Académique Documents
Professionnel Documents
Culture Documents
14 Revision Notes Endocrinology
Transféré par
emmapopeDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
14 Revision Notes Endocrinology
Transféré par
emmapopeDroits d'auteur :
Formats disponibles
14. Endocrinology 14.1 Principles of endocrinology 14.1.
1 Principles of hormone action
See revision table sheet 1 Hormones can act through several types of receptors: o GPCRs or G-protein coupled receptors have receptors on the outside of the cells that stimulate the binding of GTP in the place of GDP to different G proteins (GTP activated proteins). The G-proteins have three subunits: , and which all have different effects. Gs proteins stimulate the subunit which then phosphorylates adenylate cyclase causing an increase in intracellular cAMP. This then phosphorylates protein kinase A which phosphorylates further proteins to have a very varied effect (e.g. -adrenergic and LH receptors). Gi proteins inhibit adenylate cyclase and are used by somatostatin receptors. Gq proteins stimulate the activity of phospholipase C to convert PIP2 to IP3 and PAG. IP3 then increases the amount of intracellular calcium that is mobilised which has a number of effects including: binding to calmodulin which then activates other processes in the cell (e.g. myofilament contractions (myosin light chain kinase), calmodulin-dependent protein kinases (CaM kinase II which is multifunctioning involved in neurotransmitter release, transcription factor release and glycogen metabolism)) and acting upon calcium dependent processes e.g. protein kinases and exocytosis. o Ion channel linked proteins e.g. nicotinic Ach receptors. o Enzyme linked proteins tyrosine kinase-linked e.g. insulin, GH receptors. Ligand binds to the receptor stimulating autophosphorylating of part of the receptor, causing the activation of the enzyme. Often phosphorylate TFs e.g. GH cascade involves JAK and STAT activation. o Intracellular receptors linked to gene expression receptors are in the cytoplasm or nucleus and control gene expression e.g. thyroid and steroid receptors. They contain DNA binding zones (zinc fingers) and hormone binding sites. The zinc fingers interact with hormone-response elements (HREs) on the gene. Most of the pathways are inactivated by the removal of the stimulating signal but others are switched off by internalisation of the receptor-ligand complex for processing and recycling, desensitisation of the receptors (binding of GTP to GPCRs allows the subunit to phosphorylate the tail of the receptor, allowing it to bind to arrestin which desensitises the receptor despite the continued presence of the hormone stimulus. This is then reversed by phosphatises. Cholera toxin covalently modifies Gs so that it cant hydrolyse GTP and so becomes permanently inactivated. This causes continuous intestinal secretion, leading to diarrhoea and dehydration. Pertussis toxin (whooping cough cause) inactivates Gi unit by stabilising the GDP-bound form which is inactive. This removes the inhibitory effect on adenylate cyclase, increasing cAMP and causing hypoglycaemia. It also stops the immune system functioning correctly.
14.1.2 Main regulatory roles of hormones
14.1.3 Characteristics of main classes of hormone 14.2 Pituitary Gland
14.2.1 Components of pituitary
Posterior lobe comes from diencephalon (group of cells from bottom of embryological brain) which grows down, pulling neurons with it. Anterior lobe grows up from Rathkes pouch from the ectoderm of the roof of the mouth. Once it reaches the posterior lobe it detaches and becomes connected to the hypothalamus by a capillary portal system. Adenohypophysis is the anterior lobe made of somatotrophs, thyrotrophs, corticotrophs, gonadotrophs and lactotrophs. also contains folliculo-stellate cells which are non-secretory glial-like cells. Neurohypophysis is the posterior lobe and produces ADH and oxytocin. Adenohypophysis action is controlled by the release of specific hormones from the hypothalamus into the capillary portal systems. Also controlled by negative feedback of the hormones it produces.
14.2.2 hormones of the Adenopophysis 14.2.2.1 TSH/Thyroid Stimulating Hormone/Thyrotrophin
Glycoprotein made of an subunit common to TSH, FSH and LH and a subunit which is specific. It promotes thyroid gland growth, iodine uptake and the synthesis and secretion of thyroid hormones. Receptors are found in the thyroid gland and cause the activation of adenylyl cyclase which ends in the generation of cAMP Release is stimulated by TRH (thyroid releasing hormone) from the hypothalamus (released in response to cold and stress) and inhibited by somatostatin (injection blunts early morning spike). Is also controlled by neurosecretions e.g. dopamine physiologically inhibits TSH secretion. Released in a diurnal cycle rhythm.
14.2.2.2 ACTH/Adrenocorticotrophic hormone/Corticotrophin
Polypeptide produced by the cleavage of its precursor pro-opiomelanocortin/POMC. The other part that is cleaved from this molecule is MSH (melanocyte stimulating hormone). Receptors are GPCRs coupled to adenylyl cyclase so ACTH release causes a rise in cAMP which stimulates adrenal cortical steroid secretion and growth of the organ. Mostly affects glucocorticoid production with some increase in the adrenal sex steroids. There is hypothalamic control via CRH (corticoid releasing hormone) release in response to hypoglycaemia and stress and also negative feedback from ACTH. Released in pulses in a diurnal rhythm.
14.2.2.3 Lutenising hormone and Follicle stimulating hormone (gonadotrophins)
Both are glycoproteins with the same subunit but a different subunit. FSH stimulates follicle development and ovulation (see 13.3). LH stimulates progesterone secretion by the corpus luteum.
Both act by raising cAMP in the target cells. In men, FSH acts on Sertoli cells to initiate and maintain spermatogenesis whilst LH stimulates interstitial cells (of Leydig) to secrete testosterone. Released in pulses with cyclical variation through the menstruation cycle. Release is stimulated by hourly pulses of GnRH (Gonadotrophin releasing hormone) from the hypothalamus. Inhibited by oestrogen/testosterone by negative feedback. Switches to positive feedback at ovulation to give an LH surge. Ovarian peptides (inhibin and follistatin) inhibit FSH release. Hypergonadotrophinism can cause precocious puberty whilst hypog. Can cause infertility due to a lack of spermatogenesis.
14.2.2.4 Prolactin/mammotrophin
A protein made in Lactotroph cells which matches the receptor tyrosine kinase found in the breast. It promotes growth and development of breast and milk production Is inhibited by the release of dopamine by the hypothalamus, stimulated by circulating oestrogen, stimulated by suckling and during pregnancy. There is a large increase in the number of Lactotroph cells so that the pituitary almost doubles in size during pregnancy. Hypersecretion can be caused by a tumour (prolactinomas) and result in galactorrhoea, infertility (infertility stops the production of a new foetus whilst the first is still suckling) and impotence. Dopamine agonists (e.g. bromocryptine) are used to suppress PRL release.
14.2.2.5 Growth hormone/somatotrophin
Protein that targets tyrosine kinase receptors. Promotes protein synthesis, raises blood glucose and increases growth of long bone and soft tissue (via direct and indirect IGF mechanisms). IGFs are produced in the liver. GHs actions on metabolism is complex and works to promote amino acid uptake by the liver and muscle and so promotes protein synthesis. A chronic increase in GF will be anti-insulin and increase blood glucose. It also switches metabolism away from glucose and towards fatty acid oxidation so is important in prolonged starvation. Pulsatile release every 4 hours and on entering deep sleep. The hypothalamus releases GHRH in response to hypoglycaemia, stress and exercise and causes and increase in GH release. Somatostatin also inhibits GH release. GH insufficiency causes dwarfism and excess causes gigantism in children and Acromegaly in adults.
14.2.3 Hormones of the Neurohypophysis 14.2.3.1 ADH/vasopressin
A peptide of 9 amino acids which controls body fluid volume and osmoregularity (see 11.3.4). acts upon V2 receptors in the kidney which are GPRC to cAMP and V1 which are PLC-coupled receptors in the vasculature. The second set work to constrict peripheral arterioles and veins. Controlled by osmoreceptors which can sense blood pressure, volume and osmolarity.
Diabetes insipidus can be caused by an irregularity in ADH action. Hypothalamic DI is caused by a lack of ADH production (e.g. due to a tumour suppressing the action of the cells). Renal DI is caused by a lack of kidney function (e.g. due to a mutation in the receptors of the kidney).
14.2.3.2 Oxytocin
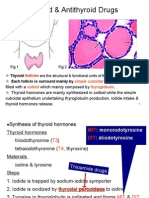
Has a role in parturition and milk-ejection. 14.3 Thyroid Gland and Iodothyronines, Calcitonin Thyroid diverticulum forms from the midline of the mouth floor between the 1st and 3rd brachial arch components of the tongue. Grows caudally over developing larynx to the anterior aspect of the trachea. Then associates with parathyroids which develop from the 3rd and 4th pharyngeal pouches and the neural crest cells that develop into the parafollicular C cells (calcitonin cells). Has two lateral lobes and a central isthmus. Most of the tissue along the developmental line disappears but some can remain resulting in ectopic thyroid tissue being found in the tongue. The isthmus is the central part of the thyroid found between the 2nd and 4th rings of the trachea and the lateral lobes extend up on either side of the trachea and larynx. Its enclosed in a pre-tracheal fascia which anchors it to the airway resulting in it moving when swallowing occurs. Big arterial and venous blood supply via system in diagram to right. Microstructure: made of circles of follicular cells around a colloid. Comes up pink on an H and E stain with the surrounding cells showing up due to purple staining nuclei. Active follicles have tall cells and small colloids with spaces where vesicles have released thyroglobulin back into the follicular cells where it is converted into T3 and t4. C cells (parafollicular cells) that release calcitonin are located at the base of the follicle epithelium. Calcitonin lowers plasma calcium The hypothalamus releases TRH in response to an increase in plasma glucose and cold. This then stimulates the release of TSH by the pituitary gland. This then travels in the blood supply to the thyroid to stimulate the release of T3 and T4. Also a negative feedback of T3 and T4 on TSH production. An Na/I symporter on the basal follicular membrane traps iodine from the plasma into the follicle where it is then used to make thyroglobulin. These are NIS and there are pendrine on the apical surface which pump I into
the colloid. This means that there is a very large store of I in the colloid to smooth out T3 and T4 production when I is low in the blood. Thyroperoxidase (TPO) is found on the apical membrane and this is the enzyme that converts thyroglobulin into T3 and T4 Production: Transport I- into cell via NIS, transport into colloid by pendrine and then convert I- to I2 and iodinate thyroglobulin by TPO. The molecule is iodinated at its tyrosine residues for storage. The colloid is then exocytosed by a TSH mediated process and proteases in lysosomes digest the thyroglobulin to produce T3 and T4 from the combined iodinated tyrosine residues. TSH stimulates TH release but also stimulates thyroid growth by binding to a GPCR linked to adenylyl cyclase which ends up activating PKA which phosphorylates transcription factors, making them stimulate DNA replication and so cell growth. T3 and T4 are transported around the body in the blood bound to certain proteins that then give them a longer half life than expected. These include thyronine-binding globulin (TBG) (higher affinity for T4 than T3), transthyretin (lower affinity) and albumin (very low affinity but high capacity). This results in T4 having a longer half life despite it being the inactive form. Peripherally, T4 is degraded to T3 by type 1 and 2 deiodinase and rT3 by type 3. Type 1 is located in the liver and kidneys and provides T3 for the blood plasma. Type 1 can also convert it to the inactive rT3 form to be cleared from the body. Type 2 is found in target tissues like the brain, adipose and pituitary and provides intracellular T3. Type 3 inactivate both T3 and T4. Iodothyronines are cleared through the kidneys as thyronine after deiodination and the iodine is salvaged for reuse by the kidneys. Deiodinases are found throughout the body in different forms: D1 and 2 produce T3 and D3 produces rT3 or T2 for inactivation and removal from the body. Thyroid hormones either work by interacting with certain enzymes, glucose transport and mitochondrial proteins, or by T3s interactions with nuclear receptors which act on response elements (TREs) which are found in gene promoter regions. Works by stimulating or inhibiting the production of different mRNAs. Sensitivity to T3 is regulated via a number of nuclear receptors. T3 actions: o Metabolic: increase protein breakdown (but RNA polymerase I and II is also stimulated, leading to an increase in protein synthesis initially), glycogenolysis, gluconeogenesis, cholesterol breakdown and lipolysis. This results in there being more substrate available for respiration. o Cardiovascular: increase cardiac output, rate and force by increasing production of myosin, beta1 receptors and Ca2+ ATPase, thus increasing contractility. o Developmental: essential for postnatal growth of CNS (stimulates production of myelin, neurotransmitters and axonal growth), bone (linear growth by chondrocyte activity) and hair, teeth and epidermis. o Others: stimulates gut mobility, increases skeletal muscle activity and increases betaadrenergic sympathetic stimulating of the heart, adipose tissue, skeletal muscle and the liver. Abnormal thyroid activity: Goitre could be caused by: o Iodine deficiency leads to lower levels of T3 and T4 secretion which means a lack of negative feedback on the hypothalamus and pituitary leading to high levels of TRH and TSH. If the enlarged thyroid then traps enough iodine then T3 and T4 levels can be normal despite the goitre.
o Graves disease when autoimmune antibodies attach to TSH receptors permanently activating them, leading to very high TH levels. o Tumour which may be functioning or non-functioning. Hyperthyroidism causes a raised BMR, raised heart rate and cardiac output, heat intolerance, weight loss despite a large appetite, increased sympathetic drive. And eye protrusion. Can be caused by graves disease or a tumour. Hypothyroidism is called cretinism which leads to mental retardation and stunting of postnatal growth if not treated correctly. It is called myxoedema in adults and results in a reduced BMR, slow mentation, hypothermia and constipation. Can be caused by many different things: o Mutation in the gene for NIS, pendrine, TPO or thyroglobulin. o Tumour in the thyroid or the pituitary (can be distinguished diagnostically by looking at TSH levels which will be low if the pituitary is affected or high if the thyroid is the problem). o Mechanical damage e.g. from a car accident o Mutation in the gene for thyroid, TSH or TRH receptors. o Damage to capillary system that transports TRH. o Iodine deficiency o Failure in development e.g. due to a mutation in the thyroid transcription factor (most common cause of cretinism in the UK) o Autoimmune destruction (most common) o With a MCT8 mutation, T3 is unable to enter cells leading to cretinism despite normal levels of thyroid hormones. o Burnt out Graves disease: if the disease is untreated, the thyroid is overworked and will eventually shut down. Treatment: hypothyroidism is treated by hormone injections (called thyroxin or T4) at different times of the day with hyperthyroidism being treated using radioactive iodide or surgery. Euthyroid sick syndrome is when there are abnormal levels of thyroid hormones (usually low T3 and T4 and high TSH and rT3) despite there being no disease in the thyroid gland. This can be due to many chronic and acute diseases such as GI, cardiovascular and pulmonary diseases which all affect the metabolic rate and stress exerted on the body and so the feedback system that controls TRH output.
14.4 Parathyroid Glands
Secrete parathyroid hormone (PTH) which is secreted in response to low plasma [Ca2+]. It is the principal control of plasma calcium and so is essential to life. PTH secreting tumours cause raised plasma calcium, bone destruction and urinary stones. There are four glands found posterior to the thyroid gland which come from neural crest mesenchyme and the third and fourth brachial arches (superior pouches from the 4th and inferior from the 3rd and swapped over in embryogenesis meaning that both are supplied by the inferior thyroid artery).
14.5 Adrenal Gland
The medulla is derived from neural crest cells (like autonomic ganglion cells) whilst the cortex comes from the intermediate mesoderm (involves the genes
Wilms tumour (WT1) and steroidogenic factor 1 (SF1)). The fetal adrenal zone is very prominent and is made up of two sections: the fetal and definitive zones. The second is very similar to the adult adrenal gland whilst the foetal zone produces weak androgens which the placenta then aromatizes to make oestrogen. The adrenal medulla is made of groups of chromaffin cells packed with catecholamine-containing cells which store large amounts of adrenaline and noradrenaline. The adrenal cortex is made up of three layers of types of cells arranged in balls and sheets interspersed with capillaries: the zona glomerulosa, fasciculate and reticularis. The adrenal gland is well vascularised by branches from the phrenic artery, the renal arteries and direct from the aorta. They drain into a single adrenal vein in each gland which then drains into the inferior vena cava on the right or the left renal vein. These blood vessels dilate under stress unlike all other blood vessels. The chromaffin cells are innervated by thoracic preganglionic sympathetic nerves that release Ach onto nicotinic receptors.
14.5.1 Adrenal Medulla
This is found in the centre of the organ and is responsible for producing adrenaline and noradrenaline which are then stored in chromaffin granules and released in response to acute stress. In the cytoplasm of the chromaffin cells, tyrosine is converted into DOPA by tyrosine hydroxylase, DOPA to dopamine by DOPA decarboxylase. The dopamine is then pumped into vesicels whre it is converted to NA by dopamine hydroxylase and the NA is then stored or diffuses out of the vesicles for conversion to adrenaline (80% of total) by pheynylethanolamine-N-methyl transferase (PNMT) in the cytoplasm of adrenaline secreting cells. Adrenaline is then pumped into vesicles for storage and release. When cortisol is released it favours the production of adrenaline over NA. Despite its role in producing adrenaline, the adrenal medulla only supplies 10% of the sympathetic response to stress so isnt vital to survival. There are many different catecholamine receptors (see 6.4.4.2) and so adrenaline has many different effects: o Cardiovascular: increases heart rate and stroke volume, increases mean arterial pressure, constricts blood vessels to most of abdomen (except adrenal gland) and dilates blood vessels to skeletal muscle. o Pulmonary: relaxes smooth muscle in respiratory tract and increases respiratory rate by effects of CNS. o Abdomen: contracts sphincter muscles in GI tract, relaxes bladder muscle wall, inhibits peristalsis. o Metabolism: increases metabolic activity, in liver it promotes glycogenolysis, gluconeogenesis and the release of glucose into the circulation; in skeletal muscle it promotes glycogenolysis and lactic acid formation; and in fat it stimulates lipolysis . o CNS: causes arousal via actions in the brainstem and this causes coarse tremor. Phaeochromocytoma is a tumour of the adrenal medulla and can secrete catecholamines causing hypertension, tremor, anxiety and a forceful heartbeat. If the medulla has to be removed to treat the cancer, the rest of the sympathetic system will compensate for it to cover the loss.
14.5.2 Adrenal cortex
The cortex is responsible for the response to prolonged stress so produces glucocorticoids (cortisol) and mineralcorticoids (aldosterone) as well as adrenal androgens from cholesterol. The cholesterol is stored in lipid droplets in all the cortex cell types, is mobilised by ACTH stimulation and is transported into specialised mitochondria by steroid acute regulatory (STAR) protein. The rate limiting step is the conversion of cholesterol into pregnenolone in the mitochondria. The enzymes necessary for the rest of the steps are found in the mitochondria and smooth endoplasmic reticulum, so the cortex cells have a lot of these organelles. The cortisol produced is transported in the blood by cortisol-binding globulin which has a high affinity and albumin which has a low affinity since it binds all steroids so is less efficient with specific molecules. Aldosterone doesnt have its own specific high affinity protein so all of it is transported by albumin and so has a shorter half life than cortisol does. The kidney filters out steroid hormones but is able to reabsorb 90% so to remove them from the system, the liver converts them to hydrophilic metabolites by hydroxylation and conjugation reactions which can then be urinated out. This is why cirrhosis causes a cortisol build up. Steroid hormones work by binding to receptors in the cytoplasm of cells which then migrate into the nucleus to regulate gene transcription and this is why they are long acting. Some are already in the nucleus. Congenital adrenal hyperplasia is a condition where the steroid synthesis is interrupted and the effects depend upon the point of interruption. For example, in the most common type (1/4500), 21hydroxylase action is interrupted so progesterone cant be converted to make cortisol or aldosterone. This not only means that the body is less able to cope with prolonged stress but that there is an excess of testosterone and other androgens secreted due to the build up of progesterone, causing precocious puberty in males and masculinisation of females. Congenital adrenal hypoplasia is a very rare condition where there is an inherited unresponsiveness to ACTH resulting in low cortisol and high ACTH. Addisons disease is a genetic condition where the T-cell community is abnormal meaning that they attack the bodys own cells. Addisons is usually accompanied by other autoimmune conditions. It results in the general symptoms of low cortisol etc including weakness, fatigue, nausea, anorexia and hypotension as well as hyperpigmentation due to the overproduction of ACTH also meaning an overproduction of MSH. Adrenoleukodystrophy is an X-linked condition where there is an abnormally high level of VLCFAs which build up in the brain, adrenals and liver resulting in congnitive dysfunction, behavioural problems, visual and gait disturbances, adrenal insufficiency (this often presents first so young men presenting with adrenal insufficiency are always screened for ALD) and infertility.
14.5.2.2 Cortisol
Produced by the zona fasciculata with receptors present in almost all cells. The glucocorticoid receptors are in the cytoplasm and when activated will them migrate into the nucleus. When inactivated in the liver it is converted to cortisone. Cortisol release is stimulated by CRF stimulating ACTH which then raises cAMP levels in the medulla and this happens within seconds though it doesnt have an effect for a couple of hours. Actions:
o Protects the body in prolonged stress so as to preserve glucose for the brain. o Stimulates the metabolism of carbohydrates (glycolysis) and so opposes insulins actions, lipids (lipolysis and ketogenesis) and proteins (protein breakdown and gluconeogenesis). This is all done through the stimulation of production of the enzymes involved. o Maintains the circulation by increasing heart contractility and increasing vascular tone. o Maintains plasma volume by preventing increased capillary permeability. o Maintains ability of skeletal muscles to produce prolonged contractions. o Promotes Na+ retention so excess cortisol can lead to too little water being excreted out of the body. o Suppresses REM sleep o Increases surfactant production in the lungs. o Increases haemopoesis o Inhibits leukocyte translocation from blood to sites of tissue damage, stimulates lymphocyte destruction. As a result, glucocorticoid selective drugs are used to treat inflammatory diseases such as asthma. o Inhibits reproduction and lactation.
14.5.3 Aldosterone
Aldosterone is produced in the zona glomerulosa and its receptors are only present in the nuclei of a few cell types (including kidney collection tubule epithelia, salivary and sweat glands, colon and some brain neurons). Cortisol also binds to mineralcorticoid receptors so in aldosterone targets, cortisol is inactivated by 11-hydroxsteroid dehydrogenase to protect the MRs from normal cortisol levels. Aldosterone regulates ion transport and so water retention in the kidney collecting tubules by stimulating Na+ reabsorption in exchange for potassium, hydrogen and ammonium ion secretion. There is a 2hr lag in response to aldosterone secretion whilst Na/K ATPase transcription is increased. In the glands and colon aldosterone also causes the retention of sodium. Aldosterone is stimulated by the renin-angiotensin system. This system is stimulated by low plasma sodium or low renal blood pressure. These stimulate the juxtaglomerular apparatus (JGA, distal tubule and mesangial cells) to release rennin which breaks down plasma angiotensinogen to produce angiotensin 1 which is then converted to angiotensin II by ACE. AII stimulates the release of aldosterone, arteriolar constriction and drinking in response to thirst. High plasma potassium can also stimulate aldosterone release and sympathetic activation can stimulate renin secretion by the JGA. Hyperaldosteronism results in sodium loss, low blood volume and low blood pressure. Hypoaldosteronism results in the opposite and is called Conns syndrome. Spronolactone can therefore be used as an anti-hypertensive since it is an aldosterone antagonist and so can ACE inhibitors. Very high levels of cortisol can also mimic this condition since this will overwhelm the deactivating enzyme and so reach the MRs.
14.5.2.4 Adrenal Androgens
These are usually a relatively small secretion of the gland but are relatively important in puberty to stimulate hair development and libido, but are most important in a foetus, where DHEA
(dehydroepiandrosterone) sulphate (as opposed to DHEA that is made in adults) is produced in copious amounts since it then travels to the placenta where it is converted into oestrogen.
14.6 Endocrine Pancreas
Composed of a head, body and tail which are supplied by the coeliac artery which then turns into the splenic artery as it enters the pancreas. Develops from the endoderm of the gut tube (not from the neural crest cells as some text books say). Activin and FGF-2 signals from local notochord suppress Shh from the endoderm which allows the expression of factors needed for pancreas formation. Abnormal proliferation (nesidioblastosis) causes neonatal hypoglycaemia. The islets of Langerhans develop from the endoderm of the pancreatic buds. There are 1 million islets embedded in the exocrine gland tissue. A rich arterial supply enters the centre of the islet with a venous drainage system to the liver via the hepatic portal vein. This means that the liver gets a much higher dose of the pancreatic hormones than the rest of the body and this can cause problems with diabetes treatment etc since in that instance there is an equal concentration of the hormone across the whole body. Also a rich sympathetic and parasympathetic supply. The islets of Langerhans are made up of 60% central cells (insulin), 15% peripheral cells (glucagon), 10% scattered cells (somatostatin) and 15% pancreatic polypeptide cells in the ventral part of the pancreas head.
14.6.1 Insulin
Insulin has the general effect of promoting anabolic reactions by: increasing glucose and amino acid uptake, suppressing glycogenolysis, suppressing lipolysis and proteolysis and supporting growth and proliferation. It is synthesised as proinsulin which is then broken down by proteolysis to form C peptide and insulin. It is released in response to high blood glucose or amino acid concentrations, GI hormones (e.g. Glucagon-like peptide/GLP which are released in response to nutrients like carbohydrates being sensed in the gut lumen. Glucose-dependent insulinotropic peptide/GIP also anticipates the arrival of more glucose. Type 2 diabetics are often not responsive to GIP. The mechanism by which glucose levels are sensed is as follows: blood glucose rises, more glucose is co-transported with Na+ into the cells through Glut2 proteins, more glucose is metabolised, more ATP produced, ATP binds to K+ channels and Na+/K+ pumps to inhibit K+ current, depolarises membrane, opens Ca2+ channels, Ca2+ bids to insulin granules and causes exocytosis. Sulphonylureas are a class of drugs used to treat some Type 2 diabetics and neonatal diabetes and works by The receptor that detects insulin is called tyrosine kinase which has two peptide tails inside the cell that cross phosphorylate each other (this is why any mutations in this gene are dominant) for activation. They then phosphorylate other molecules which set off cascade reactions e.g. phosphorylate Glut4 proteins which migrate to the cell membrane and so increase glucose transport into the cell. Insulinoma is a tumour of the beta cells. Causes unregulated release of insulin leading to hypoglycaemia.
MEN or multiple endocrine neoplasia is a class of autosomal dominant genetic conditions predisposing someone to develop multiple endocrine tumours. o MEN 1 is caused by a mutation in the MEN1 gene and usually causes tumours in the islets and the rest of the GI endocrine system, parathyroid and pituitary. o MEN 2 is mostly caused by a mutation in the RET proto-oncogene leading to phaeochromocytoma (chromaffin cells of adrenal medulla) and medullary thyroid carcinoma as well as sometimes parathyroid hyperplasia, gastrointestinal symptoms, Marfans and muscular hypotonia. Diabetes mellitus: Type I: o caused by a total loss of insulin due to an autoimmune reaction destroying cells. Treated with insulin injections which are now being developed into an artificial pancreas that will release the insulin all by itself and give more constant release to allow for better control. o 0.5% prevalence with onset usually occurring during childhood and is fatal if left untreated. o Symptoms: polyurea, thirst, dehydration, weight loss and ketosis. o If an insulin dose is missed/before the condition is diagnosed, cells are no longer able to take up glucose so switch to metabolising fats. This leads to a dangerous build up of ketone bodies which are acidic and toxic at high levels, causing ketoacidosis. Symptoms include Kussmaul breathing, dry skin and mouth, flushed face, fruity breath and vomiting. Type II: o Caused by a resistance to insulin or a disordered secretion of insulin. o 4-5% prevalence usually with mature onset and in those who are obese. o Can be caused by pancreatic amyloidosis which is when amyloid (an insoluble pleated sheet protein produced from amylin which is secreted at the same time as insulin and works to inhibit nutrient release into the blood. If the pancreas is subjected to very high levels of glucose, some of the usually soluble protein is converted into insoluble plaques instead of being passed out of the body via the kidneys as is normal.) aggregates in the pancreas causing cyotoxicity which kills the islet cells and reduces insulin production 14.6.2 Glucagon See biochem notes for detail. Polypeptide synthesized by -cells released in response to hypoglycaemia. Acts on liver via cAMP pathway to promote glycogenolysis and gluconeogenesis and promotes lipolysis in adipose tissue. Day to day glucagon stimulates glycogen release and use but during prolonged fasting it stimulates fatty acid breakdown so glucagons affects depend upon the dose. Glucagonoma causes hyperglycaemia and weight loss. Is synergistic with GH, catecholamines and glucocorticoids (cortisol opposes insulins effects so as to preserve glucose for the brain. 14.6.3 Somatostatin Paracrine peptide produced in -cells (and the stomach and intestines) which inhibits insulin and glucagon release. It also has negative effects in the gut, salivary glands and pituitary and decreases all metabolic activity in the liver. It is used to treat giantism and Acromegaly since it inhibits GH secretion.
Pandreatic polypeptide Released in response to meals and vagal stimulation (hypoglycaemia, exercise and starvation). It inhibits the secretion of pancreatic enzymes and gall bladder contraction and may suppress appetite.
Vous aimerez peut-être aussi
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5782)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Clinical Management of Thyroid Disease PDFDocument386 pagesClinical Management of Thyroid Disease PDFAnonymous ZZCCXMoRosPas encore d'évaluation
- The Thyroid and Parathyroid GlandsDocument47 pagesThe Thyroid and Parathyroid GlandsRujha Haniena Ahmad RidzuanPas encore d'évaluation
- Topic 1 Endocrine SystemDocument140 pagesTopic 1 Endocrine SystemmasdfgPas encore d'évaluation
- Thyroid Disease and Oral Health.Document6 pagesThyroid Disease and Oral Health.crookedgrange6384Pas encore d'évaluation
- Surgery 2Document27 pagesSurgery 2junaid4shaikhPas encore d'évaluation
- Graves' Disease Signs and ManagementDocument11 pagesGraves' Disease Signs and ManagementManisha Sekaran Muniandy100% (1)
- Pharmacology Tyroid and Antitiroid HormonDocument21 pagesPharmacology Tyroid and Antitiroid HormonPuterinugraha Wanca ApatyaPas encore d'évaluation
- Iodine EssayDocument3 pagesIodine Essayjflee123Pas encore d'évaluation
- Thyroid Cancer 1 Running Head: Thyroid CancerDocument9 pagesThyroid Cancer 1 Running Head: Thyroid Cancerapi-280626728Pas encore d'évaluation
- Physiology of Thyroid GlandDocument8 pagesPhysiology of Thyroid Glandأياد المشهدانيPas encore d'évaluation
- 03 Na CB GuidelinesDocument152 pages03 Na CB GuidelinesPutri Zahra ArdiyanitaPas encore d'évaluation
- Nurul Nadia Noor Azlan - Syaza Izzati Zahari: Group 84Document52 pagesNurul Nadia Noor Azlan - Syaza Izzati Zahari: Group 84Nurul NadiaPas encore d'évaluation
- DynaMed Plus - Thyroglobulin Antibody MeasurementDocument6 pagesDynaMed Plus - Thyroglobulin Antibody MeasurementGamer MadaPas encore d'évaluation
- Sodium Iodide I 131 Capsule and SolutionDocument14 pagesSodium Iodide I 131 Capsule and SolutionPham Thanh MinhPas encore d'évaluation
- Thyroid Tietz Textbook 2012 PDFDocument21 pagesThyroid Tietz Textbook 2012 PDFIvana BajunovicPas encore d'évaluation
- Analitical ErrorDocument15 pagesAnalitical Errorlabor baiturrahimPas encore d'évaluation
- Current and Emerging Autoimmune Diseases in AnimalsDocument16 pagesCurrent and Emerging Autoimmune Diseases in AnimalsMaría JesúsPas encore d'évaluation
- Laboratory Tests of Thyroid Function - Uses and Limitations PDFDocument16 pagesLaboratory Tests of Thyroid Function - Uses and Limitations PDFMansouri HichemPas encore d'évaluation
- Review: Sophie Leboulleux, R Michael Tuttle, Furio Pacini, Martin SchlumbergerDocument10 pagesReview: Sophie Leboulleux, R Michael Tuttle, Furio Pacini, Martin SchlumbergerPedro Gómez RPas encore d'évaluation
- Nejmoa 2111953Document10 pagesNejmoa 2111953Andreea VlădanPas encore d'évaluation
- QualityDocument4 pagesQualityNIGHT tubePas encore d'évaluation
- Transcript 137 Hashimotos The Root Cause With Dr. Izabella Wentz PDFDocument26 pagesTranscript 137 Hashimotos The Root Cause With Dr. Izabella Wentz PDFPopescu AndreeaPas encore d'évaluation
- Reference: Maglumi TG (Clia)Document4 pagesReference: Maglumi TG (Clia)Lidia NarbPas encore d'évaluation
- Thyroid Gland Embryology, Anatomy, and Physiology: Gerard V. Walls and Radu MihaiDocument13 pagesThyroid Gland Embryology, Anatomy, and Physiology: Gerard V. Walls and Radu MihaiKiara VelasquezPas encore d'évaluation
- Lecture Notes in Medical Technology - Lecture #5 - THYROID FUNCTION TESTSDocument14 pagesLecture Notes in Medical Technology - Lecture #5 - THYROID FUNCTION TESTSKat JornadalPas encore d'évaluation
- MTAP 100 ASSESSMENT #2 Lipids and ProteinsDocument161 pagesMTAP 100 ASSESSMENT #2 Lipids and ProteinsRoselle Joyce Arce CalubanPas encore d'évaluation
- THYROIDDocument100 pagesTHYROIDFrance PalPas encore d'évaluation
- Department of ZoologyDocument27 pagesDepartment of ZoologyAleenaPas encore d'évaluation
- Endoc, Pancreas, ThyroidDocument9 pagesEndoc, Pancreas, ThyroidKatrina Vianca DecapiaPas encore d'évaluation
- The Anatomy and Physiology of the Thyroid GlandDocument55 pagesThe Anatomy and Physiology of the Thyroid GlandMathy MtenjePas encore d'évaluation