Académique Documents
Professionnel Documents
Culture Documents
Lab Report 2
Transféré par
haroonqaiserDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Lab Report 2
Transféré par
haroonqaiserDroits d'auteur :
Formats disponibles
1 | Page
TABLE OF CONTENT:
1. Literature review
1.1Definition of Thermodynamics 1.2First law of thermodynamics 1.3Isothermal process 1.4Adiabatic process 1.5Ideal gas laws 1.5.1. Boyles law 1.5.2. Charles law 1.6Absolute zero 1.7Entropy and 2nd law of thermodynamics 1.8Thermocouple 1.9Piezoelectric material
2. Apparatus 3. Procedure 4. Analysis 5. Results and conclusions 6. Reasons of errors 7. References
2 | Page
1. Literature review:
1.1. Definition of thermodynamics:
It studies the effect of transfer of heat and the work done on or by the body or radiations on the material bodies.
1.2. 1st law of thermodynamics:
A change in internal energy of a closed thermodynamic system is equal to the differance between the heat supplied to the system and the amount of work done by the system on its surrounding. A more succinct and comprehensible definition might be something like this: Matter/energy may be altered, but not created or destroyed. It teaches that matter/energy cannot spring forth from nothing without cause, nor can it simply vanish.[1]
1.3. Isothermal process:
An isothermal process is a change of a system, in which the temperature remains constant: T = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath), and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir through heat exchange.[2] In an isothermal process, the value T = 0 but Q 0
1.4. Adiabatic process:
An adiabatic process or an isocaloric process is a thermodynamic net heat transfer to or from the working fluid is zero.[3]An adiabatic
process is where a system exchanges no heat with its surroundings (Q = 0). In other words, while in an adiabatic process, T 0 but Q = 0.
process in
which
the
1.5. Ideal gas laws:
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It is a combination of boyles law and Charles law. 1.5.1.Boyles law: Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system. Its mathematical equation is given as
v1p
1.5.2.Charles law: Charles law is stated as At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on
3 | Page the absolute temperature scale (i.e. the gas expands as the temperature increases).[4] which can be written as: where V is the volume of the gas; and T is the absolute temperature. The law can also be usefully expressed as follows:
The equation shows that, as absolute temperature increases, the volume of the gas also increases in proportion.
1.6. Absolute zero:
Temperature at which a thermodynamic system has the lowest energy, 0 kelvin (K). It corresponds to -459.67F (-273.15C) and is the lowest possible temperature theoretically achievable by a system. A gas at constant pressure contracts as the temperature is decreased. A perfect gas would reach zero volume at absolute zero. However, a real gas condenses to a liquid or a solid at a temperature higher than absolute zero. At absolute zero, the system's molecular energy is minimal and none is available for transfer to other systems. The Kelvin temperature scale has absolute zero as its zero point, and its fundamental unit is the kelvin.
[5]
1.7. Entropy and 2nd law of thermodynamics:
2nd law states that Heat cannot spontaneously flow from a colder location to a hotter location. In tern of entropy 2nd law states that: Entropy always increases or remains constant in a closed system. The entropy of an entire closed system can never decrease within that system.[1] Entropy explains the irreversibility of nature. It has the minimum value at absolute zero
1.8. Thermocouple:
It is a device consisting of two different conductors (usually metal alloys) that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a heat gradient into electricity. In contrast to most other methods of temperature measurement, thermocouples are self powered and require no external form of excitation.[6]
4 | Page
Fig 1.1 Thermocouple plugged to a multimeter displaying room temperature in C.[6]
1.9. Piezoelectric material:
A material that generates an electric charge when mechanically deformed. Conversely, when an external electric field is applied to piezoelectric materials they mechanically deform.[7]
2. Apparatus:
Heating plate Connecting tube Stand Thermocouple Multimeter Teflon tape Syringe Flask with a cork
1. Procedure:
The apparatus was set. Thermocouple was plugged into the cork of the flask and the flask was made air tight by using the Teflon tape. The syringe and the flask were connected by using the connecting tube. And this apparatus was then clamped in the stand as shown in fig. The thermocouple was plugged in the multimeter so that it can show the temperature measurements due to pressure changes in the flask. The water was boiled in the beaker by placing it on the heating plate. When the water started boiling, the flask was dipped in it.
5 | Page
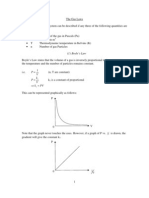
Fig 1.2 apparatus setup of verification of Charles law
2. Analysis:
As soon as the flask was dipped into the boiling water, it was noticed that the volume in the syringe tube started increasing and the temperature readings at that instant were noted. By using this procedure two reading were taken. As the volume started increasing, the temperature also showed a linear increase which verified the Charles law i.e. volume is directly proportion to temperature.
3. Results and conclusions:
Initial temperature =T1=293.15K Initial volume =V1=50cm3 Final temperature =T2=313.15K Final volume =V2=54cm3
6 | Page
Fig1.3 Linear graph representation, verifying Charles law Now, V1T1=V2T2 By placing values in above equation, we get: 50293.15=54313.15 0.170 = 0.172 Hence, the almost equal values on both sides of the above equation clearly verify Charles law.
4. Reasons of errors:
Two errors were observed during the performance of above experiment due to which the graph intercepts the x-axis at -10K.these errors are given below: The flask was not properly air tight. The syringe was not frictionless. The volume measurements were not taken immediately after taking the temperature measurements. There was a difference of a few or more seconds.
7 | Page
1. References:
[1]-http://thermodynamicstudy.net/first.html [2]-http://en.wikipedia.org/wiki/Isothermal_process [3]- http://en.wikipedia.org/wiki/Adiabatic_process [4]- http://en.wikipedia.org/wiki/Charles%27s_law [5]- http://www.answers.com/topic/absolute-zero [6]- http://en.wikipedia.org/wiki/Thermocouple [7]http://chemistry.about.com/od/chemistryglossary/a/piezoelectricdf.htm
Vous aimerez peut-être aussi
- Heat Power Engineering NotesDocument10 pagesHeat Power Engineering NotesDimple Pawan Saini100% (1)
- TPM Notes Final-2 PDFDocument247 pagesTPM Notes Final-2 PDFamarparimiPas encore d'évaluation
- Marcet BoilerDocument25 pagesMarcet BoilerNaveen Footy100% (1)
- Thermodynamic Fundamentals PDFDocument27 pagesThermodynamic Fundamentals PDFZayn AhmedPas encore d'évaluation
- Thermodynamic Fundamentals of Refrigeration, Cryogenics and Low Temperature Physics Problems For ColloquiumDocument27 pagesThermodynamic Fundamentals of Refrigeration, Cryogenics and Low Temperature Physics Problems For ColloquiumPawel WPas encore d'évaluation
- Properties Measurement/pvtDocument22 pagesProperties Measurement/pvtNurwani Hussin87% (15)
- Gas Expansion Lab Experiment 2 EH243 3C Group 3Document24 pagesGas Expansion Lab Experiment 2 EH243 3C Group 3Madihi NorhadiPas encore d'évaluation
- Specific Heat Experiment AnalysisDocument4 pagesSpecific Heat Experiment AnalysisJag MasterPas encore d'évaluation
- Thermodynamics Class 11 Notes Physics Chapter 12Document7 pagesThermodynamics Class 11 Notes Physics Chapter 12prabhat bhatiPas encore d'évaluation
- HeatDocument4 pagesHeatAnna ShanonPas encore d'évaluation
- Thermodynamic Processes Using Perfect Gas Expansion ApparatusDocument20 pagesThermodynamic Processes Using Perfect Gas Expansion ApparatusPazilah91Pas encore d'évaluation
- Latest Physics Project 2023 Class 12 2.0Document19 pagesLatest Physics Project 2023 Class 12 2.0Tusharr sgrPas encore d'évaluation
- Chapter 7 Temperature and Zeroth Law NewDocument48 pagesChapter 7 Temperature and Zeroth Law NewHarry JakePas encore d'évaluation
- Final ThermodynamicsDocument42 pagesFinal ThermodynamicsMaan LucsPas encore d'évaluation
- Gen. Physics 1 Unit 4 Lesson 12 Thermodynamics, Heat and Temp, Thermal Expansion, Specific Heat Capacity, Heat TransferDocument10 pagesGen. Physics 1 Unit 4 Lesson 12 Thermodynamics, Heat and Temp, Thermal Expansion, Specific Heat Capacity, Heat TransferMary Love JuanicoPas encore d'évaluation
- Engineering Thermodynamics Ce 360: 7.0 Second Law of ThermodynamicsDocument5 pagesEngineering Thermodynamics Ce 360: 7.0 Second Law of ThermodynamicsRyanPas encore d'évaluation
- Thermopdynamics PDFDocument12 pagesThermopdynamics PDFNillPas encore d'évaluation
- Phy-1 Mod-2Document13 pagesPhy-1 Mod-2Estiaque Arifin RishadPas encore d'évaluation
- A Definite Area or Space Where Some Thermodynamic Process Takes Place Is Known AsDocument13 pagesA Definite Area or Space Where Some Thermodynamic Process Takes Place Is Known Asrsankarganesh MECH-HICETPas encore d'évaluation
- Ideal Gas Law and ProcessesDocument8 pagesIdeal Gas Law and ProcessesPhilip Andrei CastorPas encore d'évaluation
- Lecture 2 Thermodynamic LawsDocument27 pagesLecture 2 Thermodynamic LawsRalph SotoPas encore d'évaluation
- CH-4 Thermodynamics Heat Engine CyclesDocument19 pagesCH-4 Thermodynamics Heat Engine CyclessunitbhaumikPas encore d'évaluation
- AnalysisDocument2 pagesAnalysisJosef CatiggayPas encore d'évaluation
- Experiment P2: Bomb Calorimetry: Any Question On This Document ToDocument8 pagesExperiment P2: Bomb Calorimetry: Any Question On This Document TomokilpoPas encore d'évaluation
- Thermodynamics Set TwoDocument14 pagesThermodynamics Set TwoSsemakula AllanPas encore d'évaluation
- Adiabatic DemagDocument17 pagesAdiabatic DemagvijnrajPas encore d'évaluation
- PVT Lab ReportDocument21 pagesPVT Lab ReportaminsubriPas encore d'évaluation
- Calorimetry Is The Science of Measuring The Heat of Chemical Reactions or Physical ChangesDocument10 pagesCalorimetry Is The Science of Measuring The Heat of Chemical Reactions or Physical ChangesBonaventure TuyishimePas encore d'évaluation
- Ch. 17 Biomedical Phy.Document9 pagesCh. 17 Biomedical Phy.Mahmoud Abu MayalehPas encore d'évaluation
- A New Statement of the Second Law Leads to EntropyDocument15 pagesA New Statement of the Second Law Leads to EntropyhusseinPas encore d'évaluation
- Document of Che Chapter 6 NotesDocument10 pagesDocument of Che Chapter 6 NotesEstheruPas encore d'évaluation
- ThermodynamicsDocument83 pagesThermodynamicsRhea BakiPas encore d'évaluation
- Heat and ThermodynamicsDocument15 pagesHeat and ThermodynamicsYasir KhanPas encore d'évaluation
- Thermodynamics and Its Application in RefrigerationDocument8 pagesThermodynamics and Its Application in RefrigerationYin HauPas encore d'évaluation
- Thermodynamics (Chapter-01,2 Paper) : DW PDVDocument21 pagesThermodynamics (Chapter-01,2 Paper) : DW PDVSumon HaiderPas encore d'évaluation
- Thermal PhysicsDocument24 pagesThermal PhysicsSuraj GopaulPas encore d'évaluation
- THERMAL PHYSICS 1 Ideal and Real GasesDocument36 pagesTHERMAL PHYSICS 1 Ideal and Real GasesEDENIPas encore d'évaluation
- Chemical Thermodynamics FinalDocument55 pagesChemical Thermodynamics FinalmymamforeverPas encore d'évaluation
- Part III ENTHALPY, SPONTANEITY, ENTROPY, GIBBSDocument54 pagesPart III ENTHALPY, SPONTANEITY, ENTROPY, GIBBSRalph CimanesPas encore d'évaluation
- Basic Concepts of ThermodynamicsDocument6 pagesBasic Concepts of Thermodynamicssunil kumarPas encore d'évaluation
- Physics Notes Pak CHAPTER - 11 HEATDocument13 pagesPhysics Notes Pak CHAPTER - 11 HEATflyfalcon80% (5)
- Introduction and Basic Concepts: Heat and Mass Transfer: Fundamentals & ApplicationsDocument34 pagesIntroduction and Basic Concepts: Heat and Mass Transfer: Fundamentals & ApplicationsNaveen KumarPas encore d'évaluation
- PVT Lab ReportDocument22 pagesPVT Lab Reportamirul100% (2)
- FALLSEM2020-21 MEE1003 TH VL2020210103023 Reference Material I 31-Jul-2020 First Law of Thermodynamics - IIDocument52 pagesFALLSEM2020-21 MEE1003 TH VL2020210103023 Reference Material I 31-Jul-2020 First Law of Thermodynamics - IIRahul rajelliPas encore d'évaluation
- Heat and Thermodynamics Notes PDFDocument13 pagesHeat and Thermodynamics Notes PDFRishi RajPas encore d'évaluation
- Unit II - Chemical ThermodynamicsDocument26 pagesUnit II - Chemical ThermodynamicshvacsriniPas encore d'évaluation
- Second Law of Thermodynamics For Metallurgical ProcessesDocument45 pagesSecond Law of Thermodynamics For Metallurgical ProcessesgtdomboPas encore d'évaluation
- Laboratory: Submitted To: Mabel YaconDocument16 pagesLaboratory: Submitted To: Mabel YaconMacky NoveraPas encore d'évaluation
- Introduction to Refrigeration and Air ConditioningDocument15 pagesIntroduction to Refrigeration and Air Conditioningfakhar zamanPas encore d'évaluation
- 0-Malcolm J. McPherson (Auth.) - Subsurface Ventilation and Environmental Engineering (1993, Springer Netherlands) - Libgen - LC - Compressed-65-72Document8 pages0-Malcolm J. McPherson (Auth.) - Subsurface Ventilation and Environmental Engineering (1993, Springer Netherlands) - Libgen - LC - Compressed-65-72Elisabet PasunuPas encore d'évaluation
- MUCLecture 2021 112940914Document26 pagesMUCLecture 2021 112940914Noor FarhanPas encore d'évaluation
- Egypowpd-Module2 1Document18 pagesEgypowpd-Module2 1Jas PayaPas encore d'évaluation
- Temp. MeasurementDocument9 pagesTemp. MeasurementtorikPas encore d'évaluation
- Thermodynamics 02Document31 pagesThermodynamics 02Sachin BorsePas encore d'évaluation
- Thermodynamical Fundamental ConceptsDocument25 pagesThermodynamical Fundamental ConceptsKshitij PanditPas encore d'évaluation
- Section-7 Thermodynamic LawsDocument9 pagesSection-7 Thermodynamic LawsTaiga CastañedaPas encore d'évaluation
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4D'Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Pas encore d'évaluation
- 1 The Properties of Gases 1A The Perfect Gas: Answers To Discussion QuestionsDocument21 pages1 The Properties of Gases 1A The Perfect Gas: Answers To Discussion Questions이호준Pas encore d'évaluation
- Physics & Measurement: Peter C A KamDocument333 pagesPhysics & Measurement: Peter C A KamamaryogiPas encore d'évaluation
- CHM 101 - Kinetic Theory 2021 Module B StudentsDocument5 pagesCHM 101 - Kinetic Theory 2021 Module B StudentsDanPas encore d'évaluation
- 7 E's DLL-Gas LawDocument3 pages7 E's DLL-Gas Lawcharish catungalPas encore d'évaluation
- Assignment 1Document3 pagesAssignment 1Jingwei ZhongPas encore d'évaluation
- Class 11 Physics Revision Notes ThermodynamicsDocument35 pagesClass 11 Physics Revision Notes ThermodynamicsSwastika DasPas encore d'évaluation
- States of matter and gas propertiesDocument18 pagesStates of matter and gas propertiesah_16036566Pas encore d'évaluation
- TBR GChem2 OptDocument318 pagesTBR GChem2 OptRamski80% (5)
- CH 5Document63 pagesCH 5MoPas encore d'évaluation
- Charles' Law and the Ideal Gas Law ExperimentDocument7 pagesCharles' Law and the Ideal Gas Law ExperimentshirzPas encore d'évaluation
- States of MatterDocument30 pagesStates of MatterSwapnil MandalPas encore d'évaluation
- Assignment Gaseous State JH Sir-2621Document38 pagesAssignment Gaseous State JH Sir-2621Noob Iplay100% (1)
- Che 4 BookDocument423 pagesChe 4 BookAnonymous PMF8IpBtfPas encore d'évaluation
- Satuan KOnversi PPMVDocument16 pagesSatuan KOnversi PPMVpurwakaadiPas encore d'évaluation
- Physical Chemistry 2nd Edition Ball Solution ManualDocument17 pagesPhysical Chemistry 2nd Edition Ball Solution Manualrobyn96% (27)
- Using Charles' Law and the Ideal Gas Law CHEM101LDocument5 pagesUsing Charles' Law and the Ideal Gas Law CHEM101LmePas encore d'évaluation
- Atkins 10th Ch1 PropertiesOfGasesDocument36 pagesAtkins 10th Ch1 PropertiesOfGasesPınar HıdımoğluPas encore d'évaluation
- aZD HARhN6SPkt3jDocument17 pagesaZD HARhN6SPkt3jBad JinnPas encore d'évaluation
- Fluid 9ed Solution Manual PDFDocument919 pagesFluid 9ed Solution Manual PDFDermz GayosoPas encore d'évaluation
- Ideal gas law application to air propertiesDocument12 pagesIdeal gas law application to air propertiesJuan Pablo ApazaPas encore d'évaluation
- Gaseous StateDocument45 pagesGaseous StateRajesh Das50% (2)
- CHAPTER 4 - State of Matter - Students Version CHM092 (2017)Document179 pagesCHAPTER 4 - State of Matter - Students Version CHM092 (2017)MUHAMMAD LUQMAN HAKIMI MOHD ZAMRIPas encore d'évaluation
- Tutorial experiments on solar energy conversion0.311 W= 11.6%2.67 WEffective area of the solar moduleShort-circuit currentIncident radiant powerElectrical power output at maximum power pointEfficiencyDocument40 pagesTutorial experiments on solar energy conversion0.311 W= 11.6%2.67 WEffective area of the solar moduleShort-circuit currentIncident radiant powerElectrical power output at maximum power pointEfficiencyAmélia MoreiraPas encore d'évaluation
- Termodinamika, Entropy, Dan Energi Dalam 2014Document23 pagesTermodinamika, Entropy, Dan Energi Dalam 2014Deriandra MuhyiddinPas encore d'évaluation
- XI-Chemistry 1,2,3Document64 pagesXI-Chemistry 1,2,3Muslim PhysicistPas encore d'évaluation
- Exam 3 SolutionsDocument9 pagesExam 3 SolutionsYoli ArriagaPas encore d'évaluation
- 4TH Grading Science 10Document36 pages4TH Grading Science 10Mary Grace Jerna Artazo Nozal-Cuadra50% (2)
- ch05 Lecture 8eDocument78 pagesch05 Lecture 8eJeanessa MaralangPas encore d'évaluation
- Chapter 5 Gases and The Kinetic-Molecular Theory: Follow-Up ProblemsDocument74 pagesChapter 5 Gases and The Kinetic-Molecular Theory: Follow-Up Problems원철이Pas encore d'évaluation
- SPE-174009-MS Retrieval of Misfired Perforating Systems From Shallow Well Operations: Potential Thermal Cookoff HazardDocument14 pagesSPE-174009-MS Retrieval of Misfired Perforating Systems From Shallow Well Operations: Potential Thermal Cookoff HazardSajad FalahPas encore d'évaluation