Académique Documents
Professionnel Documents
Culture Documents
Water Pollution Is The Contamination of
Transféré par
moham_idrisDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Water Pollution Is The Contamination of
Transféré par
moham_idrisDroits d'auteur :
Formats disponibles
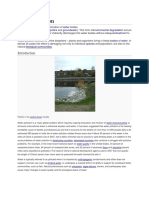
Water pollution Water pollution is the contamination of water bodies (e.g. lakes, rivers, oceans andgroundwater).
Water pollution occurs when pollutants are discharged directly or indirectly into water bodies without adequate treatment to remove harmful compounds. Water pollution affects plants and organisms living in these bodies of water. In almost all cases the effect is damaging not only to individual species and populations, but also to the naturalbiological communities. Introduction Millions depend on the polluted Gangesriver Water pollution is a major global problem which requires ongoing evaluation and revision of water resource policy at all levels (international down to individual aquifers and wells). It has been suggested that it is the leading worldwide cause of deaths and diseases,[1][2] and that it accounts for the deaths of more than 14,000 people daily. [2] An estimated 700 million Indians have no access to a proper toilet, and 1,000 Indian children die of diarrheal sickness every day.[3] Some 90% of China's cities suffer from some degree of water pollution,[4] and nearly 500 million people lack access to safe drinking water.[5] In addition to the acute problems of water pollution indeveloping countries, developed countries continue to struggle with pollution problems as well. In the most recent national report on water quality in the United States, 45 percent of assessedstream miles, 47 percent of assessed lake acres, and 32 percent of assessed bay and estuarinesquare miles were classified as polluted.[6] [edit]Categories Surface water and groundwater have often been studied and managed as separate resources, although they are interrelated.[7] Surface water seeps through the soil and becomes groundwater. Conversely, groundwater can also feed surface water sources. Sources of surface water pollution are generally grouped into two categories based on their origin. Point sources Point source water pollution refers to contaminants that enter a waterway from a single, identifiable source, such as a pipe or ditch. Examples of sources in this category include discharges from asewage treatment plant, a factory, or a city storm drain. The
U.S. Clean Water Act (CWA) defines point source for regulatory enforcement purposes.[8] The CWA definition of point source was amended in 1987 to include municipal storm sewer systems, as well as industrial stormwater, such as from construction sites.[9] [edit]Nonpoint sources Nonpoint source pollution refers to diffuse contamination that does not originate from a single discrete source. NPS pollution is often the cumulative effect of small amounts of contaminants gathered from a large area. A common example is the leaching out of nitrogen compounds from fertilized agricultural lands. Nutrient runoff in stormwater from "sheet flow" over an agricultural fieldor a forest are also cited as examples of NPS pollution. Contaminated storm water washed off of parking lots, roads and highways, called urban runoff, is sometimes included under the category of NPS pollution. However, this runoff is typically channeled into storm drain systems and discharged through pipes to local surface waters, and is a point source. However where such water is not channeled and drains directly to ground it is a non-point source. [edit]Groundwater pollution Interactions between groundwater and surface water are complex. Consequently, groundwater pollution, sometimes referred to asgroundwater contamination, is not as easily classified as surface water pollution.[7] By its very nature, groundwater aquifers are susceptible to contamination from sources that may not directly affect surface water bodies, and the distinction of point vs. non-point source may be irrelevant. A spill or ongoing releases of chemical or radionuclide contaminants into soil (located away from a surface water body) may not create point source or nonpoint source pollution, but can contaminate the aquifer below, defined as a toxin plume. The movement of the plume, called a plume front, may be analyzed through a hydrological transport model or groundwater model. Analysis of groundwater contamination may focus on the soil characteristics and site geology, hydrogeology, hydrology, and the nature of the contaminants. [edit]Causes The specific contaminants leading to pollution in water include a wide spectrum of chemicals, pathogens, and physical or sensory changes such as elevated temperature and discoloration. While many of the chemicals and substances that are
regulated may be naturally occurring (calcium, sodium, iron, manganese, etc.) the concentration is often the key in determining what is a natural component of water, and what is a contaminant. High concentrations of naturally-occurring substances can have negative impacts on aquatic flora and fauna. Oxygen-depleting substances may be natural materials, such as plant matter (e.g. leaves and grass) as well as man-made chemicals. Other natural and anthropogenic substances may cause turbidity (cloudiness) which blocks light and disrupts plant growth, and clogs the gills of some fish species.[10] Many of the chemical substances are toxic. Pathogens can produce waterborne diseases in either human or animal hosts.[11] Alteration of water's physical chemistry includes acidity (change in pH), electrical conductivity, temperature, and eutrophication. Eutrophication is an increase in the concentration of chemical nutrients in an ecosystem to an extent that increases in the primary productivity of the ecosystem. Depending on the degree of eutrophication, subsequent negative environmental effects such as anoxia (oxygen depletion) and severe reductions in water quality may occur, affecting fish and other animal populations. . [edit]Physical testing Common physical tests of water include temperature, solids concentrations (e.g., total suspended solids (TSS)) and turbidity. [edit]Chemical testing See also: water chemistry analysis and environmental chemistry Water samples may be examined using the principles of analytical chemistry. Many published test methods are available for both organic and inorganic compounds. Frequently used methods include pH, biochemical oxygen demand (BOD),[20] chemical oxygen demand(COD),[21] nutrients (nitrate and phosphorus compounds), metals (including copper, zinc, cadmium, lead and mercury), oil and grease, total petroleum hydrocarbons (TPH), and pesticides.
Air pollution Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere. The atmosphere is a complex dynamic natural gaseous system that is essential to support life on planet Earth. Stratospheric ozone depletion due to air pollution has long been recognized as a threat to human health as well as to the Earth's ecosystems. Indoor air pollution and urban air quality are listed as two of the world's worst pollution problems in the 2008 Blacksmith Institute World's Worst Polluted Places report.[1] Pollutants Before flue gas desulfurization was installed, the emissions from this power plant in New Mexicocontained excessive amounts of sulfur dioxide. Schematic drawing, causes and effects of air pollution: (1) greenhouse effect, (2) particulate contamination, (3) increased UV radiation, (4) acid rain, (5) increased ground level ozone concentration, (6) increased levels of nitrogen oxides. A substance in the air that can cause harm to humans and the environment is known as an air pollutant. Pollutants can be in the form of solid particles, liquid droplets, or gases. In addition, they may be natural or man-made.[2] Pollutants can be classified as primary or secondary. Usually, primary pollutants are directly emitted from a process, such as ash from a volcanic eruption, the carbon monoxidegas from a motor vehicle exhaust or sulfur dioxide released from factories. Secondary pollutants are not emitted directly. Rather, they form in the air when primary pollutants react or interact. An important example of a secondary pollutant is ground level ozone one of the many secondary pollutants that make up photochemical smog. Some pollutants may be both primary and secondary: that is, they are both emitted directly and formed from other primary pollutants. Major primary pollutants produced by human activity include:
Sulfur oxides (SOx) - especially sulfur dioxide, a chemical compound with the formula SO2. SO2 is produced by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain.[2] This is one of the causes
for concern over the environmental impact of the use of these fuels as power sources.
Nitrogen oxides (NOx) - especially nitrogen dioxide are emitted from high temperature combustion, and are also produced naturally duringthunderstorms by electrical discharge. Can be seen as the brown haze dome above or plume downwind of cities. Nitrogen dioxide is the chemical compound with the formula NO2. It is one of the several nitrogen oxides. This reddish-brown toxic gas has a characteristic sharp, biting odor. NO2 is one of the most prominent air pollutants. Carbon monoxide - is a colourless, odorless, non-irritating but very poisonous gas. It is a product by incomplete combustion of fuel such as natural gas, coal or wood. Vehicular exhaust is a major source of carbon monoxide. Carbon dioxide (CO2) - a colourless, odorless, non-toxic greenhouse gas also associated with ocean acidification, emitted from sources such as combustion, cement production, and respiration. It is otherwise recycled in the atmosphere in the carbon cycle. Volatile organic compounds - VOCs are an important outdoor air pollutant. In this field they are often divided into the separate categories of methane (CH4) and nonmethane (NMVOCs). Methane is an extremely efficient greenhouse gas which contributes to enhanced global warming. Other hydrocarbon VOCs are also significant greenhouse gases via their role in creating ozone and in prolonging the life of methane in the atmosphere, although the effect varies depending on local air quality. Within the NMVOCs, the aromatic compounds benzene, toluene and xylene are suspected carcinogens and may lead to leukemia through prolonged exposure. 1,3-butadiene is another dangerous compound which is often associated with industrial uses.
Particulate matter - Particulates, alternatively referred to as particulate matter (PM) or fine particles, are tiny particles of solid or liquid suspended in a gas. In contrast, aerosol refers to particles and the gas together. Sources of particulate matter can be man made or natural. Some particulates occur naturally, originating from volcanoes, dust storms, forest and grassland fires, living vegetation, and sea spray. Human activities, such as the burning of fossil fuels in vehicles, power plants and various industrial processes also generate significant amounts of aerosols. Averaged over the globe, anthropogenic aerosolsthose made by human activitiescurrently
account for about 10 percent of the total amount of aerosols in our atmosphere. Increased levels of fine particles in the air are linked to health hazards such as heart disease,[3] altered lung function and lung cancer.
Persistent free radicals connected to airborne fine particles could cause cardiopulmonary disease.[4][5] Toxic metals, such as lead, cadmium and copper. Chlorofluorocarbons (CFCs) - harmful to the ozone layer emitted from products currently banned from use. Ammonia (NH3) - emitted from agricultural processes. Ammonia is a compound with the formula NH3. It is normally encountered as a gas with a characteristic pungent odor. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to foodstuffs and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceuticals. Although in wide use, ammonia is both caustic and hazardous.
Odors such as from garbage, sewage, and industrial processes Radioactive pollutants - produced by nuclear explosions, nuclear events, war explosives, and natural processes such as the radioactive decay of radon.
Secondary pollutants include:
Particulate matter formed from gaseous primary pollutants and compounds in photochemical smog. Smog is a kind of air pollution; the word "smog" is a portmanteau of smoke and fog. Classic smog results from large amounts of coal burning in an area caused by a mixture of smoke and sulfur dioxide. Modern smog does not usually come from coal but from vehicular and industrial emissions that are acted on in the atmosphere by ultraviolet light from the sun to form secondary pollutants that also combine with the primary emissions to form photochemical smog.
Ground level ozone (O3) formed from NOx and VOCs. Ozone (O3) is a key constituent of the troposphere. It is also an important constituent of certain regions of the stratosphere commonly known as the Ozone layer. Photochemical and chemical reactions involving it drive many of the chemical processes that occur in the atmosphere by day and by night. At abnormally high concentrations brought about by human activities (largely the combustion of fossil fuel), it is a pollutant, and a constituent of smog.
Vous aimerez peut-être aussi
- Point Sources: Water Resource PolicyDocument11 pagesPoint Sources: Water Resource PolicyL.y. IshwaryaPas encore d'évaluation
- Pollution Document AnalysisDocument14 pagesPollution Document AnalysisMahendra Singh DhoniPas encore d'évaluation
- Bodies of Water Species Biological CommunitiesDocument8 pagesBodies of Water Species Biological CommunitiesVit 1997Pas encore d'évaluation
- Water Pollution: From Wikipedia, The Free EncyclopediaDocument15 pagesWater Pollution: From Wikipedia, The Free EncyclopediaAlok VishwakarmaPas encore d'évaluation
- Anjakan ParadigmaDocument3 pagesAnjakan ParadigmaLuakout ZukrinjaPas encore d'évaluation
- Water Pollution Is The Contamination ofDocument7 pagesWater Pollution Is The Contamination ofkrishna98709Pas encore d'évaluation
- Various Types of PollutionDocument4 pagesVarious Types of PollutionShubham DuaPas encore d'évaluation
- (Type The Company Name) (Type The Company Address) (Type The Phone Number) (Type The Fax Number)Document34 pages(Type The Company Name) (Type The Company Address) (Type The Phone Number) (Type The Fax Number)meenketanPas encore d'évaluation
- Water Pollution Is The Contamination ofDocument36 pagesWater Pollution Is The Contamination ofsuradotPas encore d'évaluation
- Jhadeswar Institute of Engineering & Technology: Project Name:-Global WarmingDocument7 pagesJhadeswar Institute of Engineering & Technology: Project Name:-Global WarmingAmarjit SankhuaPas encore d'évaluation
- Air Pollution: Air Is Mainly A Mixture of Various Gases Such As Oxygen, Carbon DioxideDocument14 pagesAir Pollution: Air Is Mainly A Mixture of Various Gases Such As Oxygen, Carbon Dioxidenidhichoudhary1305Pas encore d'évaluation
- Water PollutionDocument13 pagesWater PollutionvaradanPas encore d'évaluation
- New Docewument Microsoft Office WordDocument4 pagesNew Docewument Microsoft Office WordPaula PasarePas encore d'évaluation
- Major Forms and Sources of PollutionDocument4 pagesMajor Forms and Sources of PollutionakrambashirPas encore d'évaluation
- Forms of PollutionDocument3 pagesForms of PollutionMahesh TejaniPas encore d'évaluation
- Water Pollution Categories: Millions Depend On The PollutedDocument43 pagesWater Pollution Categories: Millions Depend On The PollutedthanarubanPas encore d'évaluation
- Water Pollution: Water Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans, Groundwater)Document15 pagesWater Pollution: Water Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans, Groundwater)rounzeetPas encore d'évaluation
- Water Pollution Is The Contamination of Water Bodies, Usually As ADocument11 pagesWater Pollution Is The Contamination of Water Bodies, Usually As AS V ENTERPRISESPas encore d'évaluation
- Air Pollution Is The Introduction ofDocument4 pagesAir Pollution Is The Introduction ofGuru MurthyPas encore d'évaluation
- Air PollutionDocument18 pagesAir PollutionsuryanshdundirwadePas encore d'évaluation
- Assignment Water PollutionDocument12 pagesAssignment Water PollutionM ImranPas encore d'évaluation
- Water Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans andDocument37 pagesWater Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans andFreetheist RizviPas encore d'évaluation
- Ancient Cultures: Pollution Is The Introduction ofDocument3 pagesAncient Cultures: Pollution Is The Introduction ofbbhoeePas encore d'évaluation
- Water Pollution: Water Lakes Rivers Oceans Aquifers Groundwater Environmental Degradation Pollutants TreatmentDocument11 pagesWater Pollution: Water Lakes Rivers Oceans Aquifers Groundwater Environmental Degradation Pollutants TreatmentSai RajPas encore d'évaluation
- Point Sources: Water Pollution Is The Contamination ofDocument4 pagesPoint Sources: Water Pollution Is The Contamination ofShubham DuaPas encore d'évaluation
- Water Pollution NotesDocument9 pagesWater Pollution Notessantiagofaye100% (2)
- Water PollutionDocument12 pagesWater PollutionSrikar KuthuruPas encore d'évaluation
- Pollution and Resource DepletionDocument10 pagesPollution and Resource Depletionrabz03Pas encore d'évaluation
- Types of PollutionDocument12 pagesTypes of PollutionAavika MishraPas encore d'évaluation
- Sources: Air Pollution Occurs When Harmful Substances IncludingDocument7 pagesSources: Air Pollution Occurs When Harmful Substances IncludingramasamyPas encore d'évaluation
- Water Pollution: Water Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans andDocument4 pagesWater Pollution: Water Pollution Is The Contamination of Water Bodies (E.g. Lakes, Rivers, Oceans andfaizanulhaquePas encore d'évaluation
- Water PollutionDocument23 pagesWater PollutionHimanshu DubeyPas encore d'évaluation
- Water Pollution - WikipediaDocument42 pagesWater Pollution - WikipediaChandrakala ShivakumarPas encore d'évaluation
- Air, water, soil and noise pollution: Causes and effectsDocument3 pagesAir, water, soil and noise pollution: Causes and effectsJared AllenPas encore d'évaluation
- Folio Biology Tingkatan 4Document25 pagesFolio Biology Tingkatan 4queen mira100% (1)
- Environmental Pollution Types and CausesDocument8 pagesEnvironmental Pollution Types and CausesAyush KarmakarPas encore d'évaluation
- PollutantsDocument95 pagesPollutantsAnonymous OXAn33rq100% (1)
- Chapter 1Document43 pagesChapter 1Abel MulugetaPas encore d'évaluation
- Asssesment Sheet (Autorecovered)Document22 pagesAsssesment Sheet (Autorecovered)ABHISHEK RAWATPas encore d'évaluation
- Asssesment Sheet (Autorecovered)Document22 pagesAsssesment Sheet (Autorecovered)ABHISHEK RAWATPas encore d'évaluation
- Water Pollution ReportDocument13 pagesWater Pollution Reportkishore neelagandaPas encore d'évaluation
- Water PollutionDocument13 pagesWater PollutionkiranPas encore d'évaluation
- Pollution and Enviromental DegradationDocument19 pagesPollution and Enviromental DegradationMORTAL SPas encore d'évaluation
- Pollution Is TH-WPS OfficeDocument3 pagesPollution Is TH-WPS OfficeRonweL OcsonaPas encore d'évaluation
- Water PollutionDocument16 pagesWater PollutionBelha BejiPas encore d'évaluation
- Campus Ecology PGB-4331 Assignment-Ii: K.Santhosh Kumar 23PEC23Document13 pagesCampus Ecology PGB-4331 Assignment-Ii: K.Santhosh Kumar 23PEC23logeshkannan078Pas encore d'évaluation
- Public Health Assignment on Water Pollution and Global WarmingDocument21 pagesPublic Health Assignment on Water Pollution and Global WarmingMushfiqur RahmanPas encore d'évaluation
- Lec#01 Environment Pollution ControlDocument28 pagesLec#01 Environment Pollution ControlYasir ShaikhPas encore d'évaluation
- Air Pollution Causes: Anthropogenic Sources (Human Activity) Mostly Related To Burning Different Kinds ofDocument6 pagesAir Pollution Causes: Anthropogenic Sources (Human Activity) Mostly Related To Burning Different Kinds ofSarvesh JaiswalPas encore d'évaluation
- Pollution: OllutionDocument7 pagesPollution: OllutionPhilobatir SamehPas encore d'évaluation
- Tugas Bahasa Inggris Factual Report Theme Is PollutionDocument18 pagesTugas Bahasa Inggris Factual Report Theme Is PollutionRifka AmaliaPas encore d'évaluation
- Definition: The Branch of Science Concerned With The Physical, Chemical, and Biological Conditions of The Environment and Their Effect On OrganismsDocument24 pagesDefinition: The Branch of Science Concerned With The Physical, Chemical, and Biological Conditions of The Environment and Their Effect On OrganismsAmritha ManivannanPas encore d'évaluation
- Wiki Grazing 4Document8 pagesWiki Grazing 4WolfMensch1216Pas encore d'évaluation
- Pollution Is The Introduction of Contaminants Into The Natural Environment That Cause Adverse ChangeDocument8 pagesPollution Is The Introduction of Contaminants Into The Natural Environment That Cause Adverse ChangeJatin SaxenaPas encore d'évaluation
- Lecture 14 Water Pollution-BDocument20 pagesLecture 14 Water Pollution-BMuhammad Saad KhokharPas encore d'évaluation
- EST (25 To 30) Micro ProjectDocument12 pagesEST (25 To 30) Micro ProjectVishal JadhavPas encore d'évaluation
- Environmental PollutionDocument19 pagesEnvironmental Pollutionchinedumpeters31Pas encore d'évaluation
- Environmental PollutionDocument14 pagesEnvironmental Pollutionyessi87Pas encore d'évaluation
- EARN MILLIONS WITH FAST-GROWING MELIA DUBIA TREEDocument15 pagesEARN MILLIONS WITH FAST-GROWING MELIA DUBIA TREEmoham_idrisPas encore d'évaluation
- Ec1008 High Speed Networks n9 r4 r5Document3 pagesEc1008 High Speed Networks n9 r4 r5moham_idrisPas encore d'évaluation
- Melia Dubia-Fast GrowingDocument7 pagesMelia Dubia-Fast Growingmoham_idrisPas encore d'évaluation
- Marigold 5 AcresDocument4 pagesMarigold 5 Acresmoham_idrisPas encore d'évaluation
- Answ Er Key: Hints & Solutions (Practice Paper-3)Document24 pagesAnsw Er Key: Hints & Solutions (Practice Paper-3)SaiyanfistPas encore d'évaluation
- Melia Dubea BrouchureDocument2 pagesMelia Dubea Brouchuremoham_idrisPas encore d'évaluation
- Nithil BirthdayDocument1 pageNithil BirthdayIdriBcPas encore d'évaluation
- ThirukkuralDocument67 pagesThirukkurallathapriya12375% (4)
- AffidavitDocument1 pageAffidavitmoham_idrisPas encore d'évaluation
- MABSDocument1 pageMABSmoham_idrisPas encore d'évaluation
- BE ProjectsDocument47 pagesBE Projectsmoham_idrisPas encore d'évaluation
- ARI Varmam Magazine SubscriptionDocument2 pagesARI Varmam Magazine Subscriptionmoham_idrisPas encore d'évaluation
- Pump Course Material Chapter 2Document16 pagesPump Course Material Chapter 2engr victorPas encore d'évaluation
- GERD – Definition, pathophysiology, epidemiology and diagnostic investigationsDocument132 pagesGERD – Definition, pathophysiology, epidemiology and diagnostic investigationsSorana VasilescuPas encore d'évaluation
- Solid Waste ManagementDocument26 pagesSolid Waste ManagementPamela MendozaPas encore d'évaluation
- Shanidham - In-Shanidham Pacify Lord ShaniDocument3 pagesShanidham - In-Shanidham Pacify Lord ShanisubramanyaPas encore d'évaluation
- State of The Art Penelitian - Chat GPT 2023Document137 pagesState of The Art Penelitian - Chat GPT 2023restyPas encore d'évaluation
- Buddhism Beyond ReligionDocument7 pagesBuddhism Beyond ReligionCarlos A SanchesPas encore d'évaluation
- Tennis BiomechanicsDocument14 pagesTennis BiomechanicsΒασίλης Παπατσάς100% (1)
- Dimensional Analysis Similarity Lesson2 Dimensional Parameters HandoutDocument11 pagesDimensional Analysis Similarity Lesson2 Dimensional Parameters HandoutRizqi RamadhanPas encore d'évaluation
- UPSC IFS Botany Syllabus: Paper - IDocument3 pagesUPSC IFS Botany Syllabus: Paper - IVikram Singh ChauhanPas encore d'évaluation
- Hot Tub BrochureDocument124 pagesHot Tub BrochureMai Tuan AnhPas encore d'évaluation
- Fendering For Tugs: Mike Harrison, Trelleborg Marine Systems, UKDocument5 pagesFendering For Tugs: Mike Harrison, Trelleborg Marine Systems, UKRizal RachmanPas encore d'évaluation
- Tec Relay 52GDocument3 pagesTec Relay 52Gimmer nainggolanPas encore d'évaluation
- 35.2 - ING - El Puente NewsletterDocument13 pages35.2 - ING - El Puente NewsletterIrmali FrancoPas encore d'évaluation
- BELL B40C - 872071-01 Section 2 EngineDocument38 pagesBELL B40C - 872071-01 Section 2 EngineALI AKBAR100% (1)
- Forecasting ExercisesDocument2 pagesForecasting ExercisesAsh VinaPas encore d'évaluation
- NitrocelluloseDocument7 pagesNitrocellulosejumpupdnbdjPas encore d'évaluation
- Abundance BlocksDocument1 pageAbundance BlockssunnyPas encore d'évaluation
- CH 10Document125 pagesCH 10Lisset Soraya Huamán QuispePas encore d'évaluation
- Progressing Cavity Pump Overhaul GuideDocument5 pagesProgressing Cavity Pump Overhaul Guidesdsds-54Pas encore d'évaluation
- Thank You For Taking The Week 3: Assignment 3. Week 3: Assignment 3Document3 pagesThank You For Taking The Week 3: Assignment 3. Week 3: Assignment 3DhivyaPas encore d'évaluation
- Qand ADocument5 pagesQand AJoshua PascasioPas encore d'évaluation
- Termites and Microbial Biological Control StrategiesDocument30 pagesTermites and Microbial Biological Control StrategiesMuhammad QasimPas encore d'évaluation
- Kuffner Final PresentationDocument16 pagesKuffner Final PresentationSamaa GamalPas encore d'évaluation
- Chapter 7 (Additional Notes) Thermodynamics Review (Power Plant Technology by M Wakil)Document29 pagesChapter 7 (Additional Notes) Thermodynamics Review (Power Plant Technology by M Wakil)Aries SattiPas encore d'évaluation
- IotDocument88 pagesIotLalithyaPas encore d'évaluation
- CSC-1321 Gateway User Guide: Downloaded From Manuals Search EngineDocument48 pagesCSC-1321 Gateway User Guide: Downloaded From Manuals Search EngineKislan MislaPas encore d'évaluation
- Plow Moldboard or Disc PlowDocument7 pagesPlow Moldboard or Disc PlowAdewalePas encore d'évaluation
- Presentation 123Document13 pagesPresentation 123Harishitha ManivannanPas encore d'évaluation
- Overlord - Volume 01 - The Undead KingDocument223 pagesOverlord - Volume 01 - The Undead KingPaulo FordheinzPas encore d'évaluation
- Forklift Truck Risk AssessmentDocument2 pagesForklift Truck Risk AssessmentAshis Das100% (1)