Académique Documents
Professionnel Documents
Culture Documents
Blackboard Notes Chapter 6
Transféré par
Bill CampbellDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Blackboard Notes Chapter 6
Transféré par
Bill CampbellDroits d'auteur :
Formats disponibles
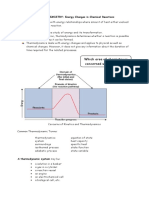
CHAPTER 6 LEARNING OBJECTIVES
To satisfy the minimum requirements for this course, you should be able to: Discuss the nature of energy by: giving examples of different forms of energy and examples of energy converting from one form to another (Section 6.1, page 230) explaining the differences between energy (E) and work (w), between thermal energy and temperature (T), and between thermal energy and heat (q) (Section 6.2-6.3, page 231239) converting between units of Joules, calories, and Calories (1 Cal is a kilocalorie = 1000 cal = 4184 J) Demonstrate an understanding of thermochemistry by: explaining the relationships among the following: system, surroundings, and universe; open system, closed system, and isolated system; exothermic process and endothermic process; internal energy (E) and enthalpy (H); E, H, qv, and qp (Section 6.3 all of it) sketching an energy diagram such as that shown in Figure 6.3 of the text given the energy changes in the processes involved associating the sign of H with whether the process is exothermic or endothermic (Section 6.4, page 241) using a thermochemical equation to calculate the quantity of heat involved in a reaction given the quantity of reactants and the enthalpy change for the reaction on a mole basis, or to calculate the amount of reactant needed to generate a given amount of heat ( Demonstrate an understanding of thermodynamics by: explaining why energy (E), pressure (P), volume (V), temperature (T), and enthalpy (H) are state functions and why heat (q) and work (w) are not state functions (Section 6.3) calculate the amount of work done when a gas changes volume at constant pressure using the equation w = PV = -RT(n) (work and heat section page 235-237, example 6.1) stating the first law of thermodynamics in words and perform calculations using the mathematical equivalent of the first law (E = q + w) explaining the sign conventions for heat and work (Table 6.1) Demonstrate an understanding of the concept of calorimetry by: explaining the difference between heat capacity (C) and specific heat (s) and converting one to the other using the relationship C = ms (Section 6.5, page 246) performing calculations using the equation q = msT or q = CT (Section 6.5, page 246) using constant pressure calorimetry data to calculate the enthalpy of reaction (Hrxn = qp) or to calculate the specific heat of a substance (page 249-250) Calculate the standard enthalpy of reaction (Horxn) using: standard enthalpies of formation (Hof ) of reactants and products (direct method, equation 6.18) (Section 6.6, page 252-254) Hess's law (indirect method) (page 254-255) Recommended additional problems: 18, 26, 34, 46, 58, 124
Worksheet Chapter 6
An OPEN SYSTEM can exchange ______ and ________. A CLOSED SYSTEM can exchange _____ but not ______. An ISOLATED SYSTEM can exchange neither ______ nor ________. Draw a beaker filled with HCl and NaOH, and label the following: system, surroundings, molecules within the system.
In an exothermic reaction, heat from the system is given off to the surroundings. The resultant energy of the system is higher/lower and the change in energy is +/- (circle the correct answers). The energy difference is the heat given off to the surroundings. In an endothermic reaction, heat from the surroundings is absorbed by the system. The resultant energy of the system is higher/lower and the change in energy is +/- (circle the correct answers). The energy difference is the heat supplied to the system by the surroundings. Key terms: Thermodynamics: study of energy and its transformations Energy (E): ability to do work or transfer heat. Units used: joules or calories (4.184 J=1 calorie) Kinetic energy (KE) energy of motion (KE=1/2 mv2) Potential energy (PE) energy of position; energy stored in chemical bonds First law of Thermodynamics energy can be neither created nor destroyed (but energy can change forms) work (w) energy used to cause an object to move against a force. (work=force*distance=PdeltaV for chemical system) heat (q) transfer of energy due to a temperature difference Temperature (T) = a measure of KE (ex. The motion of the particles in a chemical system) Internal energy sum of all KE and PE of all components of a system ( = q+w) Enthalpy (H)-heat absorbed or released under constant pressure State function: a property of a system that does not depend on the history of the system, only on its present condition
In thermodynamics, state functions are delineated with CAPITAL letters (H, E, P, V, T). q (heat) and w (work) are not state functions. But qp and qv are state functions. Why? At constant pressure, the enthalpy change ( H) of a chemical reaction equals the heat transferred from the system to the surroundings by the reaction (qp). Most reactions are carried out at constant pressure. When enthalpy is included in a chemical equation it is called a thermochemical equation. (Enthalpy is a state function, and it is an extensive property.) At constant volume, the E equals qv (because no work is done if V=0).
Is the delta H of the following reactions positive or negative? a. Combustion of methane (hint: your hands get hot): b. Evaporation of acetone (hint: your hands get cold): c. Freezing of ice (distractor: your hands are cold): A gas is allowed to expand at constant temperature from a volume of 10.0L to 20.0L against an external pressure of 1.0 atm. If the gas also absorbs 250 J of heat from the surroundings, what are the values of q, w, and E?
Heat of Enthalpy Calculation (a) and Specific Heat/Heat Capacity Calculation (b) An MRE Flameless Ration Heater weighs approx 1.5 oz, and contains a mixture of Mg (s), Fe (s), and salt. For this example, assume the packet contains 1 mole of Mg (s). To get the packet to start heating your food, you read and follow the directionsand add water! The water initiates the following reaction (using Fe and NaCl as a catalyst): Mg (s) + 2H2O (l) Mg(OH)2 (s) + H2 (g) + heat a. How many kJ are released into the surroundings (your food) in the oxidation of 1 mole of Mg (s).
b. How much water can you heat to the MILSPEC requirement (increase its temperature by 100 deg F in 12 minutes) if you were able to harness all of this energy and not dissipate it into the rest of the universe?
c. How many grams of Mg (s) would you need in your FRH to heat an 8 oz serving of chili by 100 deg F (your chili has an average specific heat of 4 kJ/(kg deg C) (assume all energy from the chemical reaction is used to heat your chili).
Calculate the standard enthalpy of formation of CS2 (l) using Hesss Law: C(graphite) + O2 (g) CO2 (g) H0 = -393.5 kJ/mol S(rhombic) + O2 (g) SO2 (g) H0 = -296.1 kJ/mol CS2(l) + 3O2 (g) CO2 (g) + 2SO2 (g) H0 = -1072 kJ/mol
A flameless ration heater, or FRH, is a water-activated exothermic chemical heater included with Meals, Ready-to-Eat (MREs), used to heat the food. US military specifications for the heater require that it be capable of raising the temperature of an eight-ounce entree by 100 F in twelve minutes, and that it display no visible flame. The ration heater contains finely powdered iron and magnesium metals, and table salt. To activate the reaction, a small amount of water is added, and the boiling point of water is quickly reached as the reaction proceeds.
Chemical reaction
Ration heaters generate heat in an electron-transfer process called an oxidation-reduction reaction. Water oxidizes magnesium metal, according to the following chemical reaction: Mg + 2H2O Mg(OH)2 + H2 + heat This reaction is analogous to iron being rusted by oxygen, and proceeds at about the same rate; that is, slowly. So on their own, the reaction between magnesium and water is simply too slow to generate usable heat. To speed up the reaction, the developers (see U.S. Patents 4,017,414 and 4,264,362) mixed metallic iron particles, and table salt (NaCl), with the magnesium particles. Iron and magnesium metals, when suspended in an electrolyte (such as salt water), form a galvanic cell -- a "battery" -- that can generate electricity. (For an example of how to build such a battery, see http://www.miniscience.com/link/Airbattery.htm) When water is added to a ration heater, it dissolves the salt to form a salt-water electrolyte, thereby turning each particle of magnesium and iron into a tiny battery. And because the magnesium and iron particles are touching each other, they become thousands of tiny shortcircuited batteries; which quickly burn out, giving up heat in a process the patent-holders called "Supercorroding Galvanic Cells". *** Find more good chemistry of MRE information at: http://chem200.tripod.com/
Zesto-Therm's Flameless Ration Heater (FRH)
Vous aimerez peut-être aussi
- A Modern Course in Statistical PhysicsD'EverandA Modern Course in Statistical PhysicsÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Chapter 5studentDocument44 pagesChapter 5studentDaniel ButenskyPas encore d'évaluation
- Intro 1a ThermochemistryDocument50 pagesIntro 1a ThermochemistryFatin IziantiPas encore d'évaluation
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4D'Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Pas encore d'évaluation
- Chapter 3 ThermodynamicsDocument92 pagesChapter 3 ThermodynamicsRaymond KakalaPas encore d'évaluation
- Unit 4: Thermochemistry and Nuclear Chemistry: Initial FinalDocument21 pagesUnit 4: Thermochemistry and Nuclear Chemistry: Initial FinalPankaj KumarPas encore d'évaluation
- Thermochemistry - Chemistry EncyclopediaDocument5 pagesThermochemistry - Chemistry EncyclopediaTwaine Angelle Villanueva SalvañaPas encore d'évaluation
- Chem 211-212 Hess's LawDocument17 pagesChem 211-212 Hess's LawRiff ShahPas encore d'évaluation
- Mew 1031 CH14Document29 pagesMew 1031 CH14Jorge Leonardo BedoyaPas encore d'évaluation
- P 7.4 ThermochemistryDocument32 pagesP 7.4 ThermochemistryFelicia GunawanPas encore d'évaluation
- Chapter 3 ThermodynamicsDocument95 pagesChapter 3 ThermodynamicsAnonymous iw2X32AwPas encore d'évaluation
- CH 6 - Thermochemistry - Notes - KeyDocument31 pagesCH 6 - Thermochemistry - Notes - KeyILEENVIRUSPas encore d'évaluation
- Chapter 6 Lecture NotesDocument10 pagesChapter 6 Lecture NotesAhmad KamalPas encore d'évaluation
- THERMODYNAMICSDocument29 pagesTHERMODYNAMICSlulunanjohndellPas encore d'évaluation
- ThermochemistryDocument25 pagesThermochemistrydanielmahsaPas encore d'évaluation
- CHAPTER 3 ThermochemistryDocument43 pagesCHAPTER 3 Thermochemistrykisan singhPas encore d'évaluation
- ThermochemistryDocument2 pagesThermochemistryJun Cueva Comeros100% (1)
- Part 1. - Chem - ThermodynamicsDocument64 pagesPart 1. - Chem - ThermodynamicsTaymeng LyPas encore d'évaluation
- Study ChemDocument13 pagesStudy ChemJanthina Rose AusteroPas encore d'évaluation
- Chapter 17 Outline Chem 1062: Probability To States of High ProbabilityDocument9 pagesChapter 17 Outline Chem 1062: Probability To States of High Probabilityaq300Pas encore d'évaluation
- AP Chem ThermodynamicsDocument58 pagesAP Chem ThermodynamicsLynda BkrPas encore d'évaluation
- THERMOCHEMISTRY Hand Outs 2023Document6 pagesTHERMOCHEMISTRY Hand Outs 2023Paul Willard GumapacPas encore d'évaluation
- Which Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsDocument19 pagesWhich Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsSahada KanapiyaPas encore d'évaluation
- Energetics and ThermochemistryDocument60 pagesEnergetics and ThermochemistryIsadora ThibauPas encore d'évaluation
- Thermodynamics, Heat & EnthalpyDocument16 pagesThermodynamics, Heat & EnthalpyTorontoAliPas encore d'évaluation
- ThermochemistryDocument6 pagesThermochemistryrskr_tPas encore d'évaluation
- Thermochemistry: Energy Flow and Chemical ChangeDocument29 pagesThermochemistry: Energy Flow and Chemical ChangeJerome LeoPas encore d'évaluation
- ThermoschemsitryDocument40 pagesThermoschemsitryHadeel IbrahimPas encore d'évaluation
- Thermo ChemistryDocument11 pagesThermo ChemistryEugenTutunaruPas encore d'évaluation
- Chemical EnergeticsDocument34 pagesChemical EnergeticsNisidini JasinghePas encore d'évaluation
- Chapter 4 - 1&2 PROCESSS CHEMISTRYDocument62 pagesChapter 4 - 1&2 PROCESSS CHEMISTRYNurakmal SyuhAdaPas encore d'évaluation
- Thermochemistry: Solutions To ExercisesDocument38 pagesThermochemistry: Solutions To ExercisessandrakristikPas encore d'évaluation
- Enthalpy of Neutralisation of Water Temperature ProbeDocument7 pagesEnthalpy of Neutralisation of Water Temperature ProbeSharanya SrinivasanPas encore d'évaluation
- 1422 Chapt 15 Thermodynamics - Good - NotesDocument40 pages1422 Chapt 15 Thermodynamics - Good - NotespatnaikdebamousumiPas encore d'évaluation
- Enthalpy and Reaction Rates NotesDocument33 pagesEnthalpy and Reaction Rates NotestausmanPas encore d'évaluation
- ThermochemistryDocument5 pagesThermochemistryjoelsantos1981Pas encore d'évaluation
- Thermochemistry or Junior IntermideateDocument48 pagesThermochemistry or Junior IntermideateEvs GoudPas encore d'évaluation
- 1422 Notes Full 2010Document365 pages1422 Notes Full 2010Sreedevi KrishnakumarPas encore d'évaluation
- Thermodynamics 0 PDFDocument11 pagesThermodynamics 0 PDFRajeev KaushikPas encore d'évaluation
- Ap Chemistry Unit 5 Notes - ThermodynamicsDocument6 pagesAp Chemistry Unit 5 Notes - Thermodynamicsapi-336799605Pas encore d'évaluation
- Electrolytic Gas: D R A F TDocument16 pagesElectrolytic Gas: D R A F TAugustine JohnPas encore d'évaluation
- Chapter 19 Chemical ThermodynamicsDocument8 pagesChapter 19 Chemical ThermodynamicsRSLPas encore d'évaluation
- 5.1 EnergeticsDocument8 pages5.1 EnergeticsEldin EnggPas encore d'évaluation
- CPC Unit IvDocument50 pagesCPC Unit IvJAVAGAR M 20CHR023Pas encore d'évaluation
- Energy BalancesDocument16 pagesEnergy BalancesEliot Kh100% (1)
- Topic 8 First Year MCAT Thermochemistry R10Document32 pagesTopic 8 First Year MCAT Thermochemistry R10Khubaib KhanPas encore d'évaluation
- Thermodynamics AllDocument141 pagesThermodynamics AllSidharth AryaPas encore d'évaluation
- Chapter 03 Thermodynamics PDFDocument101 pagesChapter 03 Thermodynamics PDFPutri Nur Aisyah Halmy AzamPas encore d'évaluation
- Ch06 SlidesDocument73 pagesCh06 SlidesbeelzeburtonPas encore d'évaluation
- Important Questions For CBSE Class 11 Chemistry Chapter 6Document14 pagesImportant Questions For CBSE Class 11 Chemistry Chapter 6Avinash KumarPas encore d'évaluation
- Thermo ReviewDocument8 pagesThermo ReviewJosh CohenPas encore d'évaluation
- Chemical EnergeticsDocument64 pagesChemical Energeticsirnihafizan6812Pas encore d'évaluation
- CO3 ThermodynamicsDocument72 pagesCO3 ThermodynamicsRonna IturaldePas encore d'évaluation
- ThermochemistryDocument13 pagesThermochemistryDrazelle PangilinanPas encore d'évaluation
- MIT ThermoDocument6 pagesMIT ThermoTinray ReyesPas encore d'évaluation
- Metallurgical Physical ChemistryDocument45 pagesMetallurgical Physical ChemistryAlvin Garcia PalancaPas encore d'évaluation
- Chapter 5 - ThermochemistryDocument54 pagesChapter 5 - ThermochemistryVarunesh MauthialaganPas encore d'évaluation
- 5 Thermochemistry: ChangesDocument53 pages5 Thermochemistry: ChangesPrashant AchariPas encore d'évaluation
- AlkanolamideDocument5 pagesAlkanolamidebellesuperPas encore d'évaluation
- Fire Fighting Foam Principles and Ethanol-Blended FuelDocument38 pagesFire Fighting Foam Principles and Ethanol-Blended FuelFrancois HamiauxPas encore d'évaluation
- Jacketed Vessel Design CalculusDocument4 pagesJacketed Vessel Design Calculusmaspiqdo100% (1)
- BNR Process BendigoDocument6 pagesBNR Process Bendigobansa79Pas encore d'évaluation
- Fundamentals of Anatomy and Physiology 4th Edition Rizzo Test BankDocument14 pagesFundamentals of Anatomy and Physiology 4th Edition Rizzo Test BankGeorgeCobbjgbcs100% (16)
- Ishrae PPT On RefrigerantDocument9 pagesIshrae PPT On RefrigerantJigar ShahPas encore d'évaluation
- Microbiology With Diseases by Body System 5th Edition Bauman Test BankDocument25 pagesMicrobiology With Diseases by Body System 5th Edition Bauman Test BankRhondaHogancank100% (50)
- Metglas - Powerlite PDFDocument5 pagesMetglas - Powerlite PDFSubramaniam AravinthPas encore d'évaluation
- D 3969 - 85 r94 - Rdm5njktodvsotqDocument3 pagesD 3969 - 85 r94 - Rdm5njktodvsotqjorge armandoPas encore d'évaluation
- Plate Girders - I: ©teaching Resource in Design of Steel Structures IIT Madras, SERC Madras, Anna Univ., INSDAGDocument38 pagesPlate Girders - I: ©teaching Resource in Design of Steel Structures IIT Madras, SERC Madras, Anna Univ., INSDAGArnoldo OlivaPas encore d'évaluation
- 0654 IGCSE Formulae (Equations)Document3 pages0654 IGCSE Formulae (Equations)BigBoiPas encore d'évaluation
- EP 1108 Photoelectric EffectDocument12 pagesEP 1108 Photoelectric EffectAryam SharmaPas encore d'évaluation
- Pre-Lab 8Document1 pagePre-Lab 8SaulS.DiazPas encore d'évaluation
- Analysis Synthesis and Design of Chemical Processes 3rd Edition Turton Solutions ManualDocument21 pagesAnalysis Synthesis and Design of Chemical Processes 3rd Edition Turton Solutions ManualdarrenrichncogbpizjkPas encore d'évaluation
- Polyaluminium Chloride: Product SpecificationsDocument2 pagesPolyaluminium Chloride: Product SpecificationsMonica Choi SeungjunhyungPas encore d'évaluation
- 8 B 829 B 1 Af 50 Aeb 45 D 91 DDocument6 pages8 B 829 B 1 Af 50 Aeb 45 D 91 Dapi-400268497Pas encore d'évaluation
- Introduction To Micro/Nano ElectronicsDocument51 pagesIntroduction To Micro/Nano ElectronicsLIAKMANPas encore d'évaluation
- Bamboo+Alumina CompositeDocument5 pagesBamboo+Alumina CompositeAmmineni Syam PrasadPas encore d'évaluation
- Thermoelectric Cooling ModulesDocument8 pagesThermoelectric Cooling ModuleshabteabPas encore d'évaluation
- Gas Arc Welding - GMAW and GTAW Gas Arc Welding - GMAW and GTAWDocument3 pagesGas Arc Welding - GMAW and GTAW Gas Arc Welding - GMAW and GTAWJulioPas encore d'évaluation
- DOT Pipeline RepairsDocument18 pagesDOT Pipeline RepairsSeng HeangPas encore d'évaluation
- A Research Project Submitted To The: DR - Naga Rathna SupriyaDocument6 pagesA Research Project Submitted To The: DR - Naga Rathna Supriyamansi bodaPas encore d'évaluation
- KIMO HQ210 BrochureDocument4 pagesKIMO HQ210 BrochurebolsjhevikPas encore d'évaluation
- Carbohydrates WorksheetDocument4 pagesCarbohydrates WorksheetNatalie Pemberton86% (7)
- 1.7 Evaporative Air Cooling EquipmentDocument8 pages1.7 Evaporative Air Cooling EquipmentRio BananPas encore d'évaluation
- Chemical Engineering CommunicationsDocument16 pagesChemical Engineering CommunicationsMichelle Quilaqueo NovoaPas encore d'évaluation
- Chapter 3 Propteries of Water HWDocument2 pagesChapter 3 Propteries of Water HWapi-521773978Pas encore d'évaluation
- Safety Data Sheet Dated 27/11/2012, Version 1Document9 pagesSafety Data Sheet Dated 27/11/2012, Version 1Radu JunePas encore d'évaluation
- Epri Chemical Cleaning PDFDocument50 pagesEpri Chemical Cleaning PDFARSALAN GOPALPas encore d'évaluation
- Elements and Compounds PowerPointDocument19 pagesElements and Compounds PowerPointRelayer 66Pas encore d'évaluation
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeD'EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeÉvaluation : 4 sur 5 étoiles4/5 (1)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincD'EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincÉvaluation : 3.5 sur 5 étoiles3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeD'EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeÉvaluation : 5 sur 5 étoiles5/5 (4)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (5)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyD'EverandSodium Bicarbonate: Nature's Unique First Aid RemedyÉvaluation : 5 sur 5 étoiles5/5 (21)
- Process Plant Equipment: Operation, Control, and ReliabilityD'EverandProcess Plant Equipment: Operation, Control, and ReliabilityÉvaluation : 5 sur 5 étoiles5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolD'EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolPas encore d'évaluation
- Chemistry for Breakfast: The Amazing Science of Everyday LifeD'EverandChemistry for Breakfast: The Amazing Science of Everyday LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (90)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilD'EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilÉvaluation : 5 sur 5 étoiles5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableD'EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableÉvaluation : 3.5 sur 5 étoiles3.5/5 (22)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsD'EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsÉvaluation : 4 sur 5 étoiles4/5 (146)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireD'EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireÉvaluation : 4 sur 5 étoiles4/5 (129)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsD'EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsÉvaluation : 5 sur 5 étoiles5/5 (3)
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersD'EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersPas encore d'évaluation
- Chemistry: a QuickStudy Laminated Reference GuideD'EverandChemistry: a QuickStudy Laminated Reference GuideÉvaluation : 5 sur 5 étoiles5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsD'EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsPas encore d'évaluation
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideD'EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuidePas encore d'évaluation
- Bioplastics: A Home Inventors HandbookD'EverandBioplastics: A Home Inventors HandbookÉvaluation : 4 sur 5 étoiles4/5 (2)
- Water-Based Paint Formulations, Vol. 3D'EverandWater-Based Paint Formulations, Vol. 3Évaluation : 4.5 sur 5 étoiles4.5/5 (6)