toxicity of the breakdown products have restricted the use of this means of eradicating aflatoxins from food and animal feeds. If alkaline treatment is mild, acidification will reverse the reaction to reform the original aflatoxin. In acid, aflatoxins B1 and G1 are converted to aflatoxins B2a and G2a by acid catalytic addition of water across the double bond of the furan ring. Oxidising reagents react and the molecules lose their fluorescence properties. Toxicity and importance Aflatoxins are both acutely and chronically toxic. Aflatoxin B1 is one of the most potent hepato-carcinogens known, and hence the long-term chronic exposure to extremely low levels of aflatoxins in the diet is an important consideration for human health. In the temperate, developed areas of the world, acute poisoning in animals is rare and in man is extremely unlikely. The outbreak of so called 'Turkey-X disease', which caused the deaths of 100,000 turkeys and other poultry in the United Kingdom in 1960, was caused by extremely high concentrations of aflatoxins in imported groundnut meal. This alerted industry and governments to the potential devastating effects of mycotoxins, particularly of the aflatoxins. Acute aflatoxin toxicity has been demonstrated in a wide range of mammals, fish, birds; rabbits, dogs and primates. Ducks, turkeys and trout are all highly susceptible. Age, sex and nutritional status all affect the degree of toxicity. Young animals are particularly susceptible and males more than females. For most species the LD50 (lethal dose) is between 0.5- and 10mg/kg body weight. The liver is the principal target organ, although the site of the hepatic effect varies with species. Effects on the lungs, myocardium and kidneys have also been observed and aflatoxin can accumulate in the brain. Teratogenic effects following administration of high doses of aflatoxin have been reported in some species.
Acute poisoning of man by aflatoxins does occur occasionally in some areas of the world. Cases of human aflatoxicosis have been reported sporadically, mainly in Africa or Asia. The majority of reported cases involve consumption of contaminated cereals - most frequently maize, rice or cassava, or cereal products such as pasta or peanut meal. A classic case occurred in Malaysia in 1990 when approximately 40 persons were affected and 13 children died after eating noodles highly contaminated with aflatoxin and boric acid. High levels of aflatoxin were found on autopsy in liver, lung, kidney, heart, brain and spleen. Aflatoxin may not always be the primary cause of death in these acute cases. Autopsy brain (cerebrum) specimens from 18 kwashiorkor children and 19 other children who had died from a variety of other diseases in Nigeria showed aflatoxin present in 81% of the cases. Aflatoxins have been implicated in sub-acute and chronic effects in humans. These effects include primary liver cancer, chronic hepatitis, jaundice, hepatomegaly and cirrhosis through repeated ingestion of low levels of aflatoxin. It is also considered that aflatoxins may place a role in a number of diseases, including Reyes syndrome, kwashiorkor and hepatitis. Aflatoxins can also affect the immune system (Pier 1991). Aflatoxin B1 is a potent mutagen causing chromosomal aberrations in a variety of plant, animal and human cells. The carcinogenicity and mutagenicity of aflatoxins B1, G1 and M1 are considered to arise as the result of the formation of a reactive epoxide at the 8, 9 position of the terminal furan ring and its subsequent covalent binding to nucleic acid, and the carcinogenicity of aflatoxin B1 has been studied in at least 12 different species. Although aflatoxins G1 and M1 have been tested less extensively, they appear to be toxicologically similar to aflatoxin B1. They are slightly less potent liver carcinogens but slightly more potent kidney carcinogens. Products affected and natural occurrence In most areas of the world, primary products such as cereals and nuts or animal products are screened routinely for aflatoxins, both by the producers and subsequently by importers or food manufacturers. Governments of many developed nations carry out surveillance on a regular basis to monitor the intake of aflatoxins by the human population so that action can be taken if this becomes necessary. Aflatoxins in peanuts moving in international trade have been found at levels of 1000 g/kg or more, and products such as peanut butter may be contaminated by smaller amounts. Other nuts particularly prone to contamination are pistachios and brazils. Climate plays a crucial part in the conditions that encourage aflatoxin, so that the problem varies in severity from year to year. Drought leading to crop stress followed by rain is particularly unwelcome for cereals producers. Sampling and analysis Moulds and aflatoxins occur in an extremely heterogeneous fashion in food commodities. It is thus crucial that sampling is carried out in a way that ensures that the analytical sample is truly representative of the consignment. Failure to do this may invalidate the subsequent analysis. Analytical methods used are based on TLC, HPLC or ELISA. Extraction with aqueous acetonitrile or methanol, followed by clean-up of the extract solutions using immunoaffinity columns, provides sensitive and selective results for a wide range of foods 3
and animal feed. Detection by TLC or HPLC is based on their fluorescence under UV radiation, although aflatoxin B1 and aflatoxin G1 need derivatisation to enhance the fluorescence to a similar level to that of aflatoxin B2 and aflatoxin G2. Limits of detection below 1 g/kg can be routinely achieved with analytical precision of 30% for foods and below 0.05g /kg for aflatoxin M1 in milk. On TLC plates, the four substances are distinguished on the basis of their colour, B standing for blue and G for green with subscripts relating to the relative chromatographic mobility. Aflatoxin B1 is usually but not exclusively found in the highest concentration. Stability and persistence Aflatoxins are quite stable in many foods and are fairly resistant to degradation. The effectiveness of some processes in reducing concentrations of aflatoxins in food can be affected by many factors, such as the presence of protein, pH, temperature and length of treatment. Commercial processing of raw commodities using cleaning regimes including the removal of broken particles, milling and sorting can reduce aflatoxin concentration considerably. Further information on decontamination will appear in the specialist fact sheet on this topic. Legislation and control The complete elimination of aflatoxins in human and animal food, while desirable, is extremely unlikely as they have the potential to arise in a wide range of agricultural products. Risk assessments have been carried out for aflatoxin. Because aflatoxin B1 is a genotoxic carcinogen, most agencies, including the Joint Expert Committee on Food Additives (JECFA) and the US Food and Drug Administration, have not set a total daily intake figure. In common with other dietary carcinogens, it is generally accepted that amounts in food should be reduced to the lowest levels that are technologically possible. Regulations have been set for human food and animal feed in many countries. The EC has established maximum permissible limits for aflatoxins in a range of commodities, including nuts, dried fruit and cereals, and for aflatoxin M1 in milk and dairy products. In the United Kingdom and elsewhere, regulation of aflatoxin B1 in animal feed has been effective in steadily reducing amounts of aflatoxin M1 in milk as shown by regular surveillance.
Vous aimerez peut-être aussi
- Aflatoxin in Food 2001 by HKSARDocument17 pagesAflatoxin in Food 2001 by HKSARMei YeePas encore d'évaluation
- FSDigest Aflatoxins EN PDFDocument5 pagesFSDigest Aflatoxins EN PDFBashar SaiidPas encore d'évaluation
- Aflatoxicosis: Etiology: Aspergillus FlavusDocument4 pagesAflatoxicosis: Etiology: Aspergillus FlavusTAMILPas encore d'évaluation
- Flavus and A. Parasiticus, Which Grow in Soil, Hay, Decaying Vegetation, and Grains. AflatoxinDocument1 pageFlavus and A. Parasiticus, Which Grow in Soil, Hay, Decaying Vegetation, and Grains. AflatoxinDiệp NguyễnPas encore d'évaluation
- Mycotoxicosis and MycetismusDocument31 pagesMycotoxicosis and MycetismusLincy JohnyPas encore d'évaluation
- Food OchratoxinDocument12 pagesFood OchratoxinAntonela ElaPas encore d'évaluation
- Antonio - Long Exam 5Document8 pagesAntonio - Long Exam 5JazMine AntonioPas encore d'évaluation
- Aflatoxin in Food: Implications On Health and Control MeasuresDocument7 pagesAflatoxin in Food: Implications On Health and Control MeasuresVi-e -minPas encore d'évaluation
- 98 Aflatoxin Reduction in Milk and Milk Products PDFDocument35 pages98 Aflatoxin Reduction in Milk and Milk Products PDFTaj 825Pas encore d'évaluation
- Laboratory Report Produced by Mr. Oko, Aloga JohnDocument4 pagesLaboratory Report Produced by Mr. Oko, Aloga JohnYashiPas encore d'évaluation
- Untitled DocumentDocument3 pagesUntitled DocumentMuhammad NasirPas encore d'évaluation
- 141923-Article Text-377588-1-10-20160812Document18 pages141923-Article Text-377588-1-10-20160812adebayo temitopePas encore d'évaluation
- Mycotoxin or Fungal ToxinDocument4 pagesMycotoxin or Fungal ToxinHely PatelPas encore d'évaluation
- Polyphenols in Chocolate: Is There A Contribution To Human Health?Document11 pagesPolyphenols in Chocolate: Is There A Contribution To Human Health?Sergio mauricio sergioPas encore d'évaluation
- Food Toxicity Ozlem GencDocument35 pagesFood Toxicity Ozlem Gencozlem gencPas encore d'évaluation
- FDADocument27 pagesFDArahul364Pas encore d'évaluation
- Mycotoxin ManualDocument77 pagesMycotoxin Manualhoria960% (1)
- Literature Review On AflatoxinDocument5 pagesLiterature Review On Aflatoxinea86yezd100% (1)
- Implication of Aflatoxins As Potent Carcinogens Implication of Aflatoxins As Potent Carcinogens Implication of Aflatoxins As Potent CarcinogensDocument7 pagesImplication of Aflatoxins As Potent Carcinogens Implication of Aflatoxins As Potent Carcinogens Implication of Aflatoxins As Potent CarcinogensFaiza NoorPas encore d'évaluation
- Mycotoxins On Poultry Production: AspergillusDocument12 pagesMycotoxins On Poultry Production: AspergillusmalwyliPas encore d'évaluation
- LauraDocument2 pagesLauraPaul DadziePas encore d'évaluation
- Aspergillus SP As Aflatoxin Producers in Grains and FeedsDocument30 pagesAspergillus SP As Aflatoxin Producers in Grains and FeedsNur QistinaPas encore d'évaluation
- FAO Fact Sheet Pyrrolizidine AlkaloidsDocument6 pagesFAO Fact Sheet Pyrrolizidine AlkaloidsMammo BerisoPas encore d'évaluation
- 052-058 PittDocument7 pages052-058 PittEm WaPas encore d'évaluation
- Occurrence of Aflatoxin in FoodDocument8 pagesOccurrence of Aflatoxin in Foodfarkad rawiPas encore d'évaluation
- Aflatoxin and CancerDocument32 pagesAflatoxin and CancerGracePas encore d'évaluation
- Biochem AssDocument18 pagesBiochem AssIshwar ChandraPas encore d'évaluation
- The Unseen EnemyDocument5 pagesThe Unseen EnemyInternational Aquafeed magazinePas encore d'évaluation
- Toxicology... 5th and 6th LecturesDocument35 pagesToxicology... 5th and 6th LecturesBushra MalikPas encore d'évaluation
- Paket Informasi Perpustakaan Bblitvet No. 10, 2016 PaketinformasibidangtoksikologidanmikologiDocument18 pagesPaket Informasi Perpustakaan Bblitvet No. 10, 2016 PaketinformasibidangtoksikologidanmikologiUka KahfianaPas encore d'évaluation
- Solanina PDFDocument9 pagesSolanina PDFEla PopaPas encore d'évaluation
- Kandungan Aflatoksin (B1, B2, G1 Dan G2) Pada Kacang Tanah (Arachis Hypogaea L) Yang Beredar Di Pasar Tradisional Daerah JabotabekDocument10 pagesKandungan Aflatoksin (B1, B2, G1 Dan G2) Pada Kacang Tanah (Arachis Hypogaea L) Yang Beredar Di Pasar Tradisional Daerah JabotabekheerawmPas encore d'évaluation
- GPL Test MycoTOX 8-PageDocument8 pagesGPL Test MycoTOX 8-PageJalya JalyaPas encore d'évaluation
- Deletarious Substances in Poultry FeedDocument4 pagesDeletarious Substances in Poultry FeedShah NawazPas encore d'évaluation
- MycotoxinsDocument4 pagesMycotoxinsnepoliyan ghimirePas encore d'évaluation
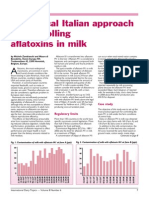
- A Practical Italian Approach To Controlling Aflatoxins in MilkDocument3 pagesA Practical Italian Approach To Controlling Aflatoxins in MilkahmrakPas encore d'évaluation
- Aflatoxins: Aspergillus. Parasiticus. They Are Produce and Widely Distributed in The Tropical and SubtropicalDocument5 pagesAflatoxins: Aspergillus. Parasiticus. They Are Produce and Widely Distributed in The Tropical and SubtropicalebenezerPas encore d'évaluation
- Aflatoxin B2Document11 pagesAflatoxin B2Akmaral KudaibergenPas encore d'évaluation
- Food Poisoning OverviewDocument16 pagesFood Poisoning Overviewupol88Pas encore d'évaluation
- PEER Stage2 10.1080/02652030701458113Document30 pagesPEER Stage2 10.1080/02652030701458113Călin RoxanaPas encore d'évaluation
- Ochratoxin A in Food (Okratoksin)Document40 pagesOchratoxin A in Food (Okratoksin)Aldi IgnielPas encore d'évaluation
- EFSA Journal - 2015 - Scientific Opinion On Acrylamide in FoodDocument321 pagesEFSA Journal - 2015 - Scientific Opinion On Acrylamide in FoodDIOMAR MILDRETH VALBUENA CARVAJALPas encore d'évaluation
- Mycotoxins: Gözde Ören Yardımcı & Büşra GültekinDocument88 pagesMycotoxins: Gözde Ören Yardımcı & Büşra GültekinBüşra GültekinPas encore d'évaluation
- A Atoxins: Carlos Humberto Corassin Carlos A F OliveiraDocument15 pagesA Atoxins: Carlos Humberto Corassin Carlos A F OliveiraFaiza NoorPas encore d'évaluation
- Flavanoids, L 3Document48 pagesFlavanoids, L 3Ammadazfar ImamPas encore d'évaluation
- Chemicals in Food: Environments and ContaminantsDocument18 pagesChemicals in Food: Environments and ContaminantsseemirrPas encore d'évaluation
- Aflatoxins in Corn: How To Sample Corn For Aflatoxin TestingDocument4 pagesAflatoxins in Corn: How To Sample Corn For Aflatoxin TestingAnonymous t5TDwdPas encore d'évaluation
- 19 - Nephrotoxic MycotoxinsDocument5 pages19 - Nephrotoxic MycotoxinsCabinet VeterinarPas encore d'évaluation
- Foodborne Illness (Also Foodborne Disease and Colloquially Referred To As Food Poisoning)Document17 pagesFoodborne Illness (Also Foodborne Disease and Colloquially Referred To As Food Poisoning)Helen McClintockPas encore d'évaluation
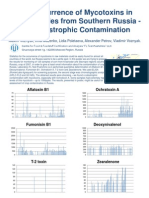
- Aflatoxins in Food - A Recent PerspectiveDocument295 pagesAflatoxins in Food - A Recent Perspectivecesar ruizPas encore d'évaluation
- Use of Anti Oxidants in Treatment of LeukoplakiaDocument30 pagesUse of Anti Oxidants in Treatment of LeukoplakiaRoja LakshmiPas encore d'évaluation
- AflatoxinsDocument16 pagesAflatoxinsdumbkidPas encore d'évaluation
- Aflatoxin Article by NKS GowdaDocument12 pagesAflatoxin Article by NKS GowdajaicmneogenPas encore d'évaluation
- Giz2019 en Aflatoxins and NutritionDocument9 pagesGiz2019 en Aflatoxins and NutritionNigusu SolomonPas encore d'évaluation
- SUBLETHAL DOSES of AflatoxinsDocument9 pagesSUBLETHAL DOSES of AflatoxinsPrecious GbemisolaPas encore d'évaluation
- Patulin: T.Pavithra 2016008021Document9 pagesPatulin: T.Pavithra 2016008021Nidharshana.SPas encore d'évaluation
- 2 Edvol 2 P 2 DDocument152 pages2 Edvol 2 P 2 DJin SiclonPas encore d'évaluation
- Dietary Exposure To Mycotoxins and Health Risk Assessment in The SecondDocument11 pagesDietary Exposure To Mycotoxins and Health Risk Assessment in The SecondTiago GraçaPas encore d'évaluation
- Tropane Alkaloids in Food: January 2010Document25 pagesTropane Alkaloids in Food: January 2010Christian ItenPas encore d'évaluation
- Lopes, 2023 MycotoxinesDocument15 pagesLopes, 2023 MycotoxinesRAFIQPas encore d'évaluation
- AgricultureandFood-ENewsLetterDocument336 pagesAgricultureandFood-ENewsLetterPinky Perez Durango100% (1)
- Applications in QC LECTUREDocument70 pagesApplications in QC LECTUREzeinab talaatPas encore d'évaluation
- Microorganisms 08 00246 v2 PDFDocument16 pagesMicroorganisms 08 00246 v2 PDFZenaw AntenbiyePas encore d'évaluation
- MycotoxinDocument57 pagesMycotoxinAshish ShresthaPas encore d'évaluation
- Korean Journal For Food Science of Animal ResourcesDocument8 pagesKorean Journal For Food Science of Animal ResourcesJelena RadivojevicPas encore d'évaluation
- Christian IdentityDocument14 pagesChristian IdentityLisaPas encore d'évaluation
- Rushing and Selim, 2019. Aflatoxin B1-A Review On Metabolism, Toxicity, Occurrence in FoodDocument21 pagesRushing and Selim, 2019. Aflatoxin B1-A Review On Metabolism, Toxicity, Occurrence in Foodsatrijo salokoPas encore d'évaluation
- Aflatoxin in Chillies-MianoDocument23 pagesAflatoxin in Chillies-Mianotanveer fatimaPas encore d'évaluation
- Eucalyptol Safety and Pharmacological ProfileDocument8 pagesEucalyptol Safety and Pharmacological ProfileTim ThomasPas encore d'évaluation
- Biosensores For LifeDocument25 pagesBiosensores For LifeTenis UnisonPas encore d'évaluation
- An Overview On Mycotoxin Contamination of Foods in Africa: REVIEW ToxicologyDocument9 pagesAn Overview On Mycotoxin Contamination of Foods in Africa: REVIEW ToxicologyTatiany RibeiroPas encore d'évaluation
- Recent Advances in Microbial Degradation 2021Document484 pagesRecent Advances in Microbial Degradation 2021Зоран ДинићPas encore d'évaluation
- Secondary Metabolites of Fungi PDFDocument19 pagesSecondary Metabolites of Fungi PDFsatriomega100% (1)
- A Review On Food Associated CarcinogenesisDocument22 pagesA Review On Food Associated CarcinogenesisJavier HernandezPas encore d'évaluation
- Peanut PasteDocument62 pagesPeanut PasteOctavia Monica CakrawinataPas encore d'évaluation
- 7.shashank M - Physical and Chemical Agents-1Document28 pages7.shashank M - Physical and Chemical Agents-1Manojna KMPas encore d'évaluation
- LauraDocument2 pagesLauraPaul DadziePas encore d'évaluation
- Update of Survey, Regulation and Toxic Effects of Mycotoxins in EuropeDocument10 pagesUpdate of Survey, Regulation and Toxic Effects of Mycotoxins in EuropeHigor MarkovicPas encore d'évaluation
- Smectite Complete BookDocument61 pagesSmectite Complete BookAbubakar Tahir RamayPas encore d'évaluation
- Voznyak IsmDocument1 pageVoznyak IsmMaxim VoznyakPas encore d'évaluation
- Aflatoxins in Food - A Recent PerspectiveDocument295 pagesAflatoxins in Food - A Recent Perspectivecesar ruizPas encore d'évaluation
- Role of N-Acetylcysteine in The Health and Production of Poultry.Document9 pagesRole of N-Acetylcysteine in The Health and Production of Poultry.Abdullah Saleem100% (1)
- Review On The Aflatoxins' Contamination of Foods and Public Health Effects Among Nigerian PopulationDocument17 pagesReview On The Aflatoxins' Contamination of Foods and Public Health Effects Among Nigerian PopulationUMYU Journal of Microbiology Research (UJMR)Pas encore d'évaluation
- Toxins 13 00148 v2Document22 pagesToxins 13 00148 v2Cristina BulgaruPas encore d'évaluation
- 2020 ProceedingsDocument299 pages2020 ProceedingsAziz El OuajidiPas encore d'évaluation
- 04.1 Commercially Available Services Mycotoxins - 2021 CEN-EN-ISO Methods (Food&feed)Document5 pages04.1 Commercially Available Services Mycotoxins - 2021 CEN-EN-ISO Methods (Food&feed)DinoPas encore d'évaluation
- Aflatoxins in PeanutsDocument32 pagesAflatoxins in PeanutsbbbbralPas encore d'évaluation
- Occurrence of Aflatoxin in FoodDocument8 pagesOccurrence of Aflatoxin in Foodfarkad rawiPas encore d'évaluation
- AgraQuantAflaMethod 4-40-020108Document4 pagesAgraQuantAflaMethod 4-40-020108Muchlas AkbarPas encore d'évaluation