Académique Documents
Professionnel Documents
Culture Documents
To Ah Yap
Transféré par
Tan Han XiDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
To Ah Yap
Transféré par
Tan Han XiDroits d'auteur :
Formats disponibles
kLAL CCkkCSICN kC8LLM
Galvanic corrosion
(http://en.wikipedia.org/wiki/Galvaniccorrosion)
OVERVIEW
Dissimilar metals and alloys have diIIerent electrode potentials and when two or more come into
contact in an electrolyte a galvanic couple is set up. The potential diIIerence between the
dissimilar metals is the driving Iorce Ior the accelerated attack on the anode member oI the
galvanic couple.
The electrolyte provides a means Ior ion migration whereby metallic ions can move Irom the
anode to the cathode. This leads to the anodic metal corroding more quickly than it otherwise
would; the corrosion oI the cathodic metal is retarded even to the point oI stopping. The presence
oI an electrolyte and a electronic conducting path between the metals is essential Ior galvanic
corrosion to occur.
In some cases, this reaction is intentionally encouraged. For example, low-cost household
batteries typically contain carbon-zinc cells. As part oI a closed circuit (the electron pathway),
the zinc within the cell will corrode preIerentially (the ion pathway). Another example is the
cathodic protection oI buried or submerged structures. In this example, sacriIicial anodes work as
part oI a galvanic couple, promoting corrosion oI the anode, rather than the protected subject
metal.
In other cases, such as HVAC systems with bimetallic plumbing/piping (most commonly copper
and cast iron) galvanic corrosion will contribute to accelerated corrosion rates oI the system.
Corrosion inhibitors such as sodium nitrite or sodium molybdate can be introduced to these
systems to reduce the galvanic potential. However, the application oI these corrosion inhibitors
must be monitored closely. II the application oI corrosion inhibitors increases the conductivity oI
the water within the system, the galvanic corrosion potential can be exponentially increased. pH
is also a major consideration with regards to closed loop bimetallic circulating systems. Should
the pH and corrosion inhibition doses be incorrect, galvanic corrosion will be accelerated. In
most HVAC systems, the concept oI anodes and cathodes are not an option as they would need
to be applied within the plumbing oI the system and, over time, would corrode and cause
potential mechanical damage to circulating pumps, heat exchangers, etc
(http://www.cdcorrosion.com/modecorrosion/corrosiongalvanic.htm)
Galvanic corrosion can be deIined simply as being the eIIect resulting Irom contact
between two diIIerent metals or alloys in a conducting corrosive environment. Another term
employed is galvanic coupling.
When a metal is immersed in any electrolytic solution, it is possible to measure its
dissolution (natural corrosion). For each solution, it is possible to establish a "galvanic series",
that is, a ranking oI diIIerent metals and alloys as a Iunction oI this measured potential. When
two diIIerent metals or alloys immersed in the same solution are joined together electrically, an
electric current will be set up between them, resulting Irom the short circuit created. The
coupling potential must oI necessity lie between the two potentials Ior the uncoupled metals and
an increase in corrosion is generally observed in the less noble alloy and a decrease or
suppression oI corrosion in the more noble material.
Due to modiIications in the electrolyte, inversions may occur in the potential series. Thus,
zinc covered with corrosion products can become more "noble" than iron in certain hot waters
(problem encountered in domestic hot water tanks); tin can become less "noble" than iron in
organic acid solutions (problem encountered in Iood cans).
For a given current between two diIIerent metals, the current density, and hence the rate oI
dissolution oI the less noble metal (anode,) will be greater the smaller the surIace area S
A
oI the
anode. The use oI unIavourable surIace area ratios has led to many expensive and oIten
spectacular Iailures.
(http://aluminumsurIace.blogspot.com/2009/04/corrosion-between-anodized-aluminum-
and.html)
Galvanic corrosion is due to an electrical contact with a more noble metal or a non-metallic
conductor in a conductive environment. The galvanic corrosion is very dependent oI the cathode
reaction and which metals are in contact which each other.
The eIIiciency oI this cathodic reaction will determine the corrosion rate. The most common
examples oI galvanic corrosion oI aluminium alloys are when they are joined to steel or copper
and exposed to a wet saline environment.
The galvanic corrosion oI aluminium is usually mild, except in highly conductive media such as
i.e. slated slush Irom road de-icing salts, sea water and other electrolytes. The contact area must
be wetted by an aqueous liquid in order to ensure ionic conduction. Otherwise there will be no
possibility oI galvanic corrosion.
The galvanic series Ior metals shows that aluminum will be the anode oI the galvanic cell in
contact with almost all other metals and hence the one which suIIer Irom galvanic corrosion.
The galvanic corrosion perIormance oI the diIIerent aluminum alloys is quite similar, so that
changing alloys cannot solve the problem.
There has to be an electrical contact either by direct contact between the two metals or by a
connection such as a bolt.
The dissolution rate depends on the surIace ratio between the two metals:
The most Iavourable case is a very large anodic surIace area and a small cathodic surIace area.
The galvanic corrosion is a local corrosion, and is thereIore limited to the contact zone.
It is unusual to see galvanic corrosion on aluminum in contact with stainless steel (passive). In
contrast contact between copper, bronze, brass and diIIerent kinds oI steel alloys (passive and
active) and aluminum can cause severe corrosion so it is advisable to provide insulation between
the two metals.
The reason Ior the conIusion oI the galvanic corrosion between aluminum and steel is that
stainless steel can be Iound as passive or active, and iI the environment contains chloride. This
will change the corrosion eIIect on aluminum substantially. Generally, the closer one metal is to
another in the series, the more compatible they will be, i.e., the galvanic eIIects will be minimal.
Conversely, the Iarther one metal is Irom another, the greater the corrosion will be.
Though prediction oI galvanic corrosion is not easy. For contact between common metals, in
particular steel and stainless steel, experience show that laboratory testing always leads to more
severe results than what is actually observed under conditions oI weathering.
ONDITION THAT AUSE GALVANI ORROSION
(http://www.corrosionist.com/GalvanicCorrosion.htm)
What Conditions are Needed
For galvanic corrosion to occur there are three conditions which must be met:
Condition 1. Metals must be Iar apart on the galvanic series
The galvanic or electrochemical series ranks metals according to their potential, generally
measured with respect to the Standard Calomel Electrode (S.C.E.). The results are oIten viewed
as a galvanic corrosion chart or galvanic corrosion table similar to that on the third page.
This chart says that the "anodic" or "less noble" metals at the negative end oI the series - at the
right oI this diagram, such as magnesium, zinc and aluminium - are more likely to be attacked
than those at the "cathodic" or "noble" end oI the series such as gold and graphite.
The critical point is the diIIerence in potential oI the two materials being considered as a joined
pair. A diIIerence oI hundreds oI millivolts is likely to result in galvanic corrosion, but only a
Iew tens oI millivolts is unlikely to be a problem.
Condition 2. The metals must be in electrical contact
The two diIIerent metals must be in electrical contact with each other. This is oI course very
common. The two metals can be bolted, welded or clamped together, or even just resting against
each other.
Condition 3. The metal junction must be bridged by an electrolyte
An electrolyte is simply an electrically conducting Iluid. Almost any Iluid Ialls into this category,
with distilled water as an exception. Even rain water is likely to become suIIiciently conducting
aIter contact with common environmental contaminants.
II the conductivity oI the liquid is high (a common example is sea water) the galvanic corrosion
oI the less noble metal will be spread over a larger area; in low conductivity liquids the corrosion
will be localised to the part oI the less noble metal near to the junction.
hotography
Mater|a| used
8olL and nuL sLalnless sLeel grade 316
SLeel plaLe low alloy sLeel
Lnv|ronment
In neutral and alkaline media:
(The environment in UTM which is quite high in huminity)
O
2
2H
2
O 4e- 4OH
Mechan|sm
nodlc
8olL and nuL SLalnless sLeel grade 316
CaLhodlc
SLeel plaLe mlld sLeel or low alloy sLeel
nodlc
round 06 v
CaLhodlc
round 01 v
ropose ways to contro|
There are several ways oI reducing and preventing this Iorm oI corrosion.
O One way is to electrically insulate the two metals Irom each other. Unless they are in
electrical contact, there can be no galvanic couple set up. This can be done using plastic
or another insulator to separate steel water pipes Irom copper-based Iittings or by using a
coat oI grease to separate aluminium and steel parts. Use oI absorbent washers that may
retain Iluid is oIten counter-productive. Piping can be isolated with a spool oI pipe made
oI plastic materials or made oI metal material internally coated or lined. It is important
that the spool has a minimum length oI approx 500 mm to be eIIective.
O Another way is to keep the metals dry and/or shielded Irom ionic compounds (salts,
acids, bases), Ior example by painting or encasing the protected metal in plastic or epoxy,
and allowing them to dry.
O Coating the two materials or iI it is not possible to coat both, the coating shall be applied
to the more noble, the material with higher potential. This is necessary because iI the
coating is applied only on the more active material, in case oI damage oI the coating there
will be a large cathode area and a very small anode area, and Ior the area eIIect the
corrosion rate will be very high.
O It is also possible to choose metals that have similar potentials. The more closely matched
the individual potentials, the lesser the potential diIIerence and hence the lesser the
galvanic current. Using the same metal Ior all construction is the most precise way oI
matching potentials.
Electroplating or other plating can also help. This tends to use more noble metals that resist
corrosion better. Chrome, nickel, silver and gold can all be used.
(http://en.wikipedia.org/wiki/Galvaniccorrosion)
Avoidance oI Galvanic Corrosion
(http://www.corrosionist.com/GalvanicCorrosion.htm)
The methods Ior avoidance oI galvanic corrosion are in general suggested by the above
descriptions oI the conditions necessary Ior its occurrence.
Don`t Mix Metals. II only one material is used in a construction the problem is avoided
(Condition 1 is not present no mixed metals) and Galvanic Corrosion will not take place. Be
particularly aware oI zinc plated or galvanised Ieasters in stainless steel sheets a common
substitution because oI perceived cost savings or better availability. These less noble Iasteners in
the galvanic series are likely to be rapidly attacked.
Prevent Electrical Contact. It is oIten practical to prevent electrical contact between the
dissimilar metals (removal oI Condition 2). This may be achieved by the use oI non conducting
(eg rubber or plastic) spacers, spool pieces or gaskets, perhaps in conjunction with sleeves
around bolts. For the same reason a gap may be leIt between galvanised rooIing and a stainless
steel down-pipe.
Corrosion potentials in Ilowing sea water at ambient temperature. The unshaded symbols show
ranges exhibited by stainless steels in acidic water such as may exist in crevices or in stagnant or
low velocity or poorly aerated water. The more Noble materials at the leIt side tend to be
cathodic and hence protected; those at the right are less Noble and tend to be anodic and hence
corroded in a galvanic couple.
Prevent the Wetted Junction.
The third Condition can be removed by ensuring that no electrolyte remains at the intermetallic
junction - this may require extra attention to drainage or to protection Irom the weather. A good
covering oI paint or sealant over the junction can be eIIective to avoid Galvanic Corrosion.
Use the Area EIIect to avoid Galvanic Corrosion.
The area eIIect should also be considered in avoiding corrosion damage, particularly in selection
oI Iastener materials.
Stainless steel Iasteners can be used to hold aluminium structures, but the area eIIect will not
apply iI the wetted area shrinks over time due to evaporation. In the situation oI 304 or 316
Iasteners used in conjunction with other less noble structural materials, the Iastener will be
galvanically protected by the surrounding large, less noble area.
Positively Use Galvanic Protection to avoid Galvanic Corrosion.
The galvanic eIIect can also be used to provide corrosion protection. For example it is prudent to
guard against possible crevices, perhaps associated with marine Iouling, or simply under bolt
heads, by speciIying slightly more noble bolt materials. An example is the use oI 316 Iasteners in
conjunction with 304 structural materials the minor galvanic protection aIIorded the Iasteners
improves their corrosion resistance.
The Area EIIect
The relative area oI the anode and cathode has a pronounced eIIect upon the amount oI corrosion
that occurs due to Galvanic Corrosion. A small anode (the less noble metal, such as aluminium)
joined to a large cathode (the more noble metal, such as stainless steel) will result in a high
current density on the aluminium, and hence a high rate oI corrosion.
The corrosion is concentrated by the area diIIerence. Conversely iI the area oI the anode is large
compared to that oI the cathode this dilutes the corrosive eIIect, in most cases to the extent that
no problem occurs. It is common practice to use stainless steel Iasteners to Iix aluminium
sheeting or signs, but iI aluminium screws were used to Iix stainless steel the screws may rapidly
corrode.
An apparent contradiction oI the area eIIect on Galvanic Corrosion occurs when the component
comprised oI the two metals is only partly wetted. Consider Ior instance a stainless steel bolt in
an aluminium plate; iI water collects in the corner at the edge oI the bolt but the remainder oI the
plate remains dry, the eIIective area oI the less noble aluminium is only the wetted region, which
may be only a similar size to that section oI the bolt that is wetted .... thus it is quite possible Ior
the aluminium plate to be galvanically attacked in the region immediately surrounding the bolt.
Only the wet 'area counts.
Crevices & Stagnant Conditions
As shown in the electrochemical series chart there are two diIIerent potentials associated with
each stainless steel grade. The less noble value shown in outlined boxes is that which applies iI a
crevice is Iormed between the two dissimilar metals or such as beneath bio-Iouling.
Such a crevice could be Irom the design or Iabrication oI the component, and Iormation oI
biological Iilms is more likely in stagnant or slow-Ilowing sea water. The result oI these stagnant
conditions is oxygen depletion and the less noble potential which can make the stainless steel
susceptible to corrosion in conditions that might otherwise be considered non-corrosive.
!reventing galvanic corrosion
(http://www.aluminiumdesign.net/corrosion-resistance.html)
The risk oI galvanic corrosion should not be exaggerated corrosion does not occur in dry,
indoor atmospheres and the risk is not great in rural atmospheres.
Electrical insulation
Where diIIerent metals are used in combination, galvanic corrosion can be prevented by
electrically insulating them Irom each other. The insulation has to break all contact between the
metals.
The illustration shows a solution Ior bolt joints.
Breaking the electrolytic bridge
In large constructions, where insulation is diIIicult, an alternative solution is to prevent an
electrolytic bridge Iorming between the metals. Painting is one way oI doing this. Here, it is
oIten best to coat the cathode surIace (i.e. the most noble metal).
A Iurther solution is to use an insulating layer between the metals.
athodic protection
Cathodic protection can be gained in two ways. The most common is to mount an anode oI a less
noble material in direct metallic contact with the aluminium object to be protected. The less
noble material 'sacriIices itselI (i.e. corrodes) Ior the aluminium. It is thus reIerred to as a
sacriIicial anode.
For the above to work, there also has to be liquid contact between the surIace to be protected and
the sacriIicial anode.
Zinc or magnesium anodes are oIten used Ior aluminium.
Another way oI obtaining cathodic protection is to connect the aluminium object to the negative
pole oI an exterior DC voltage source.
The illustration below shows the cathodic protection oI an outboard motor.
back to top
!itting
For aluminium, pitting is by Iar the most common type oI corrosion. It occurs only in the
presence oI an electrolyte (either water or moisture) containing dissolved salts, usually chlorides.
The corrosion generally shows itselI as extremely small pits that, in the open air, reach a
maximum penetration oI a minor Iraction oI the metal`s thickness. Penetration may be greater in
water and soil.
As the products oI corrosion oIten cover the points oI attack, visible pits are rarely evident on
aluminium surIaces.
!reventing pitting
Pitting is primarily an aesthetic problem that, practically speaking, never aIIects strength.
Attack is, oI course, more severe on untreated aluminium. SurIace treatment (anodising, painting
or other coating methods) counteracts pitting.
Cleaning is necessary to maintain the treated surIace`s attractive appearance and its corrosion
protection. Rinsing with water is oIten suIIicient. Alkaline detergents should be used with care.
Mild alkaline detergents are now available. These are used in, amongst other areas, the industrial
cleaning oI aluminium.
Pitting can be prevented by cathodic protection (above). It is also important to design proIiles so
that they dry easily.
vold angles and pockeLs ln
whlch waLer can collecL
lnsLead use a shape LhaL
promoLes dralnlng
SLagnanL waLer ls avolded by sulLably lncllnlng Lhe proflle
and/or provldlng draln holes 1he venLllaLlon of closed
consLrucLlons reduces Lhe rlsk of condensaLlon
Vous aimerez peut-être aussi
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (120)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Where To Eat PizzaDocument3 pagesWhere To Eat PizzaLiliancitaLcJsPas encore d'évaluation
- Psychological Attitude Towards SafetyDocument17 pagesPsychological Attitude Towards SafetyAMOL RASTOGI 19BCM0012Pas encore d'évaluation
- 4 FAR EAST BANK & TRUST COMPANY V DIAZ REALTY INCDocument3 pages4 FAR EAST BANK & TRUST COMPANY V DIAZ REALTY INCDaniellePas encore d'évaluation
- B&G 3DX LiteratureDocument2 pagesB&G 3DX LiteratureAnonymous 7xHNgoKE6ePas encore d'évaluation
- Chapter 9 Audit SamplingDocument47 pagesChapter 9 Audit SamplingYenelyn Apistar CambarijanPas encore d'évaluation
- MIami Beach City Attorney's DenialDocument7 pagesMIami Beach City Attorney's DenialDavid Arthur WaltersPas encore d'évaluation
- Mind Mapping BIOTEKDocument1 pageMind Mapping BIOTEKAdrian Muhammad RonalPas encore d'évaluation
- Alfa Laval Plate Heat Exchangers: A Product Catalogue For Comfort Heating and CoolingDocument8 pagesAlfa Laval Plate Heat Exchangers: A Product Catalogue For Comfort Heating and CoolingvictoryanezPas encore d'évaluation
- 25 - Marketing Channels - Value Networks.Document2 pages25 - Marketing Channels - Value Networks.zakavision100% (1)
- 201183-B-00-20 Part ListDocument19 pages201183-B-00-20 Part ListMohamed IsmailPas encore d'évaluation
- Order To Cash Cycle Group 1Document4 pagesOrder To Cash Cycle Group 1AswinAniPas encore d'évaluation
- BBI2002 SCL 7 WEEK 8 AdamDocument3 pagesBBI2002 SCL 7 WEEK 8 AdamAMIRUL RIDZLAN BIN RUSIHAN / UPMPas encore d'évaluation
- Item No. 6 Diary No 6856 2024 ConsolidatedDocument223 pagesItem No. 6 Diary No 6856 2024 Consolidatedisha NagpalPas encore d'évaluation
- Phet Body Group 1 ScienceDocument42 pagesPhet Body Group 1 ScienceMebel Alicante GenodepanonPas encore d'évaluation
- ShinojDocument4 pagesShinojArish BallanaPas encore d'évaluation
- Learning Activity Sheet Science 10 Second Quarter - Week 8Document4 pagesLearning Activity Sheet Science 10 Second Quarter - Week 8Eller Jansen AnciroPas encore d'évaluation
- Suggested Answers Spring 2015 Examinations 1 of 8: Strategic Management Accounting - Semester-6Document8 pagesSuggested Answers Spring 2015 Examinations 1 of 8: Strategic Management Accounting - Semester-6Abdul BasitPas encore d'évaluation
- Multiage Education in Small School SettingsDocument19 pagesMultiage Education in Small School SettingsMichelle Ronksley-PaviaPas encore d'évaluation
- CIE Physics IGCSE: General Practical SkillsDocument3 pagesCIE Physics IGCSE: General Practical SkillsSajid Mahmud ChoudhuryPas encore d'évaluation
- CRM Module 1Document58 pagesCRM Module 1Dhrupal TripathiPas encore d'évaluation
- Netaji Subhas Open UniversityDocument4 pagesNetaji Subhas Open UniversityraydipanjanPas encore d'évaluation
- The Financing Cycle Summary, Case Study, AssignmentsDocument18 pagesThe Financing Cycle Summary, Case Study, AssignmentsbernadettePas encore d'évaluation
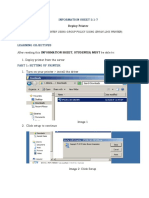
- 3.1-7 Printer Deployment - Copy (Full Permission)Document18 pages3.1-7 Printer Deployment - Copy (Full Permission)Hanzel NietesPas encore d'évaluation
- Claim Age Pension FormDocument25 pagesClaim Age Pension FormMark LordPas encore d'évaluation
- Quiz1 2, PrelimDocument14 pagesQuiz1 2, PrelimKyla Mae MurphyPas encore d'évaluation
- Pa 28 151 161 - mmv1995 PDFDocument585 pagesPa 28 151 161 - mmv1995 PDFJonatan JonatanBernalPas encore d'évaluation
- General Mathematics 2nd Quarter ExamDocument3 pagesGeneral Mathematics 2nd Quarter ExamDeped TambayanPas encore d'évaluation
- HR Q and ADocument87 pagesHR Q and Asanjeeb88Pas encore d'évaluation
- (Rajagopal) Brand Management Strategy, Measuremen (BookFi) PDFDocument317 pages(Rajagopal) Brand Management Strategy, Measuremen (BookFi) PDFSneha SinghPas encore d'évaluation
- Riba, Its Types and ImplicationsDocument37 pagesRiba, Its Types and Implicationsmahamamir012Pas encore d'évaluation